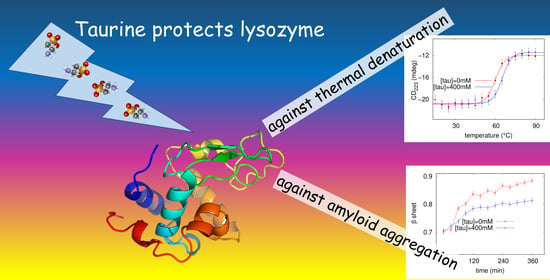
Taurine Stabilizing Effect on Lysozyme
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Small Angle X-ray Scattering
2.3. UV-Visible Spectrophotometry
2.4. Circular Dichroism
2.5. AFM
2.6. Cell Culture and Treatments
2.7. Cell Viability Assay
3. Results
3.1. Native Interactions
3.2. Thermal Unfolding
3.3. Amyloid Aggregation
4. Discussion and Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| MDPI | Multidisciplinary Digital Publishing Institute |
| SAXS | Small Angle X-ray Scattering |
| CD | circular dichroism |
References
- Dobson, C.M. Protein folding and misfolding. Nature 2003, 426, 884–890. [Google Scholar] [CrossRef] [PubMed]
- Pohl, C.; Dikic, I. Cellular quality control by the ubiquitin-proteasome system and autophagy. Science 2019, 366, 818–822. [Google Scholar] [CrossRef]
- Hartl, F.U.; Hayer-Hartl, M. Molecular Chaperones in the Cytosol: From Nascent Chain to Folded Protein. Science 2002, 295, 1852–1858. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhao, C.; Luo, F.; Liu, Z.; Gui, X.; Luo, Z.; Zhang, X.; Li, D.; Liu, C.; Li, X. Amyloid fibril structure of alpha-synuclein determined by cryoelectron microscopy. Cell Res. 2018, 28, 897–903. [Google Scholar] [CrossRef]
- Knowles, T.P.J.; Vendruscolo, M.; Dobson, C.M. The amyloid state and its association with protein misfolding diseases. Nat. Rev. Mol. Cell Biol. 2014, 15, 384–396. [Google Scholar] [CrossRef]
- Ke, P.C.; Sani, M.A.; Ding, F.; Kakinen, A.; Javed, I.; Separovic, F.; Davis, T.P.; Mezzenga, R. Implications of peptide assemblies in amyloid diseases. Chem. Soc. Rev. 2017, 46, 6492–6531. [Google Scholar] [CrossRef] [PubMed]
- Goldschmidt, L.; Teng, P.K.; Riek, R.; Eisenberg, D. Identifying the amylome, proteins capable of forming amyloid-like fibrils. Proc. Natl. Acad. Sci. USA 2010, 107, 3487–3492. [Google Scholar] [CrossRef] [PubMed]
- Swaminathan, R.; Ravi, V.K.; Kumar, S.; Kumar, M.V.S.; Chandra, N. Lysozyme: A model protein for amyloid research. In Advances in Protein Chemistry and Structural Biology; Academic Press: Cambridge, MA, USA, 2011; Volume 84, pp. 63–111. [Google Scholar] [CrossRef]
- Buell, A.K. The growth of amyloid fibrils: Rates and mechanisms. Biochem. J. 2019, 476, 2677–2703. [Google Scholar] [CrossRef] [PubMed]
- Sgarbossa, A.; Giacomazza, D.; Di Carlo, M. Ferulic acid: A hope for Alzheimer’s disease therapy from plants. Nutrients 2015, 7, 5764–5782. [Google Scholar] [CrossRef] [PubMed]
- Battisti, A.; Palumbo Piccionello, A.; Sgarbossa, A.; Vilasi, S.; Ricci, C.; Ghetti, F.; Spinozzi, F.; Marino Gammazza, A.; Giacalone, V.; Martorana, A.; et al. Curcumin-like compounds designed to modify amyloid beta peptide aggregation patterns. RSC Adv. 2017, 7, 31714–31724. [Google Scholar] [CrossRef]
- Mangione, M.R.; Palumbo Piccionello, A.; Marino, C.; Ortore, M.G.; Picone, P.; Vilasi, S.; Di Carlo, M.; Buscemi, S.; Bulone, D.; San Biagio, P.L. Photo-inhibition of A[small beta] fibrillation mediated by a newly designed fluorinated oxadiazole. RSC Adv. 2015, 5, 16540–16548. [Google Scholar] [CrossRef]
- Behl, T.; Kaur, I.; Fratila, O.; Brata, R.; Bungau, S. Exploring the Potential of Therapeutic Agents Targeted towards Mitigating the Events Associated with Amyloid-β Cascade in Alzheimer’s Disease. Int. J. Mol. Sci. 2020, 21, 7443. [Google Scholar] [CrossRef]
- Malafaia, D.; Albuquerque, H.M.; Silva, A.M. Amyloid-β and tau aggregation dual-inhibitors: A synthetic and structure-activity relationship focused review. Eur. J. Med. Chem. 2021, 214, 113209. [Google Scholar] [CrossRef]
- Kushwah, N.; Jain, V.; Yadav, D. Osmolytes: A Possible Therapeutic Molecule for Ameliorating the Neurodegeneration Caused by Protein Misfolding and Aggregation. Biomolecules 2020, 10, 132. [Google Scholar] [CrossRef] [PubMed]
- Yancey, P.; Clark, M.; Hand, S.; Bowlus, R.; Somero, G. Living with water stress: Evolution of osmolyte systems. Science 1982, 217, 1214–1222. [Google Scholar] [CrossRef] [PubMed]
- Zou, Q.; Bennion, B.; Daggett, V.; Murphy, K. The Molecular Mechanism of Stabilization of Proteins by TMAO and Its Ability to Counteract the Effects of Urea. J. Am. Chem. Soc. 2002, 124, 1192. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.T.; Manson, A.C.; DeLyser, M.R.; Noid, W.G.; Cremer, P.S. Trimethylamine N-oxide stabilizes proteins via a distinct mechanism compared with betaine and glycine. Proc. Natl. Acad. Sci. USA 2017, 114, 2479–2484. [Google Scholar] [CrossRef] [PubMed]
- Bruzdziak, P.; Panuszko, A.; Stangret, J. Influence of Osmolytes on Protein and Water Structure: A Step To Understanding the Mechanism of Protein Stabilization. J. Phys. Chem. B 2013, 117, 11502–11508. [Google Scholar] [CrossRef] [PubMed]
- Corezzi, S.; Bracco, B.; Sassi, P.; Paolantoni, M.; Comez, L. Protein Hydration in a Bioprotecting Mixture. Life 2021, 11, 995. [Google Scholar] [CrossRef] [PubMed]
- Cozzolino, S.; Tortorella, A.; Del Vecchio, P.; Graziano, G. General Counteraction Exerted by Sugars against Denaturants. Life 2021, 11, 652. [Google Scholar] [CrossRef]
- Singh, L.R.; Poddar, N.K.; Dar, T.A.; Kumar, R.; Ahmad, F. Protein and DNA destabilization by osmolytes: The other side of the coin. Life Sci. 2011, 88, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Jamal, S.; Poddar, N.K.; Singh, L.R.; Dar, T.A.; Rishi, V.; Ahmad, F. Relationship between functional activity and protein stability in the presence of all classes of stabilizing osmolytes. FEBS J. 2009, 276, 6024–6032. [Google Scholar] [CrossRef]
- Huxtable, R.J. Physiological actions of taurine. Physiol. Rev. 1992, 72, 101–163. [Google Scholar] [CrossRef] [PubMed]
- Engelborghs, S.; Marescau, B.; De Deyn, P. Amino acids and biogenic amines in cerebrospinal fluid of patients with Parkinson’s disease. Neurochem. Res. 2003, 28, 1145–1150. [Google Scholar] [CrossRef] [PubMed]
- Arai, H.; Kobayashi, K.; Ichimiya, Y.; Kosaka, K.; Iizuka, R. A preliminary study of free amino acids in the postmorten temporal cortex from Alzheimer-type dementia patients. Neurobiol. Aging 1984, 5, 319–321. [Google Scholar] [CrossRef]
- Kim, H.Y.; Kim, H.V.; Yoon, J.H.; Kang, B.R.; Cho, S.M.; Lee, S.; Kim, J.Y.; Kim, J.W.; Cho, Y.; Woo, J.; et al. Taurine in drinking water recovers learning and memory in the adult APP/PS1 mouse model of Alzheimer’s disease. Sci. Rep. 2014, 7467. [Google Scholar] [CrossRef]
- Jang, H.; Lee, S.; Choi, S.; Kim, H.; Baek, S.; Kim, Y. Taurine Directly Binds to Oligomeric Amyloid-β and Recovers Cognitive Deficits in Alzheimer Model Mice. In Taurine 10; Lee, D., Schaffer, S., Park, E., Kim, H.W., Eds.; Springer: Dordrecht, The Netherlands, 2017; p. 975. [Google Scholar] [CrossRef]
- Gorgani, S.; Jahanshahi, M.; Elyasi, L. Taurine Prevents Passive Avoidance Memory Impairment, Accumulation of Amyloid-β Plaques, and Neuronal Loss in the Hippocampus of Scopolamine-Treated Rats. Neurophysiology 2019, 51, 171–179. [Google Scholar] [CrossRef]
- Wu, G.; Ren, S.; Tang, R.; Xu, C.; Zhou, J.; Lin, S.; Feng, Y.; Yang, Q.; Hu, J.; Yang, J. Antidepressant effect of taurine in chronic unpredictable mild stress-induced depressive rats. Sci. Rep. 2017, 4989. [Google Scholar] [CrossRef] [PubMed]
- Che, Y.; Hou, L.; Sun, F.; Zhang, C.; Liu, X.; Piao, F.; Zhang, D.; Li, H.; Wang, Q. Taurine protects dopaminergic neurons in a mouse Parkinson’s disease model through inhibition of microglial M1 polarization. Cell Death Dis. 2018, 9. [Google Scholar] [CrossRef]
- Jakaria, M.; Azam, S.; Haque, M.E.; Jo, S.H.; Uddin, M.S.; Kim, I.S.; Choi, D.K. Taurine and its analogs in neurological disorders: Focus on therapeutic potential and molecular mechanisms. Redox Biol. 2019, 24, 101223. [Google Scholar] [CrossRef]
- Chaturvedi, S.K.; Alam, P.; Khan, J.M.; Siddiqui, M.K.; Kalaiarasan, P.; Subbarao, N.; Ahmad, Z.; Khan, R.H. Biophysical insight into the anti-amyloidogenic behavior of taurine. Int. J. Biol. Macromol. 2015, 80, 375–384. [Google Scholar] [CrossRef]
- Macchi, F.; Eisenkolb, M.; Kiefer, H.; Otzen, D.E. The effect of osmolytes on protein fibrillation. Int. J. Mol. Sci. 2012, 13, 3801–3819. [Google Scholar] [CrossRef] [PubMed]
- Santa-María, I.; HernÁndez, F.; Moreno, F.J.; Avila, J. Taurine, an inducer for tau polymerization and a weak inhibitor for amyloid-β-peptide aggregation. Neurosci. Lett. 2007, 429, 91–94. [Google Scholar] [CrossRef] [PubMed]
- Arakawa, T.; Timasheff, S. The stabilization of proteins by osmolytes. Biophys. J. 1985, 47, 411–414. [Google Scholar] [CrossRef]
- Brudziak, P.; Panuszko, A.; Kaczkowska, E.; Piotrowski, B.; Daghir, A.; Demkowicz, S.; Stangret, J. Taurine as a water structure breaker and protein stabilizer. Amino Acids 2018, 50, 125–140. [Google Scholar] [CrossRef] [PubMed]
- Pieraccini, S.; Burgi, L.; Genoni, A.; Benedusi, A.; Sironi, M. Atomic level description of the protecting effect of osmolytes against thermal denaturation of proteins. Chem. Phys. Lett. 2007, 438, 298–303. [Google Scholar] [CrossRef]
- Abe, Y.; Ohkuri, T.; Yoshitomi, S.; Murakami, S.; Ueda, T. Role of the osmolyte taurine on the folding of a model protein, hen egg white lysozyme, under a crowding condition. Amino Acids 2015, 47, 909–915. [Google Scholar] [CrossRef] [PubMed]
- Sassi, P.; Giugliarelli, A.; Paolantoni, M.; Morresi, A.; Onori, G. Unfolding and aggregation of lysozyme: A thermodynamic and kinetic study by FTIR spectroscopy. Biophys. Chem. 2011, 158, 46–53. [Google Scholar] [CrossRef]
- Maroufi, B.; Ranjbar, B.; Khajeh, K.; Naderi-Manesh, H.; Yaghoubi, H. Structural studies of hen egg-white lysozyme dimer: Comparison with monomer. Biochim. Biophys. Acta (BBA)—Proteins Proteom. 2008, 1784, 1043–1049. [Google Scholar] [CrossRef]
- Arnaudov, L.N.; de Vries, R. Thermally Induced Fibrillar Aggregation of Hen Egg White Lysozyme. Biophys. J. 2005, 88, 515–526. [Google Scholar] [CrossRef]
- Trautwein, E.A.; Hayes, K.C. Taurine concentrations in plasma and whole blood in humans: Estimation of error from intra- and interindividual variation and sampling technique. Am. J. Clin. Nutr. 1990, 52, 758–764. [Google Scholar] [CrossRef]
- Poniková, S.; Antošová, A.; Demjén, E.; Sedláková, D.; Marek, J.; Varhač, R.; Gažová, Z.; Sedlák, E. Lysozyme stability and amyloid fibrillization dependence on Hofmeister anions in acidic pH. J. Biol. Inorg. Chem. 2015, 20, 921–933. [Google Scholar] [CrossRef] [PubMed]
- Mari, E.; Ricci, C.; Pieraccini, S.; Spinozzi, F.; Mariani, P.; Ortore, M.G. Trehalose Effect on The Aggregation of Model Proteins into Amyloid Fibrils. Life 2020, 10, 60. [Google Scholar] [CrossRef] [PubMed]
- Amenitsch, H.; Rappolt, M.; Kriechbaum, M.; Mio, H.; Laggner, P.; Bernstorff, S. First performance assessment of the small-angle X-ray scattering beamline at ELETTRA. J. Synchrotron Radiat. 1998, 5, 506–508. [Google Scholar] [CrossRef] [PubMed]
- Haider, R.; Sartori, B.; Radeticchio, A.; Wolf, M.; Dal Zilio, S.; Marmiroli, B.; Amenitsch, H. μDrop: A system for high-throughput small-angle X-ray scattering measurements of microlitre samples. J. Appl. Crystallogr. 2021, 54, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Spinozzi, F.; Ferrero, C.; Ortore, M.G.; De Maria Antolinos, A.; Mariani, P. GENFIT: Software for the analysis of small-angle X-ray and neutron scattering data of macromolecules in solution. J. Appl. Crystallogr. 2014, 47, 1132–1139. [Google Scholar] [CrossRef] [PubMed]
- Frid, P.; Anisimov, S.V.; Popovic, N. Congo red and protein aggregation in neurodegenerative diseases. Brain Res. Rev. 2007, 53, 135–160. [Google Scholar] [CrossRef] [PubMed]
- Magi, S.; Preziuso, A.; Piccirillo, S.; Giampieri, F.; Cianciosi, D.; Orciani, M.; Amoroso, S. The Neuroprotective Effect of L-Carnitine against Glyceraldehyde-Induced Metabolic Impairment: Possible Implications in Alzheimer’s Disease. Cells 2021, 10, 2109. [Google Scholar] [CrossRef] [PubMed]
- Van Meerloo, J.; Kaspers, G.J.L.; Cloos, J. Cell sensitivity assays: The MTT assay. Methods Mol. Biol. 2011, 731, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Magi, S.; Piccirillo, S.; Maiolino, M.; Lariccia, V.; Amoroso, S. NCX1 and EAAC1 transporters are involved in the protective action of glutamate in an in vitro Alzheimer’s disease-like model. Cell Calcium 2020, 91, 102268. [Google Scholar] [CrossRef]
- Mossuto, M.F.; Dhulesia, A.; Devlin, G.; Frare, E.; Kumita, J.R.; de Laureto, P.P.; Dumoulin, M.; Fontana, A.; Dobson, C.M.; Salvatella, X. The Non-Core Regions of Human Lysozyme Amyloid Fibrils Influence Cytotoxicity. J. Mol. Biol. 2010, 402, 783–796. [Google Scholar] [CrossRef] [PubMed]
- Ortore, M.G.; Mariani, P.; Carsughi, F.; Cinelli, S.; Onori, G.; Teixeira, J.; Spinozzi, F. Preferential solvation of lysozyme in water/ethanol mixtures. J. Chem. Phys. 2011, 135, 245103. [Google Scholar] [CrossRef]
- Schroer, M.A.; Tolan, M.; Winter, R. Exploring the thermodynamic derivatives of the structure factor of dense protein solutions. Phys. Chem. Chem. Phys. 2012, 14, 9486–9491. [Google Scholar] [CrossRef]
- Diamond, R. Real-space refinement of the structure of hen egg-white lysozyme. J. Mol. Biol. 1974, 82, 371–391. [Google Scholar] [CrossRef]
- Ortore, M.G.; Spinozzi, F.; Mariani, P.; Paciaroni, A.; Barbosa, L.R.S.; Amenitsch, H.; Steinhart, M.; Ollivier, J.; Russo, D. Combining structure and dynamics: Non-denaturing high-pressure effect on lysozyme in solution. J. R. Soc. Interface R. Soc. 2009, 6 (Suppl. 5), S619–S634. [Google Scholar] [CrossRef] [PubMed]
- Spassov, V.Z.; Yan, L. A fast and accurate computational approach to protein ionization. Protein Sci. 2008, 17, 1955–1970. [Google Scholar] [CrossRef] [PubMed]
- Julius, K.; Weine, J.; Berghaus, M.; König, N.; Gao, M.; Latarius, J.; Paulus, M.; Schroer, M.A.; Tolan, M.; Winter, R. Water-Mediated Protein-Protein Interactions at High Pressures are Controlled by a Deep-Sea Osmolyte. Phys. Rev. Lett. 2018, 121, 038101. [Google Scholar] [CrossRef]
- Svergun, D.; Richard, S.; Koch, M.H.J.; Sayers, Z.; Kuprin, S.; Zaccai, G. Protein hydration in solution: Experimental obbservation by X-ray, neutron scattering. Proc. Natl. Acad. Sci. USA 1998, 95, 2267–2272. [Google Scholar] [CrossRef] [PubMed]
- Panuszko, A.; Pieloszczyk, M.; Kuffel, A.; Jacek, K.; Biernacki, K.A.; Demkowicz, S.; Stangret, J.; Bruzdziak, P. Hydration of Simple Model Peptides in Aqueous Osmolyte Solutions. Int. J. Mol. Sci. 2021, 22, 9350. [Google Scholar] [CrossRef]
- Ranjbar, B.; Gill, P. Circular Dichroism Techniques: Biomolecular and Nanostructural Analyses- A Review. Chem. Biol. Drug Des. 2009, 74, 101–120. [Google Scholar] [CrossRef] [PubMed]
- Scholtz, J.M.; Marqusee, S.; Baldwin, R.L.; York, E.J.; Stewart, J.M.; Santoro, M.; Bolen, D.W. Calorimetric determination of the enthalpy change for the alpha-helix to coil transition of an alanine peptide in water. Proc. Natl. Acad. Sci. USA 1991, 88, 2854–2858. [Google Scholar] [CrossRef]
- Kelly, C.; Gage, M.J. Protein Unfolding: Denaturant vs. Force. Biomedicines 2021, 9, 1395. [Google Scholar] [CrossRef] [PubMed]
- Manning, M.C.; Illangasekare, M.; Woody, R.W. Circular dichroism studies of distorted a-helices, twisted β-sheets, and β-turns. Biophys. Chem. 1988, 31, 77–86. [Google Scholar] [CrossRef]
- Hill, S.E.; Robinson, J.; Matthews, G.; Muschol, M. Amyloid protofibrils of lysozyme nucleate and grow via oligomer fusion. Biophys. J. 2009, 96, 3781–3790. [Google Scholar] [CrossRef]
- Vetri, V.; Fodera, V. The route to protein aggregate superstructures: Particulates and amyloid-like spherulites. FEBS Lett. 2015, 589, 2448–2463. [Google Scholar] [CrossRef] [PubMed]
- Taurine Market Growth Driven by Surging Demand for Taurine in the Food & Beverage Industry: Reports and Data. 2021. Available online: https://www.medgadget.com/2021/10/taurine-market-growth-driven-by-surging-demand-for-taurine-in-the-food-beverage-industry-reports-and-data.html (accessed on 8 December 2021).
- Niebuhr, M.; Koch, M.H.J. Effects of Urea and Trimethylamine-N-Oxide (TMAO) on the Interactions of Lysozyme in Solution. Biophys. J. 2005, 89, 1978–1983. [Google Scholar] [CrossRef]
- Ufnal, M.; Zadlo, A.; Ostaszewski, R. TMAO: A small molecule of great expectations. Nutrition 2015, 31, 1317–1323. [Google Scholar] [CrossRef] [PubMed]
- Sinibaldi, R.; Casieri, C.; Melchionna, S.; Onori, G.; Segre, A.L.; Viel, S.; Mannina, L.; De Luca, F. The Role of Water Coordination in Binary Mixtures. A Study of Two Model Amphiphilic Molecules in Aqueous Solutions by Molecular Dynamics and NMR. J. Phys. Chem. 2006, 110, 8885–8892. [Google Scholar] [CrossRef]
- Di Michele, A.; Freda, M.; Onori, G.; Paolantoni, M.; Santucci, A.; Sassi, P. Modulation of Hydrophobic Effect by Cosolutes. J. Phys. Chem. 2006, 110, 21077–21085. [Google Scholar] [CrossRef] [PubMed]
- Tsubotani, K.; Maeyama, S.; Murakami, S.; Schaffer, S.W.; Ito, T. Taurine suppresses liquid-liquid phase separation of lysozyme protein. Amino Acids 2021, 53, 745–751. [Google Scholar] [CrossRef] [PubMed]
- Belloni, L. Colloidal interactions. J. Phys. Condens. Matter 2000, 12, R549–R587. [Google Scholar] [CrossRef]







| (mM) | (C) |
|---|---|
| 0 | 60.0 ± 0.1 |
| 50 | 62.3 ± 0.5 |
| 100 | 63.1 ± 0.6 |
| 200 | 63.4 ± 0.6 |
| 400 | 65.2 ± 0.6 |
| 600 | 61.7 ± 0.1 |
| (mM) | after 180 min | after 360 min |
|---|---|---|
| 0 | 0.84 ± 0.01 | 0.88 ± 0.01 |
| 25 | 0.85 ± 0.01 | 0.83 ± 0.01 |
| 50 | 0.82 ± 0.01 | 0.84 ± 0.01 |
| 400 | 0.78 ± 0.01 | 0.81 ± 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mastrella, L.; Moretti, P.; Pieraccini, S.; Magi, S.; Piccirillo, S.; Ortore, M.G. Taurine Stabilizing Effect on Lysozyme. Life 2022, 12, 133. https://doi.org/10.3390/life12010133
Mastrella L, Moretti P, Pieraccini S, Magi S, Piccirillo S, Ortore MG. Taurine Stabilizing Effect on Lysozyme. Life. 2022; 12(1):133. https://doi.org/10.3390/life12010133
Chicago/Turabian StyleMastrella, Leonardo, Paolo Moretti, Silvia Pieraccini, Simona Magi, Silvia Piccirillo, and Maria Grazia Ortore. 2022. "Taurine Stabilizing Effect on Lysozyme" Life 12, no. 1: 133. https://doi.org/10.3390/life12010133
APA StyleMastrella, L., Moretti, P., Pieraccini, S., Magi, S., Piccirillo, S., & Ortore, M. G. (2022). Taurine Stabilizing Effect on Lysozyme. Life, 12(1), 133. https://doi.org/10.3390/life12010133