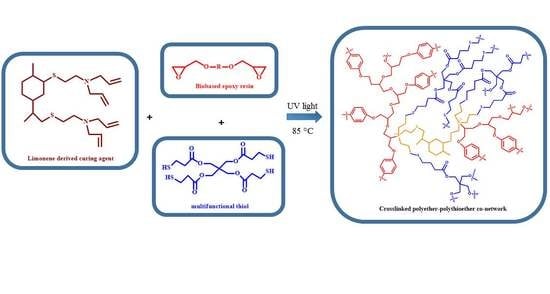
Synthesis of a Curing Agent Derived from Limonene and the Study of Its Performance to Polymerize a Biobased Epoxy Resin Using the Epoxy/Thiol-Ene Photopolymerization Technique
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Synthesis of 2-((2-(3-((2-Aminoethyl) thio)-4Methylcyclohexyl) propyl) thio) ethanamine (LC)
2.3. Synthesis of N-allyl-N-(2-((2-(3-((2-(Diallylamino)ethyl)thio)-4-Methyl cyclohexyl) propyl) thio) ethyl)prop-2-en-1-amine (LCA)
2.4. Determination of the Kinetics of the ETE Photopolymerization Using the Real-Time Fourier Transform Infrared Spectroscopy (RT-FTIR)
2.5. Bulk Photopolymerization of the ETE Formulation
2.6. Differential Scanning Calorimetry (DSC)
2.7. Dynamic Mechanical Analysis (DMA)
2.8. Thermogravimetric Analysis (TGA)
3. Results and Discussion
3.1. Synthesis of Curing Agent LCA
3.2. Kinetics of Photopolymerization by RT-FTIR
3.3. Thermal and Thermomechanical Analysis of Obtained Polymers
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Silva, A.C.Q.; Silvestre, A.J.D.; Vilela, C.; Freire, C.S.R. Natural Polymers-Based Materials: A Contribution to a Greener Future. Molecules 2022, 27, 94. [Google Scholar] [CrossRef] [PubMed]
- Piorkowska, E. Overview of biobased Polymers. Adv. Polym. Sci. 2019, 283, 1–35. [Google Scholar]
- Ganewatta, M.S.; Wang, Z.; Tang, C. Chemical syntheses of bioinspired and biomimetic polymers toward biobased materials. Nat. Rev. Chem. 2021, 5, 753–772. [Google Scholar] [CrossRef]
- Czub, P.; Sienkiewicz, A. Synthesis of biobased epoxy resins. In Bio-Based Epoxy Polymers, Blends, and Composites: Synthesis, Properties, Characterization and Applications; Parameswaranpillai, J., Rangappa, S.M., Siengchin, S.S.J., Eds.; Wiley VCH: Berlin, Germany, 2021; pp. 1–72. [Google Scholar]
- Kumar, S.; Samal, S.K.; Mohanty, S.; Nayak, S.K. Recent development of biobased epoxy resins: A review. Polym. Plast Tech. Eng. 2018, 57, 133–155. [Google Scholar] [CrossRef]
- Mashouf, R.G.; Mohanty, A.K.; Misra, M. Green Approaches To Engineer Tough Biobased Epoxies: A Review. ACS Sustain. Chem. Eng. 2017, 5, 9528–9541. [Google Scholar] [CrossRef]
- Bonamigo Moreira, V.; Rintjema, J.; Bravo, F.; Kleij, A.W.; Franco, L.; Puiggali, J.; Aleman, C.; Armelin, E. Novel Biobased Epoxy Thermosets and Coatings from Poly(limonene carbonate) Oxide and Synthetic Hardeners. ACS Sustain. Chem. Eng. 2022, 10, 2708–2719. [Google Scholar] [CrossRef]
- Feghali, E.; van de Pas, D.J.; Parrott, A.J.; Torr, K.M. Biobased Epoxy Thermoset Polymers from Depolymerized Native Hardwood Lignin. ACS Macro Lett. 2020, 9, 1155–1160. [Google Scholar] [CrossRef]
- Li, W.; Xiao, L.; Wang, Y.; Chen, J.; Nie, X. Self-healing silicon-containing eugenol-based epoxy resin based on disulfide bond exchange: Synthesis and structure-property relationships. Polymer 2021, 229, 123967. [Google Scholar] [CrossRef]
- Goncalves, F.A.M.M.; Ferreira, P.; Alvez, P. Synthesis and characterization of itaconic-based epoxy resin: Chemical and thermal properties of partially biobased epoxy resins. Polymer 2021, 235, 124285. [Google Scholar] [CrossRef]
- Fang, Z.; Nikafshar, S.; Hegg, E.L.; Nejad, M. Biobased Divanillin as a precursor for Formulating Biobased Epoxy Resin. ACS Sustain. Chem. Eng. 2020, 8, 9095–9103. [Google Scholar] [CrossRef]
- Caillol, S.; Boutevin, B.; Auvergne, R. Eugenol, a developing asset in biobased epoxy resins. Polymer 2021, 223, 123663. [Google Scholar] [CrossRef]
- Nikafshar, S.; Zabihi, O.; Hamid, S.; Moradi, Y.; Barzegar, S.; Ahmadi, M.; Naebe, M. A renewable bio-based epoxy resin with improved mechanical performance that can compete with DGEBA. RSC Adv. 2017, 7, 8694. [Google Scholar] [CrossRef] [Green Version]
- Ding, C.; Matharu, A.S. Recent Developments on Biobased Curing Agents: A Review of Their Preparation and Use. ACS Sustain. Chem. Eng. 2014, 2, 2217–2236. [Google Scholar] [CrossRef]
- Yang, X.; Wang, C.; Li, S.; Huang, K.; Li, M.; Mao, W.; Cao, S.; Xia, J. Study on the synthesis of bio-based epoxy curing agent derived from myrcene and castor oil and the properties of the cured products. RSC Adv. 2017, 7, 238. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Zhou, G. Synthesis of rosin-based imidoamine-type curing agents and curing behavior with epoxy resin. Polym. Int. 2011, 60, 557–563. [Google Scholar] [CrossRef]
- Chang, R.; Qin, J.; Gao, J. Fully biobased epoxy from isosorbide diglycidyl ether cured by biobased curing agents with enhanced properties. J. Polym. Res. 2014, 21, 501. [Google Scholar] [CrossRef]
- Liu, X.; Xin, W.; Zhang, J. Rosin-based acid anhydrides as alternatives to petrochemical curing agents. Green Chem. 2009, 11, 1018–1025. [Google Scholar] [CrossRef]
- Ibanez, M.D.; Sanchez-Ballester, N.M.; Blazquez, M.A. Encapsulated limonene: A pleasant lemon-like aroma with promising application in the agri-food industry.a review. Molecules 2020, 25, 2598. [Google Scholar] [CrossRef]
- Miyasaki, E.K.; Aoyagui dos Santos, C.; Vieira, L.R.; Ming, C.C.; Calligaris, G.A.; Cardoso, L.P.; Goncalves, L.A.G. Acceleration of polymorphic transition of cocoa butter and cocoa butter equivalent by addition of D-Limonene. Eur. J. Lipid Sci. Technol. 2016, 118, 716–723. [Google Scholar] [CrossRef]
- Chubukov, V.; Mingardon, F.; Schackwitz, W.; Baidoo, E.E.K.; Alonso-Gutierrez, J.; Hu, Q.; Lee, T.S.; Keasling, J.D.; Mukhopadhyay, A. Acute limonene toxicity in Escherichia coli is caused by limonene hydroperoxide and alleviated by a point mutation in alkyl hydroperoxidase AhpC. Appl. Environ. Microbiol. 2015, 81, 4690–4696. [Google Scholar] [CrossRef] [Green Version]
- Pourreza, N.; Naghdi, T. d-Limonene as a green bio-solvent for dispersive liquid–liquid microextraction of β-cyclodextrin followed by spectrophotometric determination. J. Ind. Eng. Chem. 2017, 51, 71–76. [Google Scholar] [CrossRef]
- Mello, N.A.; Ribeiro, A.P.B.; Bicas, J.L. Delaying crystallization in single fractionated palm olein with limonene addition. Food Res. Int. 2021, 145, 110387. [Google Scholar] [CrossRef]
- Battista, F.; Remelli, G.; Zanzoni, S.; Bolzonella, D. Valorization of Residual Orange Peels: Limonene Recovery, Volatile Fatty Acids, and Biogas Production. ACS Sustain. Chem. Eng. 2020, 8, 6834–6843. [Google Scholar] [CrossRef]
- Morinaga, H.; Haibara, S.; Ashizawa, S. Reinforcement of bio-based network polymer with wine pomace. Polym. Compos. 2021, 42, 2973–2981. [Google Scholar] [CrossRef]
- Zhu, Y.; Gao, F.; Zhong, J.; Shen, L.; Lin, Y. Renewable castor oil and DL- limonene derived fully bio-based vinylogous urethane vitrimers. Eur. Polym. J. 2020, 135, 109865. [Google Scholar] [CrossRef]
- Breloy, L.; Ouarabi, C.A.; Brosseau, A.; Dubot, P.; Brezova, V.; Abbad Andaloussi, S.; Malval, J.P.; Versace, D.-L. β-Carotene/ Limonene Derivatives /Eugenol: Green Synthesis of Antibacterial Coatings under Visible-Light Exposure. ACS Sustain. Chem. Eng. 2019, 7, 19591–19604. [Google Scholar] [CrossRef]
- Morinaga, H.; Ogawa, N.; Sakamoto, M.; Morikawa, H. Crosslinking network of bio-based bis-functional epoxides derived from trans –limonene oxide. J. Polym. Sci. Part A Polym. Chem. 2019, 57, 2466–2473. [Google Scholar] [CrossRef]
- Citrus World Markets and Trade. United States Department of Agriculture. Available online: https://apps.fas.usda.gov/psdonline/circulars/citrus.pdf (accessed on 22 May 2022).
- Pellizzeri, V.; Costa, R.; Grasso, E.; Dugo, C. Valuable products from the flowers of lemon (Citrus limon, (L.) Osbeck and grapefruit (Citrus paradise, Macfad) Italian trees. Food Bioprod. 2020, 123, 123–133. [Google Scholar] [CrossRef]
- Global D-limonene Market−Industry Trends and Forecast to 2028. Available online: https://www.databridgemarketresearch.com/reports/global-d-limonene-market (accessed on 7 April 2022).
- Lis-Balchin, M.; Ochocka, R.J.; Deans, J.; Asztemborska, M.; Hart, S. Bioactivity of the enantiomers of limonene. Med. Sci. Res. 1996, 24, 309–310. [Google Scholar]
- Aggarwal, K.K.; Khanuja, S.P.S.; Ahmad, A.; Santha Kumar, T.R.; Gupta, V.K.; Kumar, S. Antimicrobial activity profiles of the two enantiomers of limonene and carvone isolated from the oils of Mentha spicata and Anethum sowa. Flavour Fragr. J. 2002, 17, 59–63. [Google Scholar] [CrossRef]
- Causero, A.; Troll, C.; Rieger, B. (+)-Limonene Functionalization: Syntheses, Optimization, and Scaleup Procedures for Sustainable Polymer Building Blocks. Ind. Eng. Chem. Res. 2020, 59, 15464–15477. [Google Scholar] [CrossRef]
- Yadav, V.K.; Babu, K.G. A Remarkably Efficient Markovnikov Hydrochlorination of Olefins and Transformation of Nitriles into Imidates by Use of AcCl and an Alcohol. Eur. J. Org. Chem. 2005, 2005, 452–456. [Google Scholar] [CrossRef]
- Carman, R.M.; Kennard, C.H.L.; Robinson, W.T.; Smith, G.; Venzke, B.N. Halogenated Terpenoids. XXV* The Structure of Limonene Tetrabromide. Aust. J. Chem. 1986, 39, 2165–2169. [Google Scholar] [CrossRef]
- Fawzi, M.; Laamari, Y.; Koumya, Y.; Oubellaa, A.; Auhmani, A.; Itto, M.Y.A.; Abouelfida, A.; Riahi, A.; Auhmani, A. Electrochemical and theoretical studies on the corrosion inhibition performance of some synthesized d-Limonene based heterocyclic compounds. J. Mol. Struct. 2021, 1244, 130957. [Google Scholar] [CrossRef]
- Fuscaldo, R.S.; Boeira, E.O.; Stieler, R.; Lüdtke, D.S.; Gregório, J.R. Chiral Amino and Imino-Alcohols Based on (R)-Limonene. J. Braz. Chem. Soc. 2020, 31, 438–446. [Google Scholar] [CrossRef]
- Hauenstein, O.; Agarwal, S.; Greiner, A. Bio-based polycarbonate as synthetic toolbox. Nat. Commun. 2016, 7, 11862. [Google Scholar] [CrossRef]
- Pena Carrodeguas, L.; Martin, C.; Kleij, A.W. Semiaromatic Polyesters Derived from Renewable Terpene Oxides with High Glass Transitions. Macromolecules 2017, 50, 5337–5345. [Google Scholar] [CrossRef]
- Couture, G.; Granado, L.; Fanget, F.; Boutevin, B.; Caillol, S. Limonene-Based Epoxy: Anhydride Thermoset Reaction Study. Molecules 2018, 23, 2739. [Google Scholar] [CrossRef] [Green Version]
- Crivello, J.V.; Yang, B. Studies of synthesis and cationic photopolymerization of three isomeric monoterpene diepoxides. J. Polym. Sci. Part A Polym. Chem. 1995, 33, 1881–1890. [Google Scholar] [CrossRef]
- Morinaga, H.; Sakamoto, M. Synthesis of multifunctional epoxides derived from limonene oxide and its application to the network polymers. Tetrahedron Lett. 2017, 58, 2438–2440. [Google Scholar] [CrossRef]
- Mattar, N.; de Anda, A.R.; Vahabi, H.; Renard, E.; Langlois, V. Resorcinol-Based Epoxy Resins Hardened with Limonene and Eugenol Derivatives: From the Synthesis of Renewable Diamines to the Mechanical Properties of Biobased Thermosets. ACS Sustain. Chem. Eng. 2020, 8, 13064–13075. [Google Scholar] [CrossRef]
- Acosta Ortiz, R.; Garcıa Valdez, A.E. Synthesis, Reactivity and Mechanical Properties of Photocurable Epoxy-Thiol-ene Systems. In Epoxy Resins: Synthesis, Applications and Recent Developments; Cain, M., Ed.; Nova Publishers: New York, NY, USA, 2016. [Google Scholar]
- Acosta Ortiz, R.; Garcia Valdez, A.E.; Navarro Tovar, A.G.; Hilario de la Cruz, A.A.; Gonzalez Sanchez, L.F.; Trejo Garcia, J.H.; Espinoza Muñoz, J.F.; Sangermano, M. Development of an hybrid epoxy-amine/thiol-ene photocurable system. J. Polym. Res. 2014, 21, 504. [Google Scholar] [CrossRef]
- Ellis, B. Chemistry and Technology of Epoxy Resins; Springer, Science and Businees Media: Dordrecht, NL, USA, 1993. [Google Scholar]
- Sandler, S.R.; Karo, W. Epoxy Resins in Polymer Syntesis, 2nd ed.; Academic Press: Cambridge, MA, USA, 2012; pp. 87–128. [Google Scholar]
- Acosta Ortiz, R.; Garcıa Valdez, A.E.; Sangermano, M.; Hilario de la Cruz, A.A.; Aguirre Flores, R.; Espinoza Munoz, J.F. Comparison of the Performance of Two Bifunctional Curing Agents for the Photopolymerization of Epoxy Resins and the Study of the Mechanical Properties of the Obtained Polymers. Macromol. Symp. 2015, 358, 35–40. [Google Scholar] [CrossRef]
- Acosta Ortiz, R.; Garcia Valdez, A.E.; Soria Arguello, G.; Mendez Padilla, M.G.; Acosta Berlanga, O. Photocurable Shape-Memory Polyether-Polythioether/Graphene Nanocomposites and the study of their thermal conductivity. J. Polym. Res. 2018, 25, 160. [Google Scholar] [CrossRef]
- Acosta Ortiz, R.; Acosta Berlanga, O.; Garcia Valdez, A.E.; Aguirre Flores, R.; Tellez Padilla, G.; Mendez Padilla, M.G. Self-healing Photocurable epoxy/thiol-ene systems using an aromatic epoxy resin. Adv. Mater. Sci. Eng. 2016, 8245972. [Google Scholar] [CrossRef] [Green Version]
- Acosta Ortiz, R.; García Valdez, A.E.; Rodriguez Ramos, Z.Y.; Acosta Berlanga, O.; Aguirre Flores, R.; Méndez Padilla, M.G.; Espinoza Muñoz, J.F. Development of rigid toughened photocurable epoxy foams. J. Polym. Res. 2017, 24, 110. [Google Scholar] [CrossRef]
- Acosta Ortiz, R.; Garcıa Valdez, A.E.; Garcia Padilla, E.E.; Aguirre Flores, R.; Espinoza Munoz, J.F. Development of a photocurable glass-fiber reinforced epoxy-amine/thiol-ene composite. J. Polym. Res 2016, 23, 30. [Google Scholar] [CrossRef]
- Acosta Ortiz, R.; Ku Herrera, J.J.; García Santos, A.O.; García Valdez, A.E.; Soria Arguello, G. Tensile Strength and Fracture Mode I Toughness of Photocurable Carbon Fiber/Polyether-Polythioether Composites. J. Polym. Res. 2021, 28, 46. [Google Scholar] [CrossRef]
- Acosta Ortiz, R.; García Valdez, A.E.; Ku Herrera, J.J. Simultaneous reduction in situ and thiol- functionalization of Graphene Oxide during the Photopolymerization of Epoxy/Thiol-ene photocurable systems to prepare polyether-polythioether/reduced graphene oxide nanocomposites. Polym. Plast. Tech. Mat. 2020, 59, 282–293. [Google Scholar] [CrossRef]
- Acosta Ortiz, R.; García Valdez, A.E.; Hernández Cruz, D.; Nestoso Jiménez, G.; Hernández Jiménez, A.I.; Téllez Padilla, J.G.; Guerrero Santos, R. Highly Reactive Novel Biobased Cycloaliphatic Epoxy Resins Derived from Nopol and a Study of Their Cationic Photopolymerization. J. Polym. Res. 2020, 27, 144. [Google Scholar] [CrossRef]
- Acosta Ortiz, R.; Jimenez, H.; Alan, I.; García Valdez, A.E. Synthesis of tetraallylated cystamine and the study of its performanceas a curing agent for the epoxy/thiol-ene photopolymerization of biobased nopol epoxy resins. J. Polym. Res. 2021, 27, 144. [Google Scholar]
- Stemmelen, M.; Pessel, F.; Lapinte, V.; Caillol, S.; Habas, J.-P.; Robin, J.-J. A fully biobased epoxy resin from vegetable oils: From the synthesis of the precursors by thiol-ene reaction to the study of the final material. J. Polym. Sci. Part A Polym. Chem. 2011, 49, 2434–2444. [Google Scholar] [CrossRef] [Green Version]
- Firdaus, M.; Montero de Espinosa, L.; Meier, M.A.R. Terpene-Based Renewable Monomers and Polymers via Thiol-Ene Additions. Macromolecules 2011, 44, 7253–7262. [Google Scholar] [CrossRef]









| Formulation 30 mol % TES | Equivalents | Moles | Grams |
|---|---|---|---|
| Greenpoxy 28 | 1 | 7.3 × 10−4 | 0.25 |
| LCA | 0.3 | 2.2 × 10−4 | 0.10 |
| PTKMP | 0.3 | 2.2 ×10−4 | 0.11 |
| DMPA | 0.003 | 2.2 × 10−6 | 5.6 ×10−3 |
| Formulation 40 mol % TES | Equivalents | Moles | Grams |
| Greenpoxy 28 | 1 | 7.3 × 10−4 | 0.25 |
| LCA | 0.4 | 2.9 × 10−4 | 0.13 |
| PTKMP | 0.4 | 2.9 ×10−4 | 0.15 |
| DMPA | 0.004 | 2.9 × 10−6 | 7.4 × 10−3 |
| Formulation | Double Bonds (1646 cm−1) | Epoxy Groups (867 cm−1) | Thiol Groups (2552 cm−1) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Rp/M0 * (s−1) | Conversion (%) | Rp/M0 * (s−1) | Conversion | Rp/M0 * (s−1) | Conversion (%) | ||||
| 60 s | 600 s | 60 s | 600 s | 60 s | 600 s | ||||
| 40 mol % TES | 2.25 | 95 | 99 | 2.17 | 90 | 99 | 2.08 | 85 | 99 |
| 30 mol % TES | 1.67 | 85 | 99 | 1.52 | 81 | 99 | 1.50 | 70 | 99 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Acosta Ortiz, R.; Sánchez Huerta, R.S.; Ledezma Pérez, A.S.; García Valdez, A.E. Synthesis of a Curing Agent Derived from Limonene and the Study of Its Performance to Polymerize a Biobased Epoxy Resin Using the Epoxy/Thiol-Ene Photopolymerization Technique. Polymers 2022, 14, 2192. https://doi.org/10.3390/polym14112192
Acosta Ortiz R, Sánchez Huerta RS, Ledezma Pérez AS, García Valdez AE. Synthesis of a Curing Agent Derived from Limonene and the Study of Its Performance to Polymerize a Biobased Epoxy Resin Using the Epoxy/Thiol-Ene Photopolymerization Technique. Polymers. 2022; 14(11):2192. https://doi.org/10.3390/polym14112192
Chicago/Turabian StyleAcosta Ortiz, Ricardo, Rebeca Sadai Sánchez Huerta, Antonio Serguei Ledezma Pérez, and Aida E. García Valdez. 2022. "Synthesis of a Curing Agent Derived from Limonene and the Study of Its Performance to Polymerize a Biobased Epoxy Resin Using the Epoxy/Thiol-Ene Photopolymerization Technique" Polymers 14, no. 11: 2192. https://doi.org/10.3390/polym14112192
APA StyleAcosta Ortiz, R., Sánchez Huerta, R. S., Ledezma Pérez, A. S., & García Valdez, A. E. (2022). Synthesis of a Curing Agent Derived from Limonene and the Study of Its Performance to Polymerize a Biobased Epoxy Resin Using the Epoxy/Thiol-Ene Photopolymerization Technique. Polymers, 14(11), 2192. https://doi.org/10.3390/polym14112192