Spermine: Its Emerging Role in Regulating Drought Stress Responses in Plants
Abstract
:1. Introduction
2. Spermine Biosynthesis and Metabolism in Plants
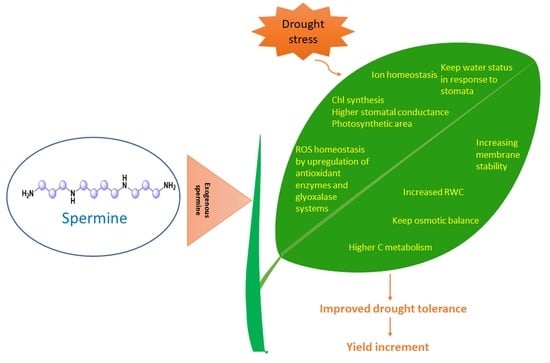
3. Spermine Induced Drought Tolerance in Plants
4. Spermine Activates Antioxidant Response in Plants under Drought Stress
5. Interaction of Spermine with Other Molecules in Drought Tolerance
6. Omics Strategies for Using Spermine to Reduce Drought-Induced Oxidative Stress
6.1. Transcriptomics
6.2. Proteomics
7. Future Prospects and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tsaniklidis, G.; Pappi, P.; Tsafouros, A.; Charova, S.N.; Nikoloudakis, N.; Roussos, P.A.; Paschalidis, K.A.; Delis, C. Polyamine Homeostasis in Tomato Biotic/Abiotic Stress Cross-Tolerance. Gene 2020, 727, 144230. [Google Scholar] [CrossRef]
- Hussain, S.S.; Ali, M.; Ahmad, M.; Siddique, K.H. Polyamines: Natural and engineered abiotic and biotic stress tolerance in plants. Biotechnol. Adv. 2011, 29, 300–311. [Google Scholar] [CrossRef] [PubMed]
- Sequera-Mutiozabal, M.; Antoniou, C.; Tiburcio, A.F.; Alcázar, R.; Fotopoulos, V. Polyamines: Emerging Hubs Promoting Drought and Salt Stress Tolerance in Plants. Curr. Mol. Bio. Rep. 2017, 3, 28–36. [Google Scholar] [CrossRef]
- Zhang, X.; Shen, L.; Li, F.; Meng, D.; Sheng, J. Methyl salicylate-induced arginine catabolism is associated with up-regulation of polyamine and nitric oxide levels and improves chilling tolerance in cherry tomato fruit. J. Agric. Food Chem. 2011, 59, 9351–9357. [Google Scholar] [PubMed]
- Tiburcio, A.F.; Altabella, T.; Bitrián, M.; Alcázar, R. The roles of polyamines during the lifespan of plants: From development to stress. Planta 2014, 240, 1–18. [Google Scholar] [CrossRef]
- Feng, H.Y.; Wang, Z.M.; Kong, F.N.; Zhang, M.J.; Zhou, S.L. Roles of carbohydrate supply and ethylene, polyamines in maize kernel set. J. Integ. Plant Biol. 2011, 53, 388–398. [Google Scholar] [CrossRef]
- Alet, A.I.; Sánchez, D.H.; Cuevas, J.C.; Marina, M.; Carrasco, P.; Altabella, T.; Tiburcio, A.F.; Ruiz, O.A. New insights into the role of spermine in Arabidopsis thaliana under long-term salt stress. Plant Sci. 2012, 182, 94–100. [Google Scholar] [CrossRef]
- Tavladoraki, P.; Cona, A.; Federico, R.; Tempera, G.; Viceconte, N.; Saccoccio, S.; Battaglia, V.; Toninello, A.; Agostinelli, E. Polyamine catabolism: Target for antiproliferative therapies in animals and stress tolerance strategies in plants. Amino Acids 2012, 42, 411–426. [Google Scholar] [CrossRef]
- Hassan, N.; Ebeed, H.; Aljaarany, A. Exogenous application of spermine and putrescine mitigate adversities of drought stress in wheat by protecting membranes and chloroplast ultra-structure. Physiol. Mol. Biol. Plants 2020, 26, 233–245. [Google Scholar] [CrossRef]
- Dawood, M.F.; Abeed, A.H. Spermine-priming restrained water relations and biochemical deteriorations prompted by water deficit on two soybean cultivars. Heliyon 2020, 6, e04038. [Google Scholar] [CrossRef]
- Liu, C.J.; Wang, H.R.; Wang, L.; Han, Y.Y.; Hao, J.H.; Fan, S.X. Effects of different types of polyamine on growth, physiological and biochemical nature of lettuce under drought stress. IOP Conf. Ser. Earth Environ. Sci. 2018, 185, 012010. [Google Scholar] [CrossRef]
- Ebeed, H.T.; Hassan, N.M.; Aljarani, A.M. Exogenous applications of polyamines modulate drought responses in wheat through osmolytes accumulation, increasing free polyamine levels and regulation of polyamine biosynthetic genes. Plant Physiol. Biochem. 2017, 118, 438–448. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Jing, W.; Peng, Y.; Zhang, X.Q.; Ma, X.; Huang, L.K. Spermine alleviates drought stress in white clover with different resistance by influencing carbohydrate metabolism and dehydrins synthesis. PLoS ONE 2015, 10, e0120708. [Google Scholar] [CrossRef] [Green Version]
- Taie, H.A.; El-Yazal, M.A.S.; Ahmed, S.M.; Rady, M.M. Polyamines modulate growth, antioxidant activity, and genomic DNA in heavy metal–stressed wheat plant. Environ. Sci. Pollut. Res. 2019, 1, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Benavides, M.P.; Groppa, M.D.; Recalde, L.; Verstraeten, S.V. Effects of polyamines on cadmium-and copper-mediated alterations in wheat (Triticum aestivum L.) and sunflower (Helianthus annuus L.) seedling membrane fluidity. Arch. Biochem. Biophys. 2018, 654, 27–39. [Google Scholar] [CrossRef]
- Rady, M.M.; Hemida, K.A. Modulation of cadmium toxicity and enhancing cadmium-tolerance in wheat seedlings by exogenous application of polyamines. Ecotoxicol. Environ. Saf. 2015, 119, 178–185. [Google Scholar]
- Fu, X.Z.; Xing, F.; Wang, N.Q.; Peng, L.Z.; Chun, C.P.; Cao, L.; Ling, L.L.; Jiang, C.L. Exogenous spermine pretreatment confers tolerance to combined high-temperature and drought stress in vitro in trifoliate orange seedlings via modulation of antioxidative capacity and expression of stress-related genes. Biotech. Biotechnol. Equip. 2014, 28, 192–198. [Google Scholar] [CrossRef] [Green Version]
- Jankovska-Bortkevič, E.; Gavelienè, V.; Šveikauskas, V.; Mockevičiutè, R.; Jankauskienè, J.; Todorova, D.; Sergiev, I.; Jurkonienè, S. Foliar application of polyamines modulates winter oilseed rape responses to increasing cold. Plants 2020, 9, 179. [Google Scholar] [CrossRef] [Green Version]
- Nahar, K.; Hasanuzzaman, M.; Alam, M.M.; Rahman, A.; Mahmud, J.A.; Suzuki, T.; Fujita, M. Insights into spermine-induced combined high temperature and drought tolerance in mung bean: Osmoregulation and roles of antioxidant and glyoxalase system. Protoplasma 2017, 254, 445–460. [Google Scholar] [CrossRef]
- Hasan, M.M.; Alharby, H.F.; Hajar, A.S.; Hakeem, K.R.; Alzahrani, Y. The effect of magnetized water on the growth and physiological conditions of Moringa species under drought stress. Pol. J. Environ. Stud. 2019, 28, 1145–1155. [Google Scholar]
- Hasan, M.M.; Alharby, H.F.; Uddin, M.N.; Ali, M.A.; Anwar, Y.; Fang, X.W.; Hakeem, K.R.; Alzahrani, Y.; Hajar, A.S. Magnetized water confers drought stress tolerance in Moringa biotype via modulation of growth, gas exchange, lipid peroxidation and antioxidant activity. Pol. J. Environ. Stud. 2020, 1, 29. [Google Scholar]
- Hasan, M.M.; Ali, M.A.; Soliman, M.H.; Alqarawi, A.A.; Abd Allah, E.F.; Fang, X.-W. Insights into 28-homobrassinolide (HBR)-mediated redox homeostasis, AsA–GSH cycle, and methylglyoxal detoxification in soybean under drought-induced oxidative stress. J. Plant Inter. 2020, 15, 371–385. [Google Scholar] [CrossRef]
- Khan, A.; Anwar, Y.; Hasan, M.; Iqbal, A.; Ali, M.; Alharby, H.F.; Hakeem, K.R.; Hasanuzzaman, M. Attenuation of drought stress in Brassica seedlings with exogenous application of Ca2+ and H2O2. Plants 2017, 6, 20. [Google Scholar] [CrossRef] [PubMed]
- Ahammed, G.J.; Li, X.; Wan, H.; Zhou, G.; Cheng, Y. SlWRKY81 reduces drought tolerance by attenuating proline biosynthesis in tomato. Scientia Hortic. 2020, 270. [Google Scholar] [CrossRef]
- Ahammed, G.J.; Li, X.; Mao, Q.; Wan, H.; Zhou, G.; Cheng, Y. The SlWRKY81 transcription factor inhibits stomatal closure by attenuating nitric oxide accumulation in the guard cells of tomato under drought. Physiol. Plantarum 2020. [Google Scholar] [CrossRef]
- Vanani, F.R.; Shabani, L.; Sabzalian, M.R.; Dehghanian, F.; Winner, L. Comparative physiological and proteomic analysis indicates lower shock response to drought stress conditions in a self-pollinating perennial ryegrass. PLoS ONE 2020, 15, e0234317. [Google Scholar] [CrossRef]
- Li, K.; Xing, C.; Yao, Z.; Huang, X. Pbr MYB 21, a novel MYB protein of Pyrus betulaefolia, functions in drought tolerance and modulates polyamine levels by regulating arginine decarboxylase gene. Plant Biotechnol. J. 2017, 15, 1186–1203. [Google Scholar] [CrossRef] [Green Version]
- Adamipour, N.; Khosh-Khui, M.; Salehi, H.; Razi, H.; Karami, A.; Moghadam, A. Role of genes and metabolites involved in polyamines synthesis pathways and nitric oxide synthase in stomatal closure on Rosa damascena Mill. under drought stress. Plant Physiol. Biochem. 2020, 148, 53–61. [Google Scholar]
- Nahar, K.; Rahman, M.; Hasanuzzaman, M.; Alam, M.M.; Rahman, A.; Suzuki, T.; Fujita, M. Physiological and biochemical mechanisms of spermine-induced cadmium stress tolerance in mung bean (Vigna radiata L.) seedlings. Environ. Sci. Pollut. Res. 2016, 23, 21206–21218. [Google Scholar] [CrossRef]
- Rai, P.K. Heavy metals/metalloids remediation from wastewater using free floating macrophytes of a natural wetland. Environ. Technol. Innovation. 2019, 15, 100393. [Google Scholar] [CrossRef]
- Shelp, B.J.; Bozzo, G.G.; Trobacher, C.P.; Zarei, A.; Deyman, K.L.; Brikis, C.J. Hypothesis/review: Contribution of putrescine to 4-aminobutyrate (GABA) production in response to abiotic stress. Plant Sci. 2012, 193, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Alcázar, R.; Altabella, T.; Marco, F.; Bortolotti, C.; Reymond, M.; Koncz, C.; Carrasco, P.; Tiburcio, A.F. Polyamines: Molecules with regulatory functions in plant abiotic stress tolerance. Planta 2010, 231, 1237–1249. [Google Scholar] [PubMed]
- Sequera-Mutiozabal, M.; Tiburcio, A.F.; Alcázar, R. Drought Stress Tolerance in Relation to Polyamine Metabolism in Plants. In Drought Stress Tolerance in Plants; Hossain, M., Wani, S., Bhattacharjee, S., Burritt, D., Tran, L.S., Eds.; Springer: Cham, Switzerland, 2016; Volume 1. [Google Scholar] [CrossRef]
- Li, H.; Guo, Y.; Cui, Q.; Zhang, Z.; Yan, X.; Ahammed, G.J.; Yang, X.; Yang, J.; Wei, C.; Zhang, X. Alkanes (C29 and C31)-Mediated Intracuticular Wax Accumulation Contributes to Melatonin- and ABA-Induced Drought Tolerance in Watermelon. J. Plant Growth Reg. 2020. [Google Scholar] [CrossRef]
- Mueller, N.D.; Gerber, J.S.; Johnston, M.; Ray, D.K.; Ramankutty, N.; Foley, J.A. Closing yield gaps through nutrient and water management. Nature 2012. [Google Scholar] [CrossRef] [PubMed]
- Iwuala, E.; Odjegba, V.; Sharma, V.; Alam, A. Drought stress modulates expression of aquaporin gene and photosynthetic efficiency in Pennisetum glaucum (L.) R. Br. genotypes. Curr. Plant Biol. 2020, 21, 100131. [Google Scholar] [CrossRef]
- Maurel, C.; Boursiac, Y.; Luu, D.T.; Santoni, V.R.; Shahzad, Z.; Verdoucq, L. Aquaporins in plants. Physiol. Rev. 2015, 95, 1321–1358. [Google Scholar] [CrossRef]
- Li, Z.; Hou, J.; Zhang, Y.; Zeng, W.; Cheng, B.; Hassan, M.J.; Zhang, Y.; Pu, Q.; Peng, Y. Spermine regulates water balance associated with Ca2+-dependent aquaporins (TrTIP2-1, TrTIP2-2, and TrPIP2-7) expression in plants under water stress. Plant Cell Physiol. 2020, 61, 1576–1589. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, J.; Liu, K.; Wang, Z.; Liu, L. Involvement of polyamines in the drought resistance of rice. J. Exp. Bot. 2007, 58, 1545–1555. [Google Scholar] [CrossRef] [Green Version]
- Tiburcio, A.F.; Alcázar, R. Potential Applications of Polyamines in Agriculture and Plant Biotechnology. In Polyamines. Methods in Molecular Biology; Alcázar, R., Tiburcio, A., Eds.; Humana Press: New York, NY, USA, 2018; Volume 1694. [Google Scholar] [CrossRef]
- Misra, B.B.; Acharya, B.R.; Granot, D.; Assmann, S.M.; Chen, S. The guard cell metabolome: Functions in stomatal movement and global food security. Front. Plant Sci. 2015, 6, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Agurla, S.; Gayatri, G.; Raghavendra, A.S. Polyamines increase nitric oxide and reactive oxygen species in guard cells of Arabidopsis thaliana during stomatal closure. Protoplasma 2018, 255, 153–162. [Google Scholar] [CrossRef]
- Fujita, Y.; Fujita, M.; Shinozaki, K.; Yamaguchi-Shinozaki, K. ABA-mediated transcriptional regulation in response to osmotic stress in plants. J. Plant Res. 2011, 124, 509–525. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liang, H.; Lv, X.; Liu, D.; Wen, X.; Liao, Y. Effect of polyamines on the grain filling of wheat under drought stress. Plant Physiol. Biochem. 2016, 100, 113–129. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Rodríguez, E.; Romero, L.; Ruiz, J. Accumulation of free polyamines enhances the antioxidant response in fruits of grafted tomato plants under water stress. J. Plant Physiol. 2016, 190, 72–78. [Google Scholar] [PubMed]
- Juzoń, K.; Czyczyło-Mysza, I.; Marcińska, I.; Dziurka, M.; Waligórski, P.; Skrzypek, E. Polyamines in yellow lupin (Lupinus luteus L.) tolerance to soil drought. Acta Physiol. Plantarum. 2017, 39, 202. [Google Scholar] [CrossRef]
- Shi, H.; Ye, T.; Chan, Z. Comparative proteomic and physiological analyses reveal the protective effect of exogenous polyamines in the bermudagrass (Cynodon dactylon) response to salt and drought stresses. J. Proteome Res. 2013, 12, 4951–4964. [Google Scholar] [CrossRef]
- Krishnan, S.; Merewitz, E.B. Polyamine application effects on gibberellic acid content in creeping bentgrass during drought stress. J. Amer. Soc. Hortic. Sci. 2017, 142, 135–142. [Google Scholar] [CrossRef]
- Yamaguchi, K.; Takahashi, Y.; Berberich, T.; Imai, A.; Takahashi, T.; Michael, A.J.; Kusano, T. A protective role for the polyamine spermine against drought stress in Arabidopsis. Biochem. Biophy.Res. Commun. 2007, 352, 486–490. [Google Scholar] [CrossRef] [PubMed]
- Arasimowicz-Jelonek, M.; Floryszak-Wieczorek, J.; Kubiś, J. Interaction between polyamine and nitric oxide signalling in adaptive responses to drought in cucumber. J. Plant Growth Reg. 2009, 28, 177–186. [Google Scholar] [CrossRef]
- Yin, Z.P.; Li, S.; Ren, J.; Song, X.S. Role of spermidine and spermine in alleviation of drought-induced oxidative stress and photosynthetic inhibition in Chinese dwarf cherry (Cerasus humilis) seedlings. Plant Growth Reg. 2014, 74, 209–218. [Google Scholar] [CrossRef]
- Talaat, N.B.; Shawky, B.T. Dual application of 24-epibrassinolide and spermine confers drought stress tolerance in maize (Zea mays L.) by modulating polyamine and protein metabolism. J. Plant Growth Reg. 2016, 35, 518–533. [Google Scholar] [CrossRef]
- Talaat, N.B.; Shawky, B.T.; Ibrahim, A.S. Alleviation of drought-induced oxidative stress in maize (Zea mays L.) plants by dual application of 24-epibrassinolide and spermine. Environ. Exp. Bot. 2015, 113, 47–58. [Google Scholar] [CrossRef]
- Farooq, M.; Wahid, A.; Lee, D.-J. Exogenously applied polyamines increase drought tolerance of rice by improving leaf water status, photosynthesis and membrane properties. Acta Physiol. Plant. 2009, 31, 937–945. [Google Scholar] [CrossRef]
- Shi, J.; Fu, X.-Z.; Peng, T.; Huang, X.-S.; Fan, Q.-J.; Liu, J.-H. Spermine pre-treatment confers dehydration tolerance of citrus in vitro plants via modulation of antioxidative capacity and stomatal response. Tree Physiol. 2010, 30, 914–922. [Google Scholar] [CrossRef] [PubMed]
- Hassan, F.A.; Ali, E.F.; Alamer, K.H. Exogenous application of polyamines alleviates water stress-induced oxidative stress of Rosa damascena Miller var. trigintipetala Dieck. S. Afr. J. Bot. 2018, 116, 96–102. [Google Scholar] [CrossRef]
- Radhakrishnan, R.; Lee, I.J. Spermine promotes acclimation to osmotic stress by modifying antioxidant, abscisic acid, and jasmonic acid signals in soybean. J. Plant Growth Reg. 2013, 32, 22–30. [Google Scholar] [CrossRef]
- Mustafavi, S.H.; Shekari, F.; Maleki, H.H. Influence of exogenous polyamines on antioxidant defence and essential oil production in valerian (Valeriana offcinalis L.) plants under drought stress. Acta Agric. Slov. 2016, 107, 81–91. [Google Scholar] [CrossRef]
- Montesinos-Pereira, D.; Barrameda-Medina, Y.; Romero, L.; Ruiz, J.M.; Sánchez-Rodríguez, E. Genotype differences in the metabolism of proline and polyamines under moderate drought in tomato plants. Plant Biol. 2014, 16, 1050–1057. [Google Scholar] [CrossRef]
- Do, P.T.; Drechsel, O.; Heyer, A.G.; Hincha, D.K.; Zuther, E. Changes in free polyamine levels, expression of polyamine biosynthesis genes, and performance of rice cultivars under salt stress: A comparison with responses to drought. Front. Plant Sci. 2014, 5, 182. [Google Scholar] [CrossRef] [Green Version]
- Seifi, H.S.; Shelp, B.J. Spermine differentially refines plant defense responses against biotic and abiotic stresses. Front. Plant Sci. 2019, 10, 117. [Google Scholar] [CrossRef] [Green Version]
- Ma, Y.; Zhang, J.; Li, X.; Zhang, S.; Lan, H. Effects of environmental stress on seed germination and seedling growth of Salsola ferganica (Chenopodiaceae). Acta Ecol. Sin. 2016, 36, 456–463. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Caverzan, A.; Casassola, A.; Brammer, S.P. Reactive oxygen species and antioxidant enzymes involved in plant tolerance to stress. In Abiotic and Biotic Stress in Plants—Recent Advances and Future Perspectives; InTech: London, UK, 2016. [Google Scholar]
- Guler, N.S.; Pehlivan, N. Exogenous low-dose hydrogen peroxide enhances drought tolerance of soybean (Glycine max L.) through inducing antioxidant system. Acta Biol. Hung. 2016, 67, 169–183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abedi, T.; Pakniyat, H. Antioxidant enzyme changes in response to drought stress in ten cultivars of oilseed rape (Brassica napus). Czech J. Genet. Plant Breed 2010, 46, 27–34. [Google Scholar] [CrossRef]
- Slabbert, M.M.; Krüger, G.H.J. Antioxidant enzyme activity, proline accumulation, leaf area and cell membrane stability in water stressed Amaranthus leaves. S. Afr. J. Bot. 2014, 95, 123–128. [Google Scholar] [CrossRef] [Green Version]
- Capell, T.; Bassie, L.; Christou, P. Modulation of the polyamine biosynthetic pathway in transgenic rice confers tolerance to drought stress. Proc. Nat. Acad. Sci. USA 2004, 101, 9909–9914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wimalasekera, R.; Tebartz, F.; Scherer, G.F. Polyamines, polyamine oxidases and nitric oxide in development, abiotic and biotic stresses. Plant Sci. 2011, 181, 593–603. [Google Scholar] [CrossRef]
- An, Z.F.; Jing, W.; Liu, Y.L.; Zhang, W.H. Hydrogen peroxide generated by copper amine oxidase is involved in abscisic acid-induced stomatal closure in Vicia faba. J. Exp. Bot. 2008, 59, 815–825. [Google Scholar] [CrossRef] [Green Version]
- Alcázar, R.; Cuevas, J.C.; Patrón, M.; Altabella, T.; Tiburcio, A.F. Abscisic acid modulates polyamine metabolism under water stress in Arabidopsis thaliana. Physiol. Plant 2006, 128, 448–455. [Google Scholar] [CrossRef]
- Marco, F.; Busó, E.; Lafuente, T.; Carrasco, P. Spermine confers stress resilience by modulating abscisic acid biosynthesis and stress responses in Arabidopsis plants. Front. Plant Sci. 2019, 10, 972. [Google Scholar] [CrossRef] [Green Version]
- Bitrián, M.; Zarza, X.; Altabella, T.; Tiburcio, A.F.; Alcázar, R. Polyamines under abiotic stress: Metabolic crossroads and hormonal crosstalks in plants. Metabolites 2012, 2, 516–528. [Google Scholar] [CrossRef] [Green Version]
- Klingler, J.P.; Batelli, G.; Zhu, J.-K. ABA receptors: The START of a new paradigm in phytohormone signalling. J. Exp.Bot. 2010, 61, 3199–3210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toumi, I.; Moschou, P.N.; Paschalidis, K.A.; Bouamama, B.; Salem-Fnayou, A.B.; Ghorbel, A.W.; Mliki, A.; Roubelakis-Angelakis, K.A. Abscisic acid signals reorientation of polyamine metabolism to orchestrate stress responses via the polyamine exodus pathway in grapevine. J. Plant Physiol. 2010, 167, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Marco, F.; Alcázar, R.; Tiburcio, A.F.; Carrasco, P. Interactions between polyamines and abiotic stress pathway responses unraveled by transcriptome analysis of polyamine overproducers. OMICS 2011, 15, 775–781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tun, N.N.; Santa-Catarina, C.; Begum, T.; Silveira, V.; Handro, W.; Floh, E.I.S.; Scherer, G.F.E. Polyamines induce rapid biosynthesis of nitric oxide (NO) in Arabidopsis thaliana seedlings. Plant Cell Physiol. 2006, 47, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Parra-Lobato, M.C.; Gomez-Jimenez, M.C. Polyamine-induced modulation of genes involved in ethylene biosynthesis and signalling pathways and nitric oxide production during olive mature fruit abscission. J. Exp. Bot. 2011, 62, 4447–4465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamasaki, H.; Cohen, M.F. NO signal at the crossroads: Polyamine-induce nitric oxide synthesis in plants? Trends Plant Sci. 2006, 11, 522–524. [Google Scholar] [CrossRef]
- Houben, M.; Van De Poel, B. 1-Aminocyclopropane-1-carboxylic acid oxidase (ACO): The enzyme that makes the plant hormone ethylene. Front. Plant Sci. 2019, 10, 695. [Google Scholar] [CrossRef] [Green Version]
- Pan, C.Z.; Zhang, H.; Ma, Q.M.; Fan, F.J.; Ahammed, G.J.; Yu, J.; Shi, K. Role of ethylene biosynthesis and signaling in elevated CO2-induced heat stress response in tomato. Planta 2019, 250, 563–572. [Google Scholar]
- Del Duca, S.; Serafini-Fracassini, D.; Cai, G. Senescence and programmed cell death in plants: Polyamine action mediated by transglutaminase. Front. Plant Sci. 2014, 5, 120. [Google Scholar] [CrossRef] [Green Version]
- Alcázar, R.; Fortes, A.M.; Tiburcio, A.F. Editorial: Polyamines in plant biotechnology, food nutrition and human health. Front. Plant Sci. 2020, 11, 120. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, Y.; Cong, R.; Sagor, G.; Niitsu, M.; Berberich, T.; Kusano, T. Characterization of five polyamine oxidase isoforms in Arabidopsis thaliana. Plant Cell Rep. 2010, 29, 955–965. [Google Scholar] [CrossRef] [PubMed]
- Pal, M.; Szalai, G.; Janda, T. Speculation: Polyamines are important in abiotic stress signaling. Plant Sci. 2015, 237, 16–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serna, M.; Coll, Y.; Zapata, P.J.; Botella, M.Á.; Pretel, M.T.; Amorós, A. A brassinosteroid analogue prevented the effect of salt stress on ethylene synthesis and polyamines in lettuce plants. Sci. Hortic. 2015, 185, 105–112. [Google Scholar] [CrossRef]
- Wang, X.-L.; Zhang, Y.-X. Regulation of salicylic acid on polyamine synthesize under NaCl stress in leaves of the yali pear. Res. J. Appl. Sci. Eng. Technol. 2012, 4, 3704–3708. [Google Scholar]
- Iqbal, M.; Ashraf, M.; Jamil, A.; Ur-Rehman, S. Does seed priming induce changes in the levels of some endogenous plant hormones in hexaploid wheat plants under salt stress? J. Integr. Plant Biol. 2006, 48, 181–189. [Google Scholar] [CrossRef]
- Echevarría-Machado, I.; Ku-González, A.; Loyola-Vargas, V.M.; Hernández-Sotomayor, S.T. Interaction of spermine with a signal transduction pathway involving phospholipase C, during the growth of Catharanthus roseus transformed roots. Physiol. Plant. 2004, 120, 140–151. [Google Scholar] [CrossRef]
- Zarza, X.; Shabala, L.; Fujita, M.; Shabala, S.; Haring, M.A.; Tiburcio, A.F. Extracellular spermine triggers a rapid intracellular phosphatidic acid response in Arabidopsis, involving PLDδ activation and stimulating ion flux. Front. Plant Sci. 2019, 10, 601. [Google Scholar] [CrossRef]
- Raman, V.P.; Rajam, M.V. Polyamine accumulation in transgenic eggplant enhances tolerance to multiple abiotic stresses and fungal resistance. Plant Biotechnol. 2007, 24, 273–282. [Google Scholar]
- Bassie, L.; Zhu, C.; Romagosa, I.; Christou, P.; Capell, T. Transgenic wheat plants expressing an oat arginine decarboxylase cDNA exhibit increases in polyamine content in vegetative tissue and seeds. Mol. Breed. 2008, 22, 39–50. [Google Scholar]
- Peremarti, A.; Bassie, L.; Christou, P.; Capell, T. Spermine facilitates recovery from drought but does not confer drought tolerance in transgenic rice plants expressing Datura stramonium S-adenosylmethionine decarboxylase. Plant Mol. Biol. 2009, 70, 253–264. [Google Scholar] [CrossRef]
- Momtaz, O.A.; Hussein, E.M.; Fahmy, E.M.; Ahmed, S.E. Expression of S-adenosyl methionine decarboxylase gene for polyamine accumulation in Egyptian cotton Giza 88 and Giza 90. GM Crops 2010, 1, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Hazarika, P.; Rajam, M.V. Biotic and abiotic stress tolerance in transgenic tomatoes by constitutive expression of S-adenosylmethionine decarboxylase gene. Physiol. Mol. Biol. Plants. 2011, 17, 115–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, L.; Geng, S.; Zhao-Hui, Z.; Xue-Lian, Z.; Ke-Jun, D. Overexpression of SAMDC gene from Salvia miltiorrhiza enhances drought tolerance in transgenic tobacco (Nicotiana tabacum). J. Agric. Biotech. 2017, 25, 729–738. [Google Scholar]
- Jiang, X.; Zhan, J.; Wang, Q.; Wu, X.; Chen, X.; Jia, B.; Liu, P.; Liu, L.; Ye, Z.; Zhu, L.; et al. Overexpression of the pear PbSPMS gene in Arabidopsis thaliana increases resistance to abiotic stress. Plant Cell Tissue Organ Cult. 2020, 140, 389–401. [Google Scholar] [CrossRef]
- Gonzalez, M.E.; Marco, F.; Minguet, E.G.; Carrasco-Sorli, P.; Blázquez, M.A.; Carbonell, J.; Ruiz, O.A.; Pieckenstain, F.L. Perturbation of spermine synthase gene expression and transcript profiling provide new insights on the role of the tetraamine spermine in Arabidopsis defense against Pseudomonas viridiflava. Plant Physiol. 2011, 156, 2266–2277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.H.; Wang, W.; Wu, H.; Gong, X.; Moriguchi, T. Polyamines function in stress tolerance: From synthesis to regulation. Front. Plant Sci. 2015, 6, 827. [Google Scholar] [CrossRef] [Green Version]
- Alcázar, R.; Bueno, M.; Tiburcio, A.F. Polyamines: Small Amines with Large Effects on Plant Abiotic Stress Tolerance. Cells 2020, 9, 2373. [Google Scholar] [CrossRef]
- Zhao, Y.; Du, H.; Wang, Z.; Huang, B. Identification of proteins associated with water-deficit tolerance in C4 perennial grass species, Cynodon dactylon× Cynodon transvaalensis and Cynodon dactylon. Physiol. Plant 2011, 141, 40–55. [Google Scholar] [CrossRef]



| Species | Stress | Spermine Treatment | Effect | Outcome | References |
|---|---|---|---|---|---|
| Arabidopsis thaliana | Drought stress (1/2 MS agar plates) | 1 mM (exogenous pretreated seedlings) | Enhanced chlorophyll content, potential role in stomatal movement | Spm protected against drought stress | [49] |
| Cynodon dactilon | Drought stress (withholding water) | 5 mM (exogenous) | Proteins involved in ROS balance stimulated by spermine. Energy-related pathways stimulated by Spm treatment | Improved drought stress tolerance | [47] |
| Cucumber | Drought | 1 mM (pretreated seed) | Reduced ion leakage from the membrane and less lipid peroxidation | Nitric oxide acts downstream of Spm during drought stress to enhance stress tolerance | [50] |
| Creeping Bentgrass (Penn G2) | Drought (withholding water) | 1 mM (exogenous) | Spermine-treated plants maintained significantly higher turf quality (TQ), relative water content (RWC), and photochemical efficiency | Protected creeping bentgrass from drought stress | [48] |
| Chinese dwarf cherry (Cerasus humilis) | Drought stress (withholding water) | 0.2 mM (exogenous) | Increased RWC and prevented lipid peroxidation | Prevented drought-induced oxidative damage | [51] |
| Lettuce | Drought (10% polyethylene glycol, PEG) | 0.1 mM (exogenous) | Increased plant height and root length. Upregulated antioxidant activity | Significantly alleviated drought stress | [11] |
| Maize | Drought (50% and 75% field capacity) | 25 mgL (exogenous) | Increased content of protein, phenolic, flavonoids, and amino acids | Improved drought tolerance by increasing ethylene and polyamine synthesis | [52] |
| Maize (Giza 10 and Giza 129 cultivars) | Drought (50% and 75% field capacity) | 25 mgL (exogenous) | Stimulated synthesis of antioxidant enzymes, and promoted ROS scavenging | Enhanced drought tolerance and reduced ROS accumulation | [53] |
| Mung bean (Vigna radiata L. cv. BARI Mung-2) | Combined drought and high temperature stress | 0.2 mM (exogenous pretreated seedlings) | Upregulated antioxidant enzymes. Reduced methylglyoxal toxicity by stimulating glyoxalase systems | Improved tolerance to drought and high temperature stress | [29] |
| Orange (Poncirus trifoliata [L.] Raf.) | Combined heat and drought | 1 mmol L-1 (exogenous pretreated seedlings) | Activated antioxidant enzymes such as CAT, SOD, and peroxidases; induced heat shock proteins and abscisic acid-response element binding factors | Enhanced drought and heat tolerance in a perennial fruit crop | [16] |
| Oryza sativa | Drought (50% field capacity) | 10 µM (seed priming treatments and foliar application) | Activated antioxidant enzymes. Enhanced ROS scavenging and stress-related gene expression | Enhanced drought and heat tolerance in rice seedlings | [54] |
| Red tangerine (Citrus reticulata Blanco) | Drought (MS agar plates) | 1 mM (pretreated seed) | Increased enzymatic antioxidant activity such as SOD and peroxidase and ROS scavenging | Prevented oxidative damage and increased drought tolerance | [55] |
| Rosa damascena Miller var. trigintipetala Dieck | Drought (50% and 100% field capacity) | 0.5 mM (exogenous) | Improved growth (RWC), photosynthetic pigments and stomatal conductance(gs) | Mitigated drought stress | [56] |
| Soybean cultivars (Giza 111 and Gazi 21) | Drought (0, −0.1, −0.5, and −1.1 MPa) | 0.2 mM (pretreated seed) | Pigment enhancement, membrane stabilization, osmolyte accumulation, and water balance | Increased drought tolerance of soybean cultivar | [10] |
| Soybean | Drought (9% PEG) | 0.2 mM (exogenous) | Enhanced CAT, SOD, and POD activities; reduced lipid peroxidation | Improved drought tolerance of soybean | [57] |
| Valerian | Drought (withholding water) | 0.1 mM (exogenous) | Increased photosynthetic pigments and antioxidant enzyme activity | Improved drought tolerance | [58] |
| Wheat | Drought (withholding water) | 100 µM (exogenous) | Increased photosynthetic pigments, antioxidants, and Rubisco | Enhanced drought tolerance of wheat by reduction of oxidative injury | [9] |
| Wheat | Drought (withholding water) | 100 µM (exogenous) | Increased cell water status and accumulation of osmoprotectants | Improved drought tolerance | [32] |
| Wheat | Drought (soil water potential at −60 ± 5 kPa) | 1 mM (exogenous) | Relieved inhibition caused by drought stress | Enhanced grain filling and drought resistance | [44] |
| White clover | Drought stress (20% PEG 6000) | 0.5 mM (exogenous) | Improved sugar metabolism and dehydrin biosynthesis | Mitigated drought stress | [33] |
| Gene | Source | Transgenic Plant | Abiotic Stress Tolerance | References |
|---|---|---|---|---|
| ADC | Datura stramonium | Oryza sativa | Drought | [68] |
| ADC | Avena sativa | Solanum meloangena | Drought, high temperature | [91] |
| ADC | Avena sativa | Triticum aestivum | Drought | [92] |
| SAMDC | Datura stramonium | Oryza sativa | Drought | [93] |
| SAMDC | Saccharomyces cerevisiae | Egyptian cotton varieties. Giza 88, Giza-90 | Drought | [94] |
| SAMDC | Saccharomyces cerevisiae | Solanum lycopersicum cv. Pusa Ruby | Drought, Salt | [95] |
| SAMDC | Sesamum indicum | Nicotiana tabacum | Drought | [96] |
| SPMS | Pyrus bretschneideri | Arabidopsis thaliana | Drought, Salt | [97] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hasan, M.M.; Skalicky, M.; Jahan, M.S.; Hossain, M.N.; Anwar, Z.; Nie, Z.-F.; Alabdallah, N.M.; Brestic, M.; Hejnak, V.; Fang, X.-W. Spermine: Its Emerging Role in Regulating Drought Stress Responses in Plants. Cells 2021, 10, 261. https://doi.org/10.3390/cells10020261
Hasan MM, Skalicky M, Jahan MS, Hossain MN, Anwar Z, Nie Z-F, Alabdallah NM, Brestic M, Hejnak V, Fang X-W. Spermine: Its Emerging Role in Regulating Drought Stress Responses in Plants. Cells. 2021; 10(2):261. https://doi.org/10.3390/cells10020261
Chicago/Turabian StyleHasan, Md. Mahadi, Milan Skalicky, Mohammad Shah Jahan, Md. Nazmul Hossain, Zunaira Anwar, Zheng-Fei Nie, Nadiyah M. Alabdallah, Marian Brestic, Vaclav Hejnak, and Xiang-Wen Fang. 2021. "Spermine: Its Emerging Role in Regulating Drought Stress Responses in Plants" Cells 10, no. 2: 261. https://doi.org/10.3390/cells10020261
APA StyleHasan, M. M., Skalicky, M., Jahan, M. S., Hossain, M. N., Anwar, Z., Nie, Z. -F., Alabdallah, N. M., Brestic, M., Hejnak, V., & Fang, X. -W. (2021). Spermine: Its Emerging Role in Regulating Drought Stress Responses in Plants. Cells, 10(2), 261. https://doi.org/10.3390/cells10020261