Polymerization and Kinetic Studies
A topical collection in Polymers (ISSN 2073-4360). This collection belongs to the section "Polymer Chemistry".
Viewed by 19674Editors
Interests: controlled/living radical polymerization; RAFT; TERP; water-soluble polymer; self-organization; polymer micelle; bioconjugate polymer
Special Issues, Collections and Topics in MDPI journals
Interests: ecological environment (carbon dioxide, biomass, etc.) polymer synthesis and utilization; olefin coordination polymerization and new catalysts; new method for structure-controlled polymerization
Special Issues, Collections and Topics in MDPI journals
Topical Collection Information
Dear Colleagues,
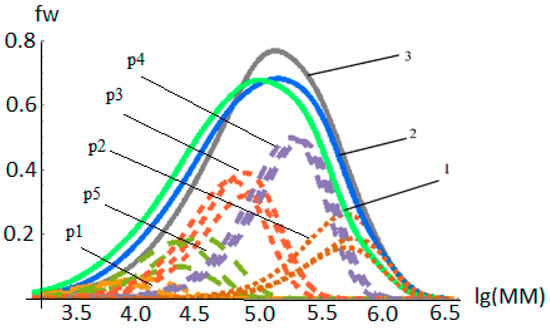
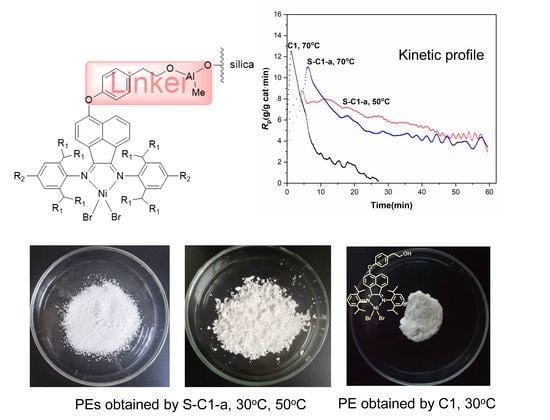
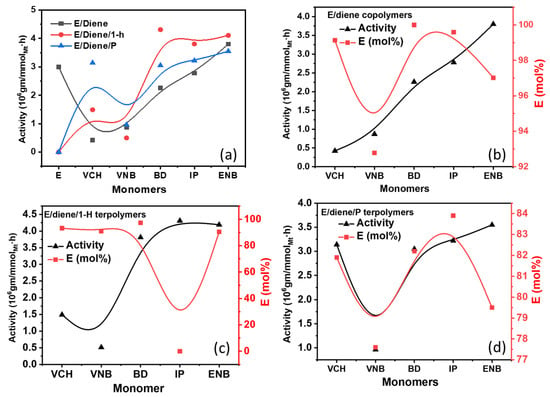
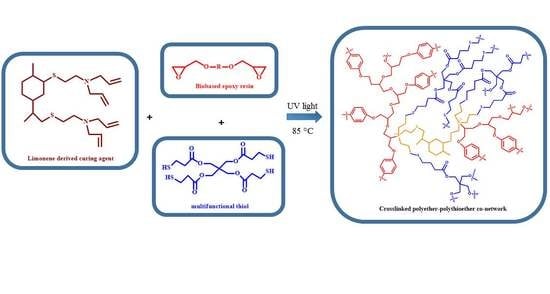
In polymer chemistry, the study of polymerization kinetics is fundamental to the production of polymers. Polymerization is an important process of combining molecules to form polymer chains or even complex topological chains, in which bifunction or multifunction monomers react to form dimers, trimers, longer oligomers, and eventually long chain polymers. Most recently, the concept of precision polymer synthesis has emerged, which seeks to control all aspects of polymer structure and needs basic and advanced knowledge about the kinetics and reaction mechanism of their formation, i.e., mechanism of polymerization in different media, effect of reaction conditions, effect of modifying additives, etc.
This Topic Collection of Polymers on “Polymerization and Kinetic Studies” will be especially focused on the application of kinetic studies to precision polymerization and the use of insights derived from polymerization kinetics to the synthesis of new polymers and copolymers.
Prof. Dr. Shin-Ichi Yusa
Prof. Dr. Binyuan Liu
Collection Editors
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Polymers is an international peer-reviewed open access semimonthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript. The Article Processing Charge (APC) for publication in this open access journal is 2700 CHF (Swiss Francs). Submitted papers should be well formatted and use good English. Authors may use MDPI's English editing service prior to publication or during author revisions.
Keywords
- precision polymerization
- reversible deactivation radical polymerization
- living/controlled polymerization
- step polymerization
- copolymerization
- branching