A Modified Ceramic-Coating Separator with High-Temperature Stability for Lithium-Ion Battery
Abstract
:1. Introduction
2. Experiments
2.1. Fabrication of the CCS-CS and CCS-CS-PDA
2.2. Electrode Preparation and Cell Assembly
2.3. Characterization of the Separators
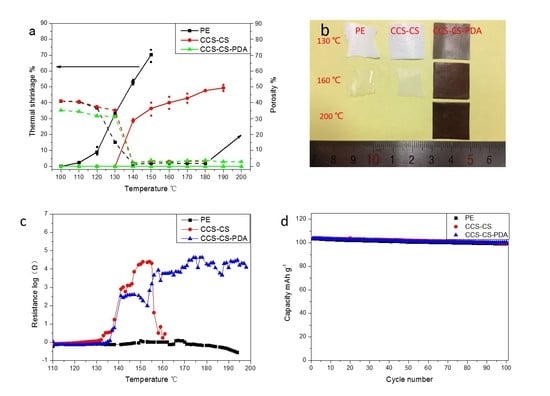
3. Results and Discussion
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Arora, P.; Zhang, Z. Battery separators. Chem. Rev. 2004, 104, 4419–4462. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.S. A review on the separators of liquid electrolyte Li-ion batteries. J. Power Sources 2007, 164, 351–364. [Google Scholar] [CrossRef]
- Shi, C.; Zhang, P.; Huang, S.; He, X.; Yang, P.; Wu, D.; Sun, D.; Zhao, J. Functional separator consisted of polyimide nonwoven fabrics and polyethylene coating layer for lithium-ion batteries. J. Power Sources 2015, 298, 158–165. [Google Scholar] [CrossRef]
- Choi, J.-A.; Kim, S.H.; Kim, D.-W. Enhancement of thermal stability and cycling performance in lithium-ion cells through the use of ceramic-coated separators. J. Power Sources 2010, 195, 6192–6196. [Google Scholar] [CrossRef]
- Woo, J.-J.; Zhang, Z.; Dietz Rago, N.L.; Lu, W.; Amine, K. A high performance separator with improved thermal stability for Li-ion batteries. J. Mater. Chem. A 2013, 1, 8538–8540. [Google Scholar] [CrossRef]
- Takemura, D.; Aihara, S.; Hamano, K.; Kise, M.; Nishimura, T.; Urushibata, H.; Yoshiyasu, H. A powder particle size effect on ceramic powder based separator for lithium rechargeable battery. J. Power Sources 2005, 146, 779–783. [Google Scholar] [CrossRef]
- Man, C.; Jiang, P.; Wong, K.-W.; Zhao, Y.; Tang, C.; Fan, M.; Lau, W.-M.; Mei, J.; Li, S.; Liu, H.; Hui, D. Enhanced wetting properties of a polypropylene separator for a lithium-ion battery by hyperthermal hydrogen induced cross-linking of poly(ethylene oxide). J. Mater. Chem. A 2014, 2, 11980–11986. [Google Scholar] [CrossRef]
- Park, J.-H.; Park, W.; Kim, J.H.; Ryoo, D.; Kim, H.S.; Jeong, Y.U.; Kim, D.-W.; Lee, S.-Y. Close-packed poly(methyl methacrylate) nanoparticle arrays-coated polyethylene separators for high-power lithium-ion polymer batteries. J. Power Sources 2011, 196, 7035–7038. [Google Scholar] [CrossRef]
- Ryou, M.-H.; Lee, D.J.; Lee, J.-N.; Lee, Y.M.; Park, J.-K.; Choi, J.W. Excellent cycle life of lithium-metal anodes in lithium-ion batteries with mussel-inspired polydopamine-coated separators. Adv. Energy Mater. 2012, 2, 645–650. [Google Scholar] [CrossRef]
- Jeong, H.-S.; Lee, S.-Y. Closely packed SiO2 nanoparticles/poly(vinylidene fluoride- hexafluoropropylene) layers-coated polyethylene separators for lithium-ion batteries. J. Power Sources 2011, 196, 6716–6722. [Google Scholar] [CrossRef]
- Kim, M.; Han, G.Y.; Yoon, K.J.; Park, J.H. Preparation of a trilayer separator and its application to lithium-ion batteries. J. Power Sources 2010, 195, 8302–8305. [Google Scholar] [CrossRef]
- Rao, M.M.; Liu, J.S.; Li, W.S.; Liao, Y.H.; Liang, Y.; Zhao, L.Z. Polyethylene-supported poly(acrylonitrile-co-methyl methacrylate)/nano-Al2O3 microporous composite polymer electrolyte for lithium ion battery. J. Sol. Stat. Electrochem. 2009, 14, 255–261. [Google Scholar] [CrossRef]
- Shin, W.-K.; Kim, D.-W. High performance ceramic-coated separators prepared with lithium ion-containing SiO2 particles for lithium-ion batteries. J. Power Sources 2013, 226, 54–60. [Google Scholar] [CrossRef]
- Zhang, S.; Xu, K.; Jow, T. An inorganic composite membrane as the separator of Li-ion batteries. J. Power Sources 2005, 140, 361–364. [Google Scholar] [CrossRef]
- Yanilmaz, M.; Zhang, X. Polymethylmethacrylate/polyacrylonitrile membranes via centrifugal spinning as separator in Li-ion batteries. Polymers 2015, 7, 629–643. [Google Scholar] [CrossRef]
- Cho, T.-H.; Tanaka, M.; Onishi, H.; Kondo, Y.; Nakamura, T.; Yamazaki, H.; Tanase, S.; Sakai, T. Battery performances and thermal stability of polyacrylonitrile nano-fiber-based nonwoven separators for Li-ion battery. J. Power Sources 2008, 181, 155–160. [Google Scholar] [CrossRef]
- Kim, J.-K.; Niedzicki, L.; Scheers, J.; Shin, C.-R.; Lim, D.-H.; Wieczorek, W.; Johansson, P.; Ahn, J.-H.; Matic, A.; Jacobsson, P. Characterization of N-butyl-N-methyl-pyrrolidinium bis(trifluoromethanesulfonyl)imide-based polymer electrolytes for high safety lithium batteries. J. Power Sources 2013, 224, 93–98. [Google Scholar] [CrossRef]
- Miao, Y.-E.; Zhu, G.-N.; Hou, H.; Xia, Y.-Y.; Liu, T. Electrospun polyimide nanofiber-based nonwoven separators for lithium-ion batteries. J. Power Sources 2013, 226, 82–86. [Google Scholar] [CrossRef]
- Zhai, Y.; Wang, N.; Mao, X.; Si, Y.; Yu, J.; Al-Deyab, S.S.; El-Newehy, M.; Ding, B. Sandwich-structured PVdF/PMIA/PVdF nanofibrous separators with robust mechanical strength and thermal stability for lithium ion batteries. J. Mater. Chem. A 2014, 2, 14511. [Google Scholar] [CrossRef]
- Zhang, X.; Ji, L.; Toprakci, O.; Liang, Y.; Alcoutlabi, M. Electrospun nanofiber-based anodes, cathodes, and separators for advanced lithium-ion batteries. Polym. Rev. 2011, 115, 239–264. [Google Scholar] [CrossRef]
- Wang, W.; Alexandridis, P. Composite polymer electrolytes: Nanoparticles affect structure and properties. Polymers 2016, 8, 387. [Google Scholar] [CrossRef]
- Costa, C.M.; Leones, R.; Silva, M.M.; Lanceros-Mendez, S. Influence of different salts in poly(vinylidene fluoride-co-trifluoroethylene) electrolyte separator membranes for battery applications. J. Electroanal. Chem. 2014, 727, 125–134. [Google Scholar] [CrossRef]
- Ito, Y.; Sakuda, A.; Ohtomo, T.; Hayashi, A.; Tatsumisago, M. Bulk-type all-solid-state lithium secondary batteries using highly ion-conductive sulfide solid electrolyte thin films. Electrochemistry 2014, 82, 591–594. [Google Scholar] [CrossRef]
- Liu, L.; Park, J.; Lin, X.; Sastry, A.M.; Lu, W. A thermal-electrochemical model that gives spatial-dependent growth of solid electrolyte interphase in a Li-ion battery. J. Power Sources 2014, 268, 482–490. [Google Scholar] [CrossRef]
- Radziuk, D.V.; Möhwald, H. Spectroscopic investigation of composite polymeric and monocrystalline systems with ionic conductivity. Polymers 2011, 3, 674–692. [Google Scholar] [CrossRef]
- Seino, Y.; Ota, T.; Takada, K. High rate capabilities of all-solid-state lithium secondary batteries using Li4Ti5O12-coated LiNi0.8Co0.15Al0.05O2 and a sulfide-based solid electrolyte. J. Power Sources 2011, 196, 6488–6492. [Google Scholar] [CrossRef]
- Shi, C.; Zhang, P.; Chen, L.; Yang, P.; Zhao, J. Effect of a thin ceramic-coating layer on thermal and electrochemical properties of polyethylene separator for lithium-ion batteries. J. Power Sources 2014, 217, 547–553. [Google Scholar] [CrossRef]
- Yang, P.; Zhang, P.; Shi, C.; Chen, L.; Dai, J.; Zhao, J. The functional separator coated with core-shell structured silica-poly(methyl methacrylate) sub-microspheres for lithium-ion batteries. J. Membr. Sci. 2015, 474, 148–155. [Google Scholar] [CrossRef]
- Dai, J.; Shi, C.; Li, C.; Shen, X.; Peng, L.; Wu, D.; Sun, D.; Zhang, P.; Zhao, J. A rational design of separator with substantially enhanced thermal features for lithium-ion batteries by the polydopamine-ceramic composite modification of polyolefin membranes. Energy Environ. Sci. 2016, 9, 3252–3261. [Google Scholar] [CrossRef]
- Cao, C.; Tan, L.; Liu, W.; Ma, J.; Li, L. Polydopamine coated electrospun poly(vinyldiene fluoride) nanofibrous membrane as separator for lithium-ion batteries. J. Power Sources 2014, 218, 224–229. [Google Scholar] [CrossRef]
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-inspired surface chemistry for multifunctional coatings. Science 2007, 318, 426–430. [Google Scholar] [CrossRef] [PubMed]
- Ryou, M.H.; Lee, Y.M.; Park, J.K.; Choi, J.W. Mussel-inspired polydopamine-treated polyethylene separators for high-power li-ion batteries. Adv. Mater. 2011, 23, 3066–3070. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zhao, Z.; Yu, L.; Zhang, K.; Na, H.; Ying, S.; Xu, D.; Zhang, G. Polydopamine hydrophilic modification of polypropylene separator for lithium ion battery. J. Appl. Polym. Sci. 2014, 131, 427–436. [Google Scholar] [CrossRef]
- Xu, Q.; Kong, Q.; Liu, Z.; Zhang, J.; Wang, X.; Liu, R.; Yue, L.; Cui, G. Polydopamine-coated cellulose microfibrillated membrane as high performance lithium-ion battery separator. RSC Adv. 2014, 4, 7845. [Google Scholar] [CrossRef]
- Shi, C.; Dai, J.; Huang, S.; Li, C.; Shen, X.; Zhang, P.; Wu, D.; Sun, D.; Zhao, J. A simple method to prepare a polydopamine modified core-shell structure composite separator for application in high-safety lithium-ion batteries. J. Membr. Sci. 2016, 518, 168–177. [Google Scholar] [CrossRef]
- Chen, S.; Yu, M.; Han, W.-P.; Yan, X.; Liu, Y.-C.; Zhang, J.-C.; Zhang, H.-D.; Yu, G.-F.; Long, Y.-Z. Electrospun anatase TiO2 nanorods for flexible optoelectronic devices. RSC Adv. 2014, 4, 46152–46156. [Google Scholar] [CrossRef]
- Huang, X.; Hitt, J. Lithium ion battery separators: Development and performance characterization of a composite membrane. J. Membr. Sci. 2013, 425, 163–168. [Google Scholar] [CrossRef]
- Jeong, H.-S.; Hong, S.C.; Lee, S.-Y. Effect of microporous structure on thermal shrinkage and electrochemical performance of Al2O3/poly(vinylidene fluoride-hexafluoropropylene) composite separators for lithium-ion batteries. J. Membr. Sci. 2010, 36, 177–182. [Google Scholar] [CrossRef]







| Separator | PE separator | CCS-CS | CCS-CS-PDA |
|---|---|---|---|
| Weight mg | 3.1 | 4.3 | 4.6 ± 0.1 |
| Porosity % | 41.5 ± 0.5 | 41.2 ± 0.5 | 35.3 ± 0.5 |
| Average uptake % | 54 ± 1 | 71.2 ± 2 | 70.3 ± 2 |
| Contact angle with electrolyte | 35 | 0 | 0 |
| AC impedance mS·cm–1 | 0.78 ± 0.01 | 1.10 ± 0.01 | 0.71 ± 0.01 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, C.; Dai, J.; Li, C.; Shen, X.; Peng, L.; Zhang, P.; Wu, D.; Sun, D.; Zhao, J. A Modified Ceramic-Coating Separator with High-Temperature Stability for Lithium-Ion Battery. Polymers 2017, 9, 159. https://doi.org/10.3390/polym9050159
Shi C, Dai J, Li C, Shen X, Peng L, Zhang P, Wu D, Sun D, Zhao J. A Modified Ceramic-Coating Separator with High-Temperature Stability for Lithium-Ion Battery. Polymers. 2017; 9(5):159. https://doi.org/10.3390/polym9050159
Chicago/Turabian StyleShi, Chuan, Jianhui Dai, Chao Li, Xiu Shen, Longqing Peng, Peng Zhang, Dezhi Wu, Daoheng Sun, and Jinbao Zhao. 2017. "A Modified Ceramic-Coating Separator with High-Temperature Stability for Lithium-Ion Battery" Polymers 9, no. 5: 159. https://doi.org/10.3390/polym9050159
APA StyleShi, C., Dai, J., Li, C., Shen, X., Peng, L., Zhang, P., Wu, D., Sun, D., & Zhao, J. (2017). A Modified Ceramic-Coating Separator with High-Temperature Stability for Lithium-Ion Battery. Polymers, 9(5), 159. https://doi.org/10.3390/polym9050159