Preparation and Phytotoxicity Evaluation of Cellulose Acetate Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Nanoparticles
2.3. Hydrodynamic Particles Size and Zeta Potential Characterization
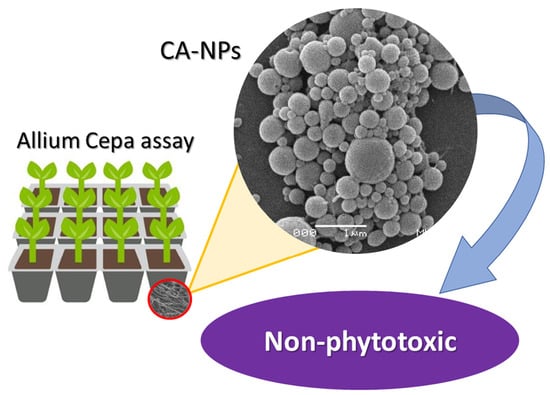
2.4. NPs’ Morphology Characterization
2.5. NPs’ Identification—Molecular Infrared Spectra
2.6. Allium Cepa Assay
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Joseph, B.; Sagarika, V.K.; Sabu, C.; Kalarikkal, N.; Thomas, S. Cellulose nanocomposites: Fabrication and biomedical applications. J. Bioresour. Bioprod. 2020, 5, 223–237. [Google Scholar] [CrossRef]
- Sajid, M.; Płotka-Wasylka, J. Nanoparticles: Synthesis, characteristics, and applications in analytical and other sciences. Microchem. J. 2020, 154, 104623. [Google Scholar] [CrossRef]
- Kulterer, M.R.; Reichel, V.E.; Kargl, R.; Köstler, S.; Sarbova, V.; Heinze, T.; Stana-Kleinschek, K.; Ribitsch, V. Functional polysaccharide composite nanoparticles from cellulose acetate and potential applications. Adv. Funct. Mater. 2012, 22, 1749–1758. [Google Scholar] [CrossRef]
- Jin, X.; Xu, J.; Wang, X.; Xie, Z.; Liu, Z.; Liang, B.; Chen, D.; Shen, G. Flexible TiO2/cellulose acetate hybrid film as a recyclable photocatalyst. RSC Adv. 2014, 4, 12640–12648. [Google Scholar] [CrossRef]
- Zulfiqar, S.; Rafique, U.; Akhtar, M.J. Removal of pirimicarb from agricultural wastewater using cellulose acetate—Modified ionic liquid membrane. Environ. Sci. Pollut. Res. 2019, 26, 15795–15802. [Google Scholar] [CrossRef] [PubMed]
- Barud, H.S.; de Araújo Júnior, A.M.; Santos, D.B.; de Assunção, R.M.N.; Meireles, C.S.; Cerqueira, D.A.; Rodrigues Filho, G.; Ribeiro, C.A.; Messaddeq, Y.; Ribeiro, S.J.L. Thermal behavior of cellulose acetate produced from homogeneous acetylation of bacterial cellulose. Thermochim. Acta 2008, 471, 61–69. [Google Scholar] [CrossRef]
- Hamed, O.A.; Jodeh, S. Cellulose acetate from biomass waste of olive industry. J. Wood Sci. 2015, 61, 45–52. [Google Scholar] [CrossRef]
- Karl, M. Toxicity of cellulose acetate sheets to plants and fish. Science 1952, 115, 236. [Google Scholar]
- Spasova, M.; Manolova, N.; Rashkov, I.; Tsekova, P.; Georgieva, A.; Toshkova, R.; Markova, N. Cellulose Acetate-Based Electrospun Materials with a Variety of Biological Potentials: Antibacterial, Antifungal and Anticancer. Polymers 2021, 13, 1631. [Google Scholar] [CrossRef]
- Sayyed, A.J.; Pinjari, D.V.; Sonawane, S.H.; Bhanvase, B.A.; Sheikh, J.; Sillanpaa, M. Cel-lulose-based nanomaterials for water and wastewater treatments: A review. J. Environ. Chem. Eng. 2021, 9, 106626. [Google Scholar] [CrossRef]
- Ruiz-Palomero, C.; Laura Soriano, M.; Valcarcel, M. Nanocellulose as analyte and analytical tool: Opportuni-ties and challenges. TRAC-Trends Anal. Chem. 2017, 87, 1–18. [Google Scholar] [CrossRef]
- Senna, A.M.; Botaro, V.R. Biodegradable hydrogel derived from cellulose acetate and EDTA as a reduction substrate of leaching NPK compound fertilizer and water retention in soil. J. Control. Release 2017, 260, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, Y.; Wang, L.; Fang, G.; Cang, L.; Herath, H.M.S.K.; Zhou, D. Reducing the bioavailability of PCBs in soil to plant by biochars assessed with triolein-embedded cellulose acetate membrane technique. Environ. Pollut. 2013, 174, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Bifaria, E.N.; Khanb, S.B.; Alamryb, K.A.; Asirib, A.M.; Akhtard, K. Cellulose Acetate Based Nanocomposites for Biomedical Applications: A Review. Curr. Pharm. Des. 2016, 22, 3007–3019. [Google Scholar] [CrossRef] [PubMed]
- Varshosaz, J.; Taymouri, S.; Jafari, E.; Jahanian-najafabadi, A.; Taheri, A. Formulation and characterization of cellulose acetate butyrate nanoparticles loaded with nevirapine for HIV treatment. J. Drug Deliv. Sci. Technol. 2018, 48, 9–20. [Google Scholar] [CrossRef]
- Liakos, A.I.L.; Iordache, F.; Scarpellini, A.; Oneto, M.; Bianchini, P.; Grumezescu, A.M.; Holban, A.M. Cellulose acetate—Essential oil nanocapsules with antimicrobial activity for biomedical applications. Colloids Surf. B Biointerfaces 2018, 172, 471–479. [Google Scholar] [CrossRef]
- Marrez, D.A.; Abdelhamid, A.E.; Darwesh, O.M. Eco-friendly cellulose acetate green synthesized silver nano-composite as antibacterial packaging system for food safety. Food Packag. Shelf Life 2019, 20, 100302. [Google Scholar] [CrossRef]
- Huda, E.; Rahmi; Khairan. Preparation and characterization of cellulose acetate from cotton. IOP Conf. Ser. Earth Environ. Sci. 2019, 364, 012021. [Google Scholar] [CrossRef]
- Scherer, M.D.; Sposito, J.C.V.; Falco, W.F.; Grisolia, A.B.; Andrade, L.H.C.; Lima, S.M.; Machado, G.; Nascimento, V.A.; Gonçalves, D.A.; Wender, H.; et al. Cytotoxic and genotoxic effects of silver nanoparticles on meristematic cells of Allium cepa roots: A close analysis of particle size dependence. Sci. Total Environ. 2019, 660, 459–467. [Google Scholar] [CrossRef]
- Pathiratne, A.; Hemachandra, C.K.; De Silva, N. Efficacy of Allium cepa test system for screening cytotoxicity and genotoxicity of industrial effluents originated from different industrial activities. Environ. Monit. Assess. 2015, 187, 730. [Google Scholar] [CrossRef]
- Leme, D.M.; Marin-Morales, M.A. Allium cepa test in environmental monitoring: A review on its application. Mutat. Res./Rev. Mutat. Res. 2009, 682, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Rafiei, P.; Haddadi, A. Docetaxel-loaded PLGA and PLGA-PEG nanoparticles for intravenous application: Pharmacokinetics and biodistribution profile. Int. J. Nanomed. 2017, 12, 935–947. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Margarida Cardoso, M.; Peça, I.N.; Raposo, C.D.; Petrova, K.T.; Teresa Barros, M.; Gardner, R.; Bicho, A. Doxorubicin-loaded galactose-conjugated poly(d,l-lactide-co-glycolide) nanoparticles as hepatocyte-targeting drug carrier. J. Microencapsul. 2016, 33, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Fodor-Kardos, A.; Kiss, Á.F.; Monostory, K.; Feczkó, T. Sustained: In vitro interferon-beta release and in vivo toxicity of PLGA and PEG-PLGA nanoparticles. RSC Adv. 2020, 10, 15893–15900. [Google Scholar] [CrossRef] [Green Version]
- Candido, R.G.; Godoy, G.G.; Gonçalves, A. Characterization and application of cellulose acetate synthesized from sugarcane bagasse. Carbohydr. Polym. 2017, 167, 280–289. [Google Scholar] [CrossRef] [Green Version]
- Culica, M.E.; Chibac-Scutaru, A.L.; Melinte, V.; Coseri, S. Cellulose Acetate Incorporating Organically Functionalized CeO2 NPs: Efficient Materials for UV Filtering Applications. Materials 2020, 13, 2955. [Google Scholar] [CrossRef]
- Rodrigues Filho, G.; Monteiro, D.S.; da Silva Meireles, C.; de Assunção, R.M.N.; Cerqueira, D.A.; Barud, H.S.; Ribeiro, S.J.L.; Messadeq, Y. Synthesis and characterization of cellulose acetate produced from recycled newspaper. Carbohydr. Polym. 2008, 73, 74–82. [Google Scholar] [CrossRef]
- Kumari, M.; Mukherjee, A.; Chandrasekaran, N. Genotoxicity of silver nanoparticles in Allium cepa. Sci. Total Environ. 2009, 407, 5243–5246. [Google Scholar] [CrossRef]
- Magnusson, J.; Ramel, C.; Eriksson, A. Mutagenic effects of chlorinated phenoxyacetic acids in Drosophila melanogaster. Hereditas 1977, 87, 121–123. [Google Scholar] [CrossRef]
- Jogaiah, S.; Paidi, M.K.; Venugopal, K.; Geetha, N.; Mujtaba, M.; Udikeri, S.S.; Govarthanan, M. Phytotoxicological effects of engineered nanoparticles: An emerging nanotoxicology. Sci. Total Environ. 2021, 801, 149809. [Google Scholar] [CrossRef]
- Tong, Y.; Wu, Y.; Zhao, C.; Xu, Y.; Lu, J.; Xiang, S.; Zong, F.; Wu, X. Polymeric Nanoparticles as a Metolachlor Carrier: Water-Based Formulation for Hydrophobic Pesticides and Absorption by Plants. J. Agric. Food Chem. 2017, 65, 7371–7378. [Google Scholar] [CrossRef] [PubMed]
- Zielińska, A.; Costa, B.; Ferreira, M.V.; Miguéis, D.; Louros, J.M.S.; Durazzo, A.; Lucarini, M.; Eder, P.; Chaud, M.V.; Morsink, M.; et al. Nanotoxicology and nanosafety: Safety-by-design and testing at a glance. Int. J. Environ. Res. Public Health 2020, 17, 4657. [Google Scholar] [CrossRef] [PubMed]
- Viana, J.L.; França, T.; Cena, C. Solution blow spinning poly(Vinyl alcohol) sub-microfibers produced from different solvents. Orbital 2020, 12, 1–6. [Google Scholar] [CrossRef]
- Sobral, F.; Silva, M.J.; Canassa, T.; Goncalves, A.M.; Cena, C. PVDF/KNO3 Composite Sub-Microfibers Produced by Solution Blow Spinning as a Hydrophobic Matrix for Fertilizer Delivery System. Polymers 2022, 14, 1000. [Google Scholar] [CrossRef] [PubMed]
- Cena, C.R.; Silva, M.J.; Malmonge, L.F.; Malmonge, J.A. Poly(vinyl pyrrolidone) sub-microfibers produced by solution blow spinning. J. Polym. Res. 2018, 25, 238. [Google Scholar] [CrossRef]
- Esler, D.; Trust, K.A.; Ballachey, B.E.; Iverson, S.A.; Lewis, T.L.; Rizzolo, D.J.; Mulcahy, D.M.; Miles, A.K.; Woodin, B.B.R.; Stegeman, C.J.J.; et al. Cytochrome p4501a biomarker indication of oil exposure in harlequin ducks up to 20 years after the exxon valdez oil spill. Environ. Toxicol. Chem. 2010, 29, 1138–1145. [Google Scholar] [PubMed]
- Nandini, B.; Puttaswamy, H.; Prakash, H.S.; Adhikari, S.; Jogaiah, S.; Nagaraja, G. Elicitation of novel trichogenic-lipid nanoemulsion signaling resistance against pearl millet downy mildew disease. Biomolecules 2020, 10, 25. [Google Scholar] [CrossRef] [PubMed]



| Particles | DSEM (nm) | PDI | DSize-DLS (nm) | DZ-Average-DLS (nm) | Zeta (mV) |
|---|---|---|---|---|---|
| CA-NPs | 216 ± 8.21 | 0.933 | 508.3 ± 49.7 | 1945 ± 87.6 | −2.52 |
| Treatment | MI (%) | CAI (%) | MNI (%) | IG (%) | REI (mm) |
|---|---|---|---|---|---|
| control (DI Water) | 1.64 ± 0.38 | 0.08 ± 0.04 | 0.04 ± 0.04 | 70.00 ± 16.67 | 9.51 ± 0.88 |
| 12.5 µg·m | 1.38 ± 0.44 | 0.12 ± 0.07 | 0.04 ± 0.02 | 74.44 ± 10.11 | 9.45 ± 0.46 |
| 25 µg·m | 1.36 ± 0.64 | 0.08 ± 0.06 | 0 ± 0.00 | 65.56 ± 7.70 | 9.12 ± 2.30 |
| 50 µg·m | 0.90 ± 0.22 | 0.04 ± 0.02 | 0.02 ± 0.02 | 65.56 ± 10.18 | 11.72 ± 2.15 |
| 100 µg·m | 0.74 ± 0.28 * | 0 ± 0.00 | 0.10 ± 0.06 | 67.78 ± 10.18 | 11.39 ± 1.51 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lima, R.G.; Maranni, M.; Araujo, L.O.; Maciel, B.M.; Canassa, T.; Caires, A.R.L.; Cena, C. Preparation and Phytotoxicity Evaluation of Cellulose Acetate Nanoparticles. Polymers 2022, 14, 5022. https://doi.org/10.3390/polym14225022
Lima RG, Maranni M, Araujo LO, Maciel BM, Canassa T, Caires ARL, Cena C. Preparation and Phytotoxicity Evaluation of Cellulose Acetate Nanoparticles. Polymers. 2022; 14(22):5022. https://doi.org/10.3390/polym14225022
Chicago/Turabian StyleLima, Regiane G., Maria Maranni, Leandro O. Araujo, Bruno Marinho Maciel, Thalita Canassa, Anderson R. L. Caires, and Cícero Cena. 2022. "Preparation and Phytotoxicity Evaluation of Cellulose Acetate Nanoparticles" Polymers 14, no. 22: 5022. https://doi.org/10.3390/polym14225022
APA StyleLima, R. G., Maranni, M., Araujo, L. O., Maciel, B. M., Canassa, T., Caires, A. R. L., & Cena, C. (2022). Preparation and Phytotoxicity Evaluation of Cellulose Acetate Nanoparticles. Polymers, 14(22), 5022. https://doi.org/10.3390/polym14225022