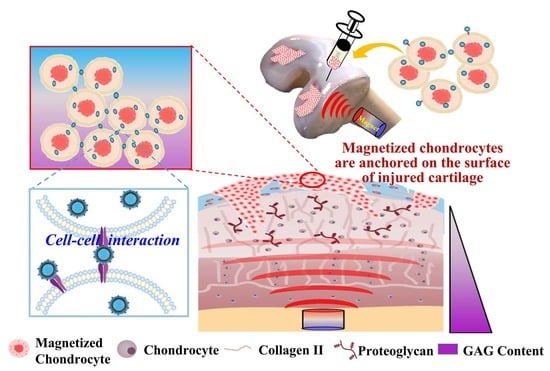
High-Density Horizontal Stacking of Chondrocytes via the Synergy of Biocompatible Magnetic Gelatin Nanocarriers and Internal Magnetic Navigation for Enhancing Cartilage Repair
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Amphiphilic Gelatin (AG)
2.3. Synthesis of Superparamagnetic Iron Oxide Nanoparticles (SPIONs)
2.4. Preparation of MAGNCs
2.5. Analysis of MAGNC Characteristics
2.6. Chondrocyte Isolation and Culture
2.7. Cell Viability Test
2.8. Evaluation of Magnetic-Guiding Chondrocytes
2.9. N-Sulfated-Glycosaminoglycans (sGAG) Quantification and Alcian Blue Staining
2.10. Real-Time Quantitative Polymerase Chain Reaction (RT-qPCR)
2.11. Animal Study
2.12. Immunohistochemical Staining
2.13. Simulation of the Magnetic Field
2.14. Statistical Analysis
3. Results
3.1. Synthesis and Characterization of MAGNCs
3.2. Evaluation of Magnetized Chondrocytes Efficiency
3.3. Magnetic Guidance of Cells
3.4. sGAG Content Assay
3.5. RT-qPCR
3.6. Animal Study
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Martel-Pelletier, J. Pathophysiology of osteoarthritis. Osteoarthr. Cartil. 2004, 12, 31–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Correa, D.; Lietman, S.A. Articular cartilage repair: Current needs, methods and research directions. Semin. Cell Dev. Biol. 2017, 62, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Sophia Fox, A.J.; Bedi, A.; Rodeo, S.A. The basic science of articular cartilage: Structure, composition, and function. Sports Health 2009, 1, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Grässel, S.; Aszodi, A. Osteoarthritis and cartilage regeneration: Focus on pathophysiology and molecular mechanisms. Int. J. Mol. Sci. 2019, 20, 6156. [Google Scholar] [CrossRef] [Green Version]
- Bannuru, R.R.; Osani, M.C.; Vaysbrot, E.E.; Arden, N.K.; Bennell, K.; Bierma-Zeinstra, S.M.A.; Kraus, V.B.; Lohmander, L.S.; Abbott, J.H.; Bhandari, M.; et al. Oarsi guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthr. Cartil. 2019, 27, 1578–1589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ngadimin, K.D.; Stokes, A.; Gentile, P.; Ferreira, A.M. Biomimetic hydrogels designed for cartilage tissue engineering. Biomater. Sci. 2021, 9, 4246–4259. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.; Wang, F.; Huang, Y.; Lin, X.; Chen, C.; Wang, F.; Yang, L. Magnetic-targeting of polyethylenimine-wrapped iron oxide nanoparticle labeled chondrocytes in a rabbit articular cartilage defect model. RSC Adv. 2018, 8, 7633–7640. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Liu, W.; Li, J.J.; Chai, S.; Xing, D.; Yu, H.; Zhang, Y.; Yan, W.; Xu, Z.; Zhao, B.; et al. A low dose cell therapy system for treating osteoarthritis: In vivo study and in vitro mechanistic investigations. Bioact. Mater. 2022, 7, 478–490. [Google Scholar] [CrossRef]
- Nikolova, M.P.; Chavali, M.S. Recent advances in biomaterials for 3d scaffolds: A review. Bioact. Mater. 2019, 4, 271–292. [Google Scholar] [CrossRef]
- Roelofs, A.J.; Rocke, J.P.; De Bari, C. Cell-based approaches to joint surface repair: A research perspective. Osteoarthr. Cartil. 2013, 21, 892–900. [Google Scholar] [CrossRef] [Green Version]
- Blümler, P.; Friedrich, R.P.; Pereira, J.; Baun, O.; Alexiou, C.; Mailänder, V. Contactless nanoparticle-based guiding of cells by controllable magnetic fields. Nanotechnol. Sci. Appl. 2021, 14, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Zhi, D.; Yang, T.; Yang, J.; Fu, S.; Zhang, S. Targeting strategies for superparamagnetic iron oxide nanoparticles in cancer therapy. Acta Biomater. 2020, 102, 13–34. [Google Scholar] [CrossRef] [PubMed]
- Bull, E.; Madani, S.Y.; Sheth, R.; Seifalian, A.; Green, M.; Seifalian, A.M. Stem cell tracking using iron oxide nanoparticles. Int. J. Nanomed. 2014, 9, 1641–1653. [Google Scholar]
- Chen, S.; Zhang, J.; Jiang, S.; Lin, G.; Luo, B.; Yao, H.; Lin, Y.; He, C.; Liu, G.; Lin, Z. Self-assembled superparamagnetic iron oxide nanoclusters for universal cell labeling and mri. Nanoscale Res. Lett. 2016, 11, 263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, H.; Beck, A.M.; Shimomura, K.; Sohn, J.; Fritch, M.R.; Deng, Y.; Kilroy, E.J.; Tang, Y.; Alexander, P.G.; Tuan, R.S. Optimization of photocrosslinked gelatin/hyaluronic acid hybrid scaffold for the repair of cartilage defect. J. Tissue Eng. Regen. Med. 2019, 13, 1418–1429. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Zeng, H.; Robinson, D.B.; Raoux, S.; Rice, P.M.; Wang, S.X.; Li, G. Monodisperse mfe2o4 (m = fe, co, mn) nanoparticles. J. Am. Chem. Soc. 2004, 126, 273–279. [Google Scholar] [CrossRef]
- Hou, K.T.; Liu, T.Y.; Chiang, M.Y.; Chen, C.Y.; Chang, S.J.; Chen, S.Y. Cartilage tissue-mimetic pellets with multifunctional magnetic hyaluronic acid-graft-amphiphilic gelatin microcapsules for chondrogenic stimulation. Polymers 2020, 12, 785. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.W.; Ku, K.C.; Chen, S.Y.; Kuo, S.M.; Chen, I.F.; Wang, T.Y.; Chang, S.J. Development of chondrocyte-seeded electrosprayed nanoparticles for repair of articular cartilage defects in rabbits. J. Biomater. Appl. 2018, 32, 800–812. [Google Scholar] [CrossRef]
- Ahamed, M.; Khan, M.A.M.; Akhtar, M.J.; Alhadlaq, H.A.; Alshamsan, A. Ag-doping regulates the cytotoxicity of TiO2 nanoparticles via oxidative stress in human cancer cells. Sci. Rep. 2017, 7, 17662. [Google Scholar] [CrossRef]
- Ahamed, M.; Alhadlaq, H.A.; Alam, J.; Khan, M.A.M.; Ali, D.; Alarafi, S. Iron Oxide Nanoparticle-induced Oxidative Stress and Genotoxicity in Human Skin Epithelial and Lung Epithelial Cell Lines. Curr. Pharm. Des. 2013, 19, 6681–6690. [Google Scholar] [CrossRef]
- Baseer, A.; Koenneke, A.; Zapp, J.; Khan, S.A.; Schneider, M. Design and characterization of surface-crosslinked gelatin nanoparticles for the delivery of hydrophilic macromolecular drugs. Macromol. Chem. Phys. 2019, 220, 1900260. [Google Scholar] [CrossRef] [Green Version]
- Ahamed, M.; Akhtar, M.J.; Khan, M.A.M.; Alhadlaq, H.A. Novel Green Preparation of Ag/RGO Nanocomposites with Highly Effective Anticancer Performance. Polymers 2021, 13, 3350. [Google Scholar] [CrossRef] [PubMed]
- Ahamed, M.; Akhtar, M.J.; Khan, M.A.M.; Alhadlaq, H.A. Facile green synthesis of ZnO-RGO nanocomposites with enhanced anticancer efficacy. Methods 2021, 4, 20. [Google Scholar] [CrossRef] [PubMed]
- Ahamed, M.; Akhtar, M.J.; Khan, M.A.M.; Alhadlaq, H.A. SnO2-Doped ZnO/Reduced Graphene Oxide Nanocomposites: Synthesis, Characterization, and Improved Anticancer Activity via Oxidative Stress Pathway. Int. J. Nanomed. 2021, 16, 89–104. [Google Scholar] [CrossRef] [PubMed]
- Ahamed, M.; Akhtar, M.J.; Khan, M.A.M.; Alhadlaq, H.A. Facile Synthesis of Zn-Doped Bi2O3 Nanoparticles and Their Selective Cytotoxicity toward Cancer Cells. ACS Omega 2021, 27, 17353–17361. [Google Scholar] [CrossRef]
- Cao, B.; Li, Z.; Peng, R.; Ding, J. Effects of cell–cell contact and oxygen tension on chondrogenic differentiation of stem cells. Biomaterials 2015, 64, 21–32. [Google Scholar] [CrossRef]
- Kwon, H.J.; Yasuda, K.; Ohmiya, Y.; Honma, K.; Chen, Y.M.; Gong, J.P. In vitro differentiation of chondrogenic ATDC5 cells is enhanced by culturing on synthetic hydrogels with various charge densities. Acta Biomater. 2010, 6, 494–501. [Google Scholar] [CrossRef] [Green Version]
- Lian, C.; Wang, X.; Qiu, X.; Wu, Z.; Gao, B.; Liu, L.; Liang, G.; Zhou, H.; Yang, X.; Peng, Y.; et al. Collagen type ii suppresses articular chondrocyte hypertrophy and osteoarthritis progression by promoting integrin β1−smad1 interaction. Bone Res. 2019, 7, 8. [Google Scholar] [CrossRef] [Green Version]
- Wu, L.; Gonzalez, S.; Shah, S.; Kyupelyan, L.; Petrigliano, F.A.; McAllister, D.R.; Adams, J.S.; Karperien, M.; Tuan, T.L.; Benya, P.D.; et al. Extracellular matrix domain formation as an indicator of chondrocyte dedifferentiation and hypertrophy. Tissue Eng. Part C Methods 2014, 20, 160–168. [Google Scholar] [CrossRef] [Green Version]
- Hattori, T.; Müller, C.; Gebhard, S.; Bauer, E.; Pausch, F.; Schlund, B.; Bösl, M.R.; Hess, A.; Surmann-Schmitt, C.; von der Mark, H.; et al. Sox9 is a major negative regulator of cartilage vascularization, bone marrow formation and endochondral ossification. Development 2010, 137, 901–911. [Google Scholar] [CrossRef] [Green Version]
- Orfanidou, T.; Iliopoulos, D.; Malizos, K.N.; Tsezou, A. Involvement of sox-9 and fgf-23 in runx-2 regulation in osteoarthritic chondrocytes. J. Cell Mol. Med. 2009, 13, 3186–3194. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.K.; Masters, T.E.; Hu, J.C.; Athanasiou, K.A. Engineering a fibrocartilage spectrum through modulation of aggregate redifferentiation. Cell Transplant. 2015, 24, 235–245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borzí, R.M.; Olivotto, E.; Pagani, S.; Vitellozzi, R.; Neri, S.; Battistelli, M.; Falcieri, E.; Facchini, A.; Flamigni, F.; Penzo, M.; et al. Matrix metalloproteinase 13 loss associated with impaired extracellular matrix remodeling disrupts chondrocyte differentiation by concerted effects on multiple regulatory factors. Arthritis Rheum. 2010, 62, 2370–2381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arpino, V.; Brock, M.; Gill, S.E. The role of timps in regulation of extracellular matrix proteolysis. Matrix Biol. 2015, 44–46, 247–254. [Google Scholar] [CrossRef]
- Zhang, C.; Cai, Y.-Z.; Lin, X.-J.; Wang, Y. Magnetically actuated manipulation and its applications for cartilage defects: Characteristics and advanced therapeutic strategies. Front. Cell Dev. Biol. 2020, 8, 526. [Google Scholar] [CrossRef]
- Yang, X.; Hong, H.; Grailer, J.J.; Rowland, I.J.; Javadi, A.; Hurley, S.A.; Xiao, Y.; Yang, Y.; Zhang, Y.; Nickles, R.J.; et al. Crgd-functionalized, dox-conjugated, and ⁶⁴cu-labeled superparamagnetic iron oxide nanoparticles for targeted anticancer drug delivery and pet/mr imaging. Biomaterials 2011, 32, 4151–4160. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.; Hou, Y.; Bu, B.; Wang, W.; Ma, T.; Liu, C.; Lin, L.; Ma, L.; Lou, X.; Gao, M. Timely visualization of the collaterals formed during acute ischemic stroke with fe3o4 nanoparticle-based mr imaging probe. Small 2018, 14, 1800573. [Google Scholar] [CrossRef]
- Crist, R.M.; Dasa, S.S.K.; Liu, C.H.; Clogston, J.D.; Dobrovolskaia, M.A.; Stern, S.T. Challenges in the development of nanoparticle-based imaging agents: Characterization and biology. WIREs Nanomed. Nanobiotechnol. 2021, 13, e1665. [Google Scholar] [CrossRef]
- Rastegari, E.; Hsiao, Y.J.; Lai, W.Y.; Lai, Y.H.; Yang, T.C.; Chen, S.J.; Huang, P.I.; Chiou, S.H.; Mou, C.Y.; Chien, Y. An update on mesoporous silica nanoparticle applications in nanomedicine. Pharmaceutics 2021, 13, 1067. [Google Scholar] [CrossRef]
- Hersel, U.; Dahmen, C.; Kessler, H. Rgd modified polymers: Biomaterials for stimulated cell adhesion and beyond. Biomaterials 2003, 24, 4385–4415. [Google Scholar] [CrossRef]
- Gaihre, B.; Hee Lee, Y.; Khil, M.S.; Yi, H.K.; Kim, H.Y. In-vitro cytotoxicity and cell uptake study of gelatin-coated magnetic iron oxide nanoparticles. J. Microencapsul. 2011, 28, 240–247. [Google Scholar] [CrossRef]
- Shevkoplyas, S.S.; Siegel, A.C.; Westervelt, R.M.; Prentiss, M.G.; Whitesides, G.M. The force acting on a superparamagnetic bead due to an applied magnetic field. Lab Chip 2007, 7, 1294–1302. [Google Scholar] [CrossRef]
- Baun, O.; Blümler, P. Permanent magnet system to guide superparamagnetic particles. J. Magn. Magn. Mater. 2017, 439, 294–304. [Google Scholar] [CrossRef] [Green Version]
- Singamaneni, S.; Bliznyuk, V.N.; Binek, C.; Tsymbal, E.Y. Magnetic nanoparticles: Recent advances in synthesis, self-assembly and applications. J. Mater. Chem. 2011, 21, 16819–16845. [Google Scholar] [CrossRef] [Green Version]
- Davis, S.; Roldo, M.; Blunn, G.; Tozzi, G.; Roncada, T. Influence of the mechanical environment on the regeneration of osteochondral defects. Front. Bioeng. Biotechnol. 2021, 9, 603408. [Google Scholar] [CrossRef] [PubMed]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, S.-W.; Chen, Y.-J.; Chen, C.-J.; Liu, J.-T.; Yang, C.-Y.; Tsai, J.-H.; Lu, H.-E.; Chen, S.-Y.; Chang, S.-J. High-Density Horizontal Stacking of Chondrocytes via the Synergy of Biocompatible Magnetic Gelatin Nanocarriers and Internal Magnetic Navigation for Enhancing Cartilage Repair. Polymers 2022, 14, 809. https://doi.org/10.3390/polym14040809
Yang S-W, Chen Y-J, Chen C-J, Liu J-T, Yang C-Y, Tsai J-H, Lu H-E, Chen S-Y, Chang S-J. High-Density Horizontal Stacking of Chondrocytes via the Synergy of Biocompatible Magnetic Gelatin Nanocarriers and Internal Magnetic Navigation for Enhancing Cartilage Repair. Polymers. 2022; 14(4):809. https://doi.org/10.3390/polym14040809
Chicago/Turabian StyleYang, Shan-Wei, Yong-Ji Chen, Ching-Jung Chen, Jen-Tsai Liu, Chin-Yi Yang, Jen-Hao Tsai, Huai-En Lu, San-Yuan Chen, and Shwu-Jen Chang. 2022. "High-Density Horizontal Stacking of Chondrocytes via the Synergy of Biocompatible Magnetic Gelatin Nanocarriers and Internal Magnetic Navigation for Enhancing Cartilage Repair" Polymers 14, no. 4: 809. https://doi.org/10.3390/polym14040809
APA StyleYang, S. -W., Chen, Y. -J., Chen, C. -J., Liu, J. -T., Yang, C. -Y., Tsai, J. -H., Lu, H. -E., Chen, S. -Y., & Chang, S. -J. (2022). High-Density Horizontal Stacking of Chondrocytes via the Synergy of Biocompatible Magnetic Gelatin Nanocarriers and Internal Magnetic Navigation for Enhancing Cartilage Repair. Polymers, 14(4), 809. https://doi.org/10.3390/polym14040809