Starch Chemical Composition and Molecular Structure in Relation to Physicochemical Characteristics and Resistant Starch Content of Four Thai Commercial Rice Cultivars Differing in Pasting Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
2.2. Measurements of Grain Starch and Reducing Sugar Content
2.3. Starch Isolation and Chemical Composition Analyses
2.4. Determination of Chain Length Distribution (CLD) of Amylopectin Branches
2.5. Determination of Starch Particle Size Distribution
2.6. Analysis of Relative Crystallinity
2.7. Measurements of Swelling Power and Water Solubility
2.8. Determination of Thermal Properties
2.9. Determination of Pasting Properties
2.10. Resistant Starch (RS) Determination
2.11. Statistical Analysis
3. Results
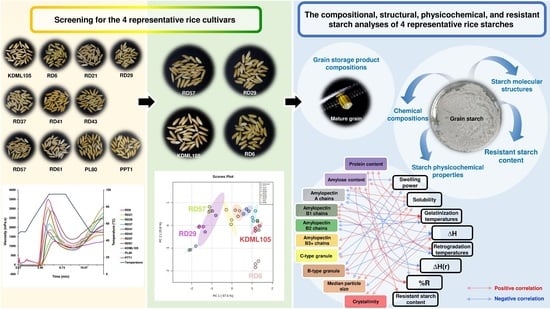
3.1. Screening of Rice Cultivars with Distinctive Starch Pasting Properties
3.2. Reducing Sugar and Total Starch Contents of Rice Grains
3.3. Chemical Composition of Isolated Rice Starches
3.4. Starch Molecular Structure
3.4.1. Chain-Length Distribution (CLD) of Amylopectin Branches
3.4.2. Starch Granule Size and Size Distribution
3.4.3. Starch Granule Crystalline Structure
3.5. Starch Physicochemical and Functional Properties
3.5.1. Swelling Power and Water Solubility
3.5.2. Thermal Properties
3.6. Resistant Starch (RS) Content
3.7. Correlation Analysis between Starch Compositional and Structural Characteristics and Starch Physicochemical and Functional Characteristics
4. Discussion
4.1. Variations in the Chemical Composition of the Four Thai Commercial Rice Starches with Different Pasting Properties
4.2. Differences in the Structural Features of the Four Thai Commercial Rice Starches with Different Pasting Properties
4.3. Starch Compositional and Structural Characteristics in Relation to Physicochemical and Functional Properties and Resistant Starch Content
4.3.1. Swelling Power and Water Solubility
4.3.2. Gelatinization Properties
4.3.3. Retrogradation Properties
4.3.4. Resistant Starch Content
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, Z.; Li, W.; Qi, J.; Shi, P.; Yin, Y. Starch accumulation, activities of key enzyme and gene expression in starch synthesis of wheat endosperm with different starch contents. J. Food Sci. Technol. 2014, 51, 419–429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sangwongchai, W.; Tananuwong, K.; Krusong, K.; Thitisaksakul, M. Yield, Grain Quality and Starch Physicochemical Properties of 2 Elite Thai Rice Cultivars Grown under Varying Production Systems and Soil Characteristics. Foods 2021, 10, 2601. [Google Scholar] [CrossRef] [PubMed]
- Macrotrends. Thailand Population 1950–2022. Available online: https://www.macrotrends.net/countries/THA/thailand/population (accessed on 2 July 2022).
- Huang, L.; Tan, H.; Zhang, C.; Li, Q.; Liu, Q. Starch biosynthesis in cereal endosperms: An updated review over the last decade. Plant Commun. 2021, 2, 100237. [Google Scholar] [CrossRef] [PubMed]
- Wanichthanarak, K.; Thitisaksakul, M. ThRSDB: A database of Thai rice starch composition, molecular structure and functionality. Database 2020, 2020, baaa068. [Google Scholar] [CrossRef]
- Sirikeratikul, S. TH2022-0024; Food Processing Ingredients; The United States Department of Agriculture (USDA): Washington, DC, USA, 2022; p. 10.
- Sangwongchai, W.; Krusong, K.; Thitisaksakul, M. Salt tolerance at vegetative stage is partially associated with changes in grain quality and starch physicochemical properties of rice exposed to salinity stress at reproductive stage. J. Sci. Food Agric. 2022, 102, 370–382. [Google Scholar] [CrossRef]
- Tangsrianugul, N.; Wongsagonsup, R.; Suphantharika, M. Physicochemical and rheological properties of flour and starch from Thai pigmented rice cultivars. Int. J. Biol. Macromol. 2019, 137, 666–675. [Google Scholar] [CrossRef]
- Thitisaksakul, M.; Sangwongchai, W.; Mungmonsin, U.; Promrit, P.; Krusong, K.; Wanichthanarak, K.; Tananuwong, K. Granule morphological and structural variability of Thai certified glutinous rice starches in relation to thermal, pasting, and digestible properties. Cereal Chem. 2021, 98, 492–506. [Google Scholar] [CrossRef]
- Chung, H.-J.; Liu, Q.; Lee, L.; Wei, D. Relationship between the structure, physicochemical properties and in vitro digestibility of rice starches with different amylose contents. Food Hydrocoll. 2011, 25, 968–975. [Google Scholar] [CrossRef]
- Wang, W.; Ge, J.; Xu, K.; Gao, H.; Liu, G.; Wei, H.; Zhang, H. Differences in starch structure, thermal properties, and texture characteristics of rice from main stem and tiller panicles. Food Hydrocoll. 2020, 99, 105341. [Google Scholar] [CrossRef]
- Yanjie, X.; Yining, Y.; Shuhong, O.; Xiaoliang, D.; Hui, S.; Shukun, J.; Shichen, S.; Jinsong, B. Factors affecting sensory quality of cooked japonica rice. Rice Sci. 2018, 25, 330–339. [Google Scholar] [CrossRef]
- Patindol, J.A.; Siebenmorgen, T.J.; Wang, Y.J. Impact of environmental factors on rice starch structure: A review. Starch-Stärke 2015, 67, 42–54. [Google Scholar] [CrossRef]
- Kong, X.; Zhu, P.; Sui, Z.; Bao, J. Physicochemical properties of starches from diverse rice cultivars varying in apparent amylose content and gelatinisation temperature combinations. Food Chem. 2015, 172, 433–440. [Google Scholar] [CrossRef]
- Magallanes-Cruz, P.A.; Flores-Silva, P.C.; Bello-Perez, L.A. Starch structure influences its digestibility: A review. J. Food Sci. 2017, 82, 2016–2023. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Xie, B.; Shi, J.; Xue, S.; Deng, Q.; Wei, Y.; Tian, B. Physicochemical properties and structure of starches from Chinese rice cultivars. Food Hydrocoll. 2010, 24, 208–216. [Google Scholar] [CrossRef]
- Li, C.; Wu, A.; Yu, W.; Hu, Y.; Li, E.; Zhang, C.; Liu, Q. Parameterizing starch chain-length distributions for structure-property relations. Carbohydr. Polym. 2020, 241, 116390. [Google Scholar] [CrossRef]
- Asante, M.D. Breeding rice for improved grain quality. In Advances in International Rice Research; InTech: Rijeka, Croatia, 2017; pp. 69–89. [Google Scholar]
- RD. Rice Knowledge Bank. Available online: http://webold.ricethailand.go.th/rkb3/Varieties.htm (accessed on 19 March 2022).
- Custodio, M.C.; Cuevas, R.P.; Ynion, J.; Laborte, A.G.; Velasco, M.L.; Demont, M. Rice quality: How is it defined by consumers, industry, food scientists, and geneticists? Trends Food Sci. Technol. 2019, 92, 122–137. [Google Scholar] [CrossRef]
- Jiamyangyuen, S.; Wichaphon, J.; Boonmeejoy, J. Classification of rice cultivars by using chemical, physicochemical, thermal, hydration properties, and cooking quality. Food Appl. Biosci. J. 2019, 7, 42–62. [Google Scholar]
- Yoon, M.-R.; Koh, H.-J.; Kang, M.-Y. Pasting and amylose component characteristics of seven rice cultivars. J. Korean Soc. Appl. Biol. Chem. 2009, 52, 63–69. [Google Scholar] [CrossRef]
- Kesarwani, A.; Chiang, P.Y.; Chen, S.S. Rapid visco analyzer measurements of japonica rice cultivars to study interrelationship between pasting properties and farming system. Int. J. Agron. 2016, 2016, 3595326. [Google Scholar] [CrossRef] [Green Version]
- Chumsri, P.; Chaijan, M.; Panpipat, W. A comparison of nutritional values, physicochemical features and in vitro bioactivities of Southern Thai short-grain brown rice with commercial long-grain varieties. Int. J. Food Sci. Technol. 2021, 56, 6515–6526. [Google Scholar] [CrossRef]
- Gani, A.; Ashwar, B.A.; Akhter, G.; Shah, A.; Wani, I.A.; Masoodi, F.A. Physico-chemical, structural, pasting and thermal properties of starches of fourteen Himalayan rice cultivars. Int. J. Biol. Macromol. 2017, 95, 1101–1107. [Google Scholar] [CrossRef] [PubMed]
- Khoomtong, A.; Noomhorm, A. Development of a simple portable amylose content meter for rapid determination of amylose content in milled rice. Food Bioprocess Technol. 2015, 8, 1938–1946. [Google Scholar] [CrossRef]
- Wani, A.A.; Singh, P.; Shah, M.A.; Schweiggert-Weisz, U.; Gul, K.; Wani, I.A. Rice starch diversity: Effects on structural, morphological, thermal, and physicochemical properties—A review. Compr. Rev. Food Sci. Food Saf. 2012, 11, 417–436. [Google Scholar] [CrossRef]
- AOAC. AOAC Official Method 920.87 Protein (Total) in Flour. In Official Methods of Analysis AOAC International, 16th ed.; Cunniff, P., Ed.; AOAC International: Gaithersburg, MD, USA, 1999. [Google Scholar]
- AACC. Approved Methods of the American Association of Cereal Chemists, 10th ed.; Methods 32-40.01 and 44-19 ed.; The Association: St. Paul, MN, USA, 2000. [Google Scholar]
- Lee, C.-K.; Le, Q.-T.; Kim, Y.-H.; Shim, J.-H.; Lee, S.-J.; Park, J.-H.; Lee, K.-P.; Song, S.-H.; Auh, J.H.; Lee, S.-J. Enzymatic synthesis and properties of highly branched rice starch amylose and amylopectin cluster. J. Agric. Food Chem. 2008, 56, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Ramadoss, B.R.; Gangola, M.P.; Agasimani, S.; Jaiswal, S.; Venkatesan, T.; Sundaram, G.R.; Chibbar, R.N. Starch granule size and amylopectin chain length influence starch in vitro enzymatic digestibility in selected rice mutants with similar amylose concentration. J. Food Sci. Technol. 2019, 56, 391–400. [Google Scholar] [CrossRef]
- Rueden, C.T.; Schindelin, J.; Hiner, M.C.; DeZonia, B.E.; Walter, A.E.; Arena, E.T.; Eliceiri, K.W. ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinform. 2017, 18, 529. [Google Scholar] [CrossRef] [Green Version]
- Shujun, W.; Jinglin, Y.; Wenyuan, G. Use of X-ray diffractometry (XRD) for identification of Fritillaria according to geographical origin. Am. J. Biochem. Biotechnol. 2005, 1, 207–211. [Google Scholar] [CrossRef]
- Tananuwong, K.; Malila, Y. Changes in physicochemical properties of organic hulled rice during storage under different conditions. Food Chem. 2011, 125, 179–185. [Google Scholar] [CrossRef]
- AACC. Method 76-21.01, general pasting method for wheat or rye flour or starch using the rapid visco analyser. Approv. Methods Anal. 1999. [Google Scholar]
- Zhu, F. Relationships between amylopectin internal molecular structure and physicochemical properties of starch. Trends Food Sci. Technol. 2018, 78, 234–242. [Google Scholar] [CrossRef]
- Srichuwong, S.; Jane, J.-I. Physicochemical properties of starch affected by molecular composition and structures: A review. Food Sci. Biotechnol. 2007, 16, 663–674. [Google Scholar]
- Noda, T.; Tohnooka, T.; Taya, S.; Suda, I. Relationship between physicochemical properties of starches and white salted noodle quality in Japanese wheat flours. Cereal Chem. 2001, 78, 395–399. [Google Scholar] [CrossRef]
- Koroteeva, D.A.; Kiseleva, V.I.; Krivandin, A.V.; Shatalova, O.V.; Błaszczak, W.; Bertoft, E.; Piyachomkwan, K.; Yuryev, V.P. Structural and thermodynamic properties of rice starches with different genetic background: Part 2. Defectiveness of different supramolecular structures in starch granules. Int. J. Biol. Macromol. 2007, 41, 534–547. [Google Scholar] [CrossRef]
- Koroteeva, D.A.; Kiseleva, V.I.; Sriroth, K.; Piyachomkwan, K.; Bertoft, E.; Yuryev, P.V.; Yuryev, V.P. Structural and thermodynamic properties of rice starches with different genetic background: Part 1. Differentiation of amylopectin and amylose defects. Int. J. Biol. Macromol. 2007, 41, 391–403. [Google Scholar] [CrossRef]
- Kozlov, S.; Noda, T.; Bertoft, E.; Yuryev, V. Structure of starches extracted from near isogenic wheat lines: Part I. Effect of different GBSS I combinations. J. Therm. Anal. Calorim. 2006, 86, 291–301. [Google Scholar] [CrossRef]
- Pearce, M.; Marks, B.; Meullenet, J.F. Effects of postharvest parameters on functional changes during rough rice storage. Cereal Chem. 2001, 78, 354–357. [Google Scholar] [CrossRef]
- Varavinit, S.; Shobsngob, S.; Varanyanond, W.; Chinachoti, P.; Naivikul, O. Effect of amylose content on gelatinization, retrogradation and pasting properties of flours from different cultivars of Thai rice. Starch-Stärke 2003, 55, 410–415. [Google Scholar] [CrossRef]
- Cornejo-Ramírez, Y.I.; Martínez-Cruz, O.; Del Toro-Sánchez, C.L.; Wong-Corral, F.J.; Borboa-Flores, J.; Cinco-Moroyoqui, F.J. The structural characteristics of starches and their functional properties. CyTA-J. Food 2018, 16, 1003–1017. [Google Scholar] [CrossRef]
- Koch, K.; Jane, J.L. Morphological changes of granules of different starches by surface gelatinization with calcium chloride. Cereal Chem. 2000, 77, 115–120. [Google Scholar] [CrossRef]
- Wang, S.; Li, C.; Copeland, L.; Niu, Q.; Wang, S. Starch retrogradation: A comprehensive review. Compr. Rev. Food Sci. Food Saf. 2015, 14, 568–585. [Google Scholar] [CrossRef]
- Wiruch, P.; Naruenartwongsakul, S.; Chalermchart, Y. Textural properties, resistant starch, and in vitro starch digestibility as affected by parboiling of brown glutinous rice in a retort pouch. Curr. Res. Nutr. Food Sci. J. 2019, 7, 555–567. [Google Scholar] [CrossRef]


| Cultivars | Reducing Sugar Content (mg gFW−1) | Starch Content (mg gFW−1) | N Content (%) | Protein Content (N × 5.95) (%) | Amylose Content (%) | Moisture Content (%) |
|---|---|---|---|---|---|---|
| RD57 | 0.76 ± 0.02 ab | 713.61 ± 9.54 b | 0.25 ± 0.03 a | 1.51 ± 0.10 a | 23.60 ± 0.77 a | 9.22 ± 0.27 a |
| RD29 | 0.82 ± 0.03 a | 761.77 ± 12.46 ab | 0.23 ± 0.01 a | 1.39 ± 0.04 a | 22.80 ± 1.17 a | 9.53 ± 0.52 a |
| KDML105 | 0.81 ± 0.01 a | 791.43 ± 12.03 ab | 0.21 ± 0.03 a | 1.23 ± 0.10 a | 13.74 ± 0.69 b | 8.20 ± 0.34 a |
| RD6 | 0.65 ± 0.05 b | 794.86 ± 31.56 a | 0.20 ± 0.01 a | 1.19 ± 0.03 a | 1.65 ± 0.04 c | 9.58 ± 0.65 a |
| Cultivars | Amylopectin Branch Chain Length Distribution (%) | CL (AGU) | |||
|---|---|---|---|---|---|
| DP6–12 (A Chains) | DP13–24 (B1 Chains) | DP25–36 (B2 Chains) | DP ≥ 37 (B3+ Chains) | ||
| RD57 | 27.42 ± 0.28 b | 57.31 ± 0.10 a | 8.73 ± 0.02 b | 6.54 ± 0.34 ab | 18.12 ± 0.12 a |
| RD29 | 33.72 ± 0.29 a | 52.15 ± 0.09 b | 9.57 ± 0.06 a | 4.56 ± 0.33 b | 17.11 ± 0.12 b |
| KDML105 | 32.48 ± 0.39 a | 50.85 ± 0.30 c | 9.22 ± 0.02 b | 7.46 ± 0.68 a | 18.05 ± 0.22 a |
| RD6 | 32.48 ± 0.12 a | 51.16 ± 0.47 bc | 9.21 ± 0.11 b | 7.16 ± 0.60 a | 17.96 ± 0.18 a |
| Cultivars | Volume Distributions of Starch Granule (%) | d(0.5) m) | Relative Crystallinity (%) | SP (g/g) | S (%) | ||
|---|---|---|---|---|---|---|---|
| C-Type m) | B-Type m) | A-Type m) | |||||
| RD57 | 39.88 ± 2.78 b | 60.12 ± 2.78 a | 0.00 ± 0.00 b | 5.59 ± 0.27 a | 36.76 ± 0.86 ab | 17.66 ± 0.76 c | 27.01 ± 1.15 a |
| RD29 | 33.89 ± 2.05 b | 66.02 ± 1.98 a | 0.87 ± 0.07 b | 6.08 ± 0.10 a | 33.05 ± 0.74 c | 15.75 ± 0.55 c | 26.28 ± 1.60 a |
| KDML105 | 39.23 ± 2.06 b | 56.74 ± 1.99 a | 4.03 ± 0.28 a | 5.75 ± 0.17 a | 35.41 ± 0.46 bc | 28.14 ± 0.63 b | 14.24 ± 0.71 b |
| RD6 | 55.98 ± 3.27 a | 44.02 ± 3.27 b | 0.00 ± 0.00 b | 4.62 ± 0.21 b | 38.33 ± 0.55 a | 39.60 ± 0.78 a | 1.47 ± 0.12 c |
| Cultivars | Gelatinization Properties | ||||
|---|---|---|---|---|---|
| To(g) (°C) | Tp(g) (°C) | Tc(g) (°C) | ∆T(g) (°C) | ∆H(g) * (J/g) | |
| RD57 | 63.90 ± 0.36 a | 73.34 ± 0.21 a | 80.58 ± 0.11 a | 16.68 ± 0.32 d | 11.89 ± 0.11 b |
| RD29 | 57.28 ± 0.21 b | 65.64 ± 0.12 c | 76.03 ± 0.31 c | 18.75 ± 0.45 c | 10.74 ± 0.09 c |
| KDML105 | 57.23 ± 0.24 b | 68.14 ± 0.15 b | 79.46 ± 0.13 b | 22.23 ± 0.20 b | 11.65 ± 0.29 b |
| RD6 | 54.96 ± 0.15 c | 67.55 ± 0.39 b | 79.65 ± 0.26 b | 24.69 ± 0.33 a | 14.28 ± 0.12 a |
| Cultivars | Retrogradation Properties | |||||
|---|---|---|---|---|---|---|
| To(r) (°C) | Tp(r) (°C) | Tc(r) (°C) | ∆T(r) (°C) | ∆H(r) (J/g) | R% | |
| RD57 | 38.36 ± 0.15 b | 56.02 ± 0.11 a | 71.02 ± 0.16 a | 32.65 ± 0.31 a | 8.07 ± 0.11 a | 68.09 ± 1.64 a |
| RD29 | 42.36 ± 0.11 a | 54.24 ± 0.11 b | 67.27 ± 0.12 b | 24.90 ± 0.17 b | 3.52 ± 0.10 b | 32.79 ± 0.56 b |
| KDML105 | 42.71 ± 0.18 a | 54.56 ± 0.05 b | 67.39 ± 0.22 b | 24.69 ± 0.13 b | 3.08 ± 0.06 b | 26.46 ± 0.61 c |
| RD6 | 42.59 ± 0.29 a | 54.44 ± 0.17 b | 64.14 ± 0.23 c | 21.54 ± 0.32 c | 2.50 ± 0.13 c | 17.44 ± 0.74 d |
| Cultivars | Non-RS (%) | RS (%) |
|---|---|---|
| RD57 | 97.61 ± 0.51 a | 0.150 ± 0.01 a |
| RD29 | 97.83 ± 0.87 a | 0.153 ± 0.01 a |
| KDML105 | 98.36 ± 0.51 a | 0.027 ± 0.00 b |
| RD6 | 96.80 ± 0.81 a | 0.025 ± 0.00 b |
| Parameters | Sugar | Starch | NC | Protein | AC | MC | DP6–12 | DP13–24 | DP25–36 | DP≥ 37 | CL | C-Type | B-Type | A-Type | d(0.5) | RC |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SP | −0.600 * | 0.561 * | −0.590 * | −0.590 * | −0.967 ** | −0.031 | 0.284 | −0.577 * | 0.004 | 0.615 * | 0.415 | 0.812 ** | −0.869 ** | 0.187 | −0.757 ** | 0.657 ** |
| S | 0.551 * | −0.551 * | 0.706 * | 0.706 * | 0.962 ** | −0.040 | −0.378 | 0.633 * | −0.112 | −0.529 | −0.310 | −0.805 ** | 0.849 ** | −0.128 | 0.741 ** | −0.550 * |
| To(g) | 0.202 | −0.683 ** | 0.705 * | 0.705 * | 0.707 ** | −0.026 | −0.880 ** | 0.942 ** | −0.740 ** | −0.070 | 0.255 | −0.393 | 0.440 | −0.193 | 0.331 | −0.007 |
| Tp(g) | −0.075 | −0.470 | 0.471 | 0.471 | 0.309 | −0.025 | −0.968 ** | 0.870 ** | −0.936 ** | 0.279 | 0.579 * | −0.072 | 0.093 | −0.099 | 0.002 | 0.387 |
| Tc(g) | −0.427 | −0.027 | 0.033 | 0.033 | −0.284 | −0.181 | −0.710 ** | 0.395 | −0.853 ** | 0.721 ** | 0.859 ** | 0.399 | −0.447 | 0.193 | −0.404 | 0.756 ** |
| ∆T(g) | −0.456 | 0.716 ** | −0.755 ** | −0.755 ** | −0.916 ** | −0.074 | 0.570 | −0.813 ** | 0.337 | 0.478 | 0.199 | 0.654 * | −0.732 ** | 0.320 | −0.589 * | 0.433 |
| ∆H(g) | −0.759 ** | 0.279 | −0.470 | −0.470 | −0.864 ** | 0.222 | −0.004 | −0.199 | −0.239 | 0.454 | 0.384 | 0.900 ** | −0.877 ** | −0.228 | −0.860 ** | 0.759 ** |
| To(r) | 0.127 | 0.593 * | −0.646 * | −0.646 * | −0.554 | −0.121 | 0.948 ** | −0.955 ** | 0.794 ** | −0.043 | −0.373 | 0.179 | −0.260 | 0.387 | −0.114 | −0.262 |
| Tp(r) | −0.058 | −0.636 * | 0.558 | 0.558 | 0.424 | −0.163 | −0.948 ** | 0.925 ** | −0.827 ** | 0.106 | 0.428 | −0.079 | 0.122 | −0.209 | 0.006 | 0.369 |
| Tc(r) | 0.324 | −0.538 | 0.692 * | 0.692 * | 0.842 ** | −0.133 | −0.761 ** | 0.848 ** | −0.563 | −0.145 | 0.150 | −0.565 | 0.579 * | −0.008 | 0.511 | −0.086 |
| ∆T(r) | 0.136 | −0.586 * | 0.702 * | 0.702 * | 0.750 ** | −0.025 | −0.878 ** | 0.933 ** | −0.692 * | −0.067 | 0.256 | −0.417 | 0.462 | −0.178 | 0.356 | 0.066 |
| ∆H(r) | 0.012 | −0.638 * | 0.662 * | 0.662 * | 0.658 * | 0.082 | −0.926 ** | 0.972 ** | −0.761 ** | −0.039 | 0.297 | −0.289 | 0.360 | −0.322 | 0.217 | 0.157 |
| R% | 0.108 | −0.632 * | 0.706 * | 0.706 * | 0.746 ** | 0.067 | −0.883 ** | 0.957 ** | −0.689 * | −0.105 | 0.227 | −0.390 | 0.458 | −0.295 | 0.313 | 0.059 |
| Non-RS | 0.311 | 0.192 | 0.206 | 0.206 | 0.209 | 0.396 | 0.072 | 0.039 | 0.026 | −0.214 | −0.210 | −0.782 ** | 0.783 ** | 0.092 | 0.690 * | −0.452 |
| RS | 0.315 | −0.545 * | 0.675 * | 0.675 * | 0.818 ** | 0.295 | −0.339 | 0.676 * | −0.068 | −0.693 * | −0.461 | −0.637 * | 0.758 ** | −0.533 | 0.583 * | −0.470 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sangwongchai, W.; Tananuwong, K.; Krusong, K.; Natee, S.; Thitisaksakul, M. Starch Chemical Composition and Molecular Structure in Relation to Physicochemical Characteristics and Resistant Starch Content of Four Thai Commercial Rice Cultivars Differing in Pasting Properties. Polymers 2023, 15, 574. https://doi.org/10.3390/polym15030574
Sangwongchai W, Tananuwong K, Krusong K, Natee S, Thitisaksakul M. Starch Chemical Composition and Molecular Structure in Relation to Physicochemical Characteristics and Resistant Starch Content of Four Thai Commercial Rice Cultivars Differing in Pasting Properties. Polymers. 2023; 15(3):574. https://doi.org/10.3390/polym15030574
Chicago/Turabian StyleSangwongchai, Wichian, Kanitha Tananuwong, Kuakarun Krusong, Supidcha Natee, and Maysaya Thitisaksakul. 2023. "Starch Chemical Composition and Molecular Structure in Relation to Physicochemical Characteristics and Resistant Starch Content of Four Thai Commercial Rice Cultivars Differing in Pasting Properties" Polymers 15, no. 3: 574. https://doi.org/10.3390/polym15030574
APA StyleSangwongchai, W., Tananuwong, K., Krusong, K., Natee, S., & Thitisaksakul, M. (2023). Starch Chemical Composition and Molecular Structure in Relation to Physicochemical Characteristics and Resistant Starch Content of Four Thai Commercial Rice Cultivars Differing in Pasting Properties. Polymers, 15(3), 574. https://doi.org/10.3390/polym15030574