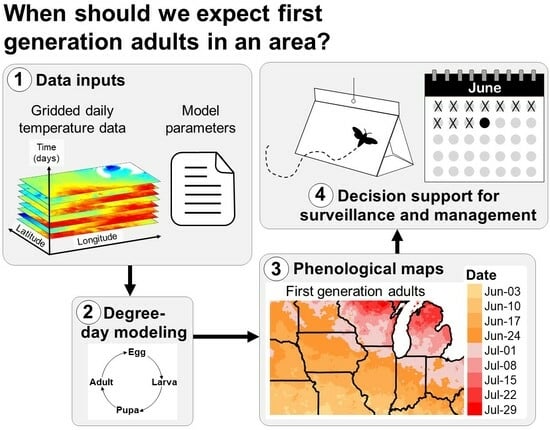
Phenological Mapping of Invasive Insects: Decision Support for Surveillance and Management
Abstract
:Simple Summary
Abstract
1. Introduction
2. Data Requirements for Degree-Day Models
3. Types of Phenological (Degree-Day) Maps
3.1. Generic Degree-Day Map
3.2. Degree-Day Lookup Table Map
- A relatively straightforward workflow. The workflow of generating a degree-day lookup table map involves using gridded daily Tmin and Tmax data to calculate degree-day accumulations between a start date (usually 1 January, although some models use other start dates, such as 1 March) and a specified end date. Degree-day lookup tables are then used to associate degree-day accumulations with life stages, and output maps depict the results with color tables and legends.
- The use of common base thresholds for multiple species. As degree-day lookup table maps are relatively simple and generic, there is the potential to use the same lower temperature threshold base maps for multiple species. This contrasts with more complex models that would require a separate base map for each case because of the application of different parameter values, including lower and upper thresholds, calculation methods, start dates, or diapause. The use of upper thresholds is relatively rare, at least for degree-day lookup table maps. For example, the SAFARIS FO Weekly maps produced by models for the old world bollworm [Helicoverpa armigera (Hübner)] and brown marmorated stink bug [Halyomorpha halys (Stål)] are constructed using the same 54 °F base maps, with no upper threshold. At least three other species in the FO Weekly map series share the same map.
- An ability to provide a “snapshot in time” for a single date. This allows, for example, regular updates that provide a gradually changing view of the current or near-future status of insect phenology. For example, degree-day lookup table maps produced by DDRP every 2−3 days depict the life stage and generation of insects on the map issue date. The USA National Phenology Network’s Pheno Forecast maps take advantage of 7-day National Digital Forecast Database (NDFD) forecasts to provide a 1-week “look ahead” prediction for CONUS [47]. SAFARIS PestCAST maps include a 1-month forecast using a 7-day NDFD forecast followed by three weeks of recent 20-year average PRISM data [15].
- Relatively simple design requirements. Degree-day lookup table maps can be designed as very simple visualization tools, such as by designing legend items to display only the stage or activity of interest (e.g., adult flight or egg hatch). Other stages and activities can then be represented as merged entries. The practice of focusing end users on a single target event represents a clear trade-off in reducing complexity (of multiple life stages) for users who may need clear directions in implementing surveillance or management actions.
3.3. Phenological Event Map
- Standardization. Mapping dates of phenological events allows for the standardization of legends and color tables across multiple species and events. For example, the colors assigned to each range of dates in the legend (e.g., 1–8 January = dark blue, 9–16 January = medium blue, etc.) can be applied to several events within a species, as well the same or different events in other species. For example, a prototype phenological event map developed for the codling moth in Oregon in the 1990s [62] depicts dates of egg hatch using uniquely colored two- or three-day intervals that span 17 May to 11 June (Figure 4). An alternative approach is to depict dates relative to the map issue date (Figure 5). For example, Pheno Forecast maps produce by the USA National Phenology Network use a single target event, such as “adult emergence”, while earlier events are ignored or replaced by approximations of time to the target event, such as “adults expected in 1–2 weeks” and “declining activity”.Figure 4. Phenological event map predicting dates of egg hatch for the codling moth [Cydia pomonella (L.)] for Hood River, Oregon. The map was used to illustrate use of GIS and phenological modeling for the first U.S. Department of Agriculture supported “Areawide IPM Project For Pome Fruit Crops in the Western US” [62] but has not been previously published.Figure 4. Phenological event map predicting dates of egg hatch for the codling moth [Cydia pomonella (L.)] for Hood River, Oregon. The map was used to illustrate use of GIS and phenological modeling for the first U.S. Department of Agriculture supported “Areawide IPM Project For Pome Fruit Crops in the Western US” [62] but has not been previously published.Figure 5. Pheno Forecast produced by the USA National Phenology Network (USA NPN) for emerald ash borer (Agrilus planipennis Fairmaire) [63]. The map depicts the time to first adult emergence of the emerald ash borer in the contiguous United States relative to the map issue date (13 April 2023). Maps are updated every two days, which allows decision-makers to stay up to date on when this phenological event is expected. Reproduced with permission from the USA NPN, Emerald Ash Borer Adult Forecast; published by the USA NPN, 2023. Available online: https://www.usanpn.org/data/forecasts/EAB (accessed on 19 December 2023).Figure 5. Pheno Forecast produced by the USA National Phenology Network (USA NPN) for emerald ash borer (Agrilus planipennis Fairmaire) [63]. The map depicts the time to first adult emergence of the emerald ash borer in the contiguous United States relative to the map issue date (13 April 2023). Maps are updated every two days, which allows decision-makers to stay up to date on when this phenological event is expected. Reproduced with permission from the USA NPN, Emerald Ash Borer Adult Forecast; published by the USA NPN, 2023. Available online: https://www.usanpn.org/data/forecasts/EAB (accessed on 19 December 2023).
- Operationally ready. Phenological event maps could be considered a more operational (tactical) product than degree-day lookup tables because they predict dates of events for a particular life stage, potentially up to weeks or months into the future. For example, a decision-maker may want to start planning their trap-setting operations several weeks before the estimated date of the first spring flight. Phenological event maps are more straightforward to interpret than degree-day lookup table maps because they do not require a mental conversion from stages into dates of events, which may reduce the learning curve for their use in decision support and allow for more direct communication of operational support. For example, phenological event map predictions could be merged with a calendar scheduling of monitoring or management activities, such as the dates of trap placement and removal. Nonetheless, operational readiness is presumptive, as most phenological event maps, at least for most invasive species, have not been formally tested in actual field use at this time.
- Simpler comparisons and expression of error rates. Phenological event maps allow for more direct comparisons of year-to-year variations of events than generic degree-day and degree-day lookup table maps. It is a relatively simple recordkeeping and reporting exercise to express differences in dates in the form of days difference [12,27,28]. This approach can also be used to express errors between predicted and observed events as discussed below (“8. Model validation”).
4. Applications of Phenological Maps
5. Gridded Climate Data
6. Potential Sources of Error and Uncertainty
7. Increasing Model Realism While Maintaining Simplicity
8. Model Validation
9. Recommendations for Future Research
10. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A. Minimum R Code to Calculate Degree-Days from PRISM Daily Climate Data
- #Load the required standard R libraries
- require(sp)
- require(rgdal)
- require(raster)
- #Set the lower developmental threshold (LDT) in Celsius for this group of species
- LDT <- 10
- #Search and load PRISM daily temperature grids that user has downloaded.pattern = paste("(PRISM_tmin_)(.*)(_bil.bil)$", sep="")
- files <- list.files(pattern=pattern, all.files=FALSE, full.names=TRUE)
- numlist <- vector()
- for (file in files) {
- num <- strsplit(file,split="_")[[1]][5]
- numlist <- c(numlist,num)
- }
- #Order by date starting with first downloaded date. Assume starting date Jan 01
- sortedlist <- sort(numlist)
- filelist <- vector()
- #Read in first raster as a template and initialize tracking rasters as 0 using the template
- template <- raster(files[1])
- template[!is.na(template)] <- 0
- dd_today, DDaccum <- template
- #Read in data, calculate and accumulate degree-days
- for (d in sortedlist) {
- #Read in that day’s PRISM raster files
- pattern <- paste("(PRISM_tmin_)(.*)(",d,")(_bil.bil)$", sep="")
- temp <- list.files(pattern=pattern,all.files=FALSE, full.names=TRUE)
- tmin <- raster(temp)
- pattern <- paste("(PRISM_tmax_)(.*)(",d,")(_bil.bil)$", sep="")
- temp <- list.files(pattern=pattern,all.files=FALSE, full.names=TRUE)
- tmax <- raster(temp)
- rm(pattern,temp)
- #Use simple average DDs, more complex formulas are available and preferable
- dd_today <- ((tmax+tmin/2) - LDT)
- DDaccum <- DDaccum + dd_today
- }
- #Note: DDaccum is current cumulative DDs for day d, can be saved to a data stack and used for
- #DD lookup maps for species with same LDT, and can be used to derive pest event maps by saving
- #the date of each data cell equal to or greater than the pest event DD amount.
References
- Bowers, J.H.; Malayer, J.R.; Martínez-López, B.; LaForest, J.; Bargeron, C.; Neeley, A.D.; Coop, L.; Barker, B.S.; Mastin, A.J.; Parnell, S.; et al. Surveillance for early detection of high-consequence pests and pathogens. In Tactical Sciences for Biosecurity of Animal and Plant Systems; Cardwell, K.F., Bailey, K.L., Eds.; IGI Global: Hershey, PN, USA, 2022; pp. 120–177. [Google Scholar]
- Reaser, J.K.; Veatch, S.D.; Burgiel, S.W.; Kirkey, J.; Brantley, K.A.; Burgos-Rodri, J. The early detection of and rapid response (EDRR) to invasive species: A conceptual framework and federal capacities assessment. Biol. Invasions 2020, 22, 1–19. [Google Scholar] [CrossRef]
- Vänninen, I. Advances in Insect Pest and Disease Monitoring and Forecasting in Horticulture; Burleigh Dodds Science Publishing: Sawston, UK, 2022. [Google Scholar] [CrossRef]
- Wilson, L.T.; Barnett, W.W. Degree-days: An aid in crop and pest management. Calif. Agric. 1983, 37, 4–7. [Google Scholar]
- Herms, D.A. Using degree-days and plant phenology to predict pest activity. In IPM (Integrated Pest Management) of Midwest Landscapes, Minnesota Agricultural Experiment Station Publication SB-07645; Krischik, V., Davidson, J., Eds.; Minnesota Agricultural Experiment Station: St. Paul, MN, USA, 2004; pp. 49–59. [Google Scholar]
- Ascerno, M.E. Insect phenology and integrated pest management. J. Arboric. 1991, 17, 13–15. [Google Scholar] [CrossRef]
- Zalom, F.G.; Goodell, P.B.; Wilson, L.T.; Barnett, W.W.; Bentley, W.J. Degree-Days: The Calculation and Use of Heat Units in Pest Management; University of California, Division of Agriculture and Natural Resources: Berkeley, CA, USA, 1983. [Google Scholar]
- Gage, S.H.; Whalon, M.E.; Miller, D.J. Pest event scheduling system for biological monitoring and pest management. Environ. Entomol. 1982, 11, 1127–1133. [Google Scholar] [CrossRef]
- Ferguson, A.W.; Skellern, M.P.; Johnen, A.; Richthofen, V.; Watts, N.P.; Bardsley, E.; Murray, A.; Cook, S.M. The potential of decision support systems to improve risk assessment for pollen beetle management in winter oilseed rape. Pest Manag. Sci. 2016, 72, 609–617. [Google Scholar] [CrossRef] [PubMed]
- Jones, V.P.; Brunner, J.F.; Grove, G.G.; Petit, B.; Tangren, G.V.; Jones, W.E. A web-based decision support system to enhance IPM programs in Washington tree fruit. Pest Manag. Sci. 2010, 66, 587–595. [Google Scholar] [CrossRef] [PubMed]
- Rossi, V.; Sperandio, G.; Caffi, T.; Simonetto, A.; Gilioli, G. Critical success factors for the adoption of decision tools in IPM. Agronomy 2019, 9, 710. [Google Scholar] [CrossRef]
- Cormier, D.; Chouinard, G.; Pelletier, F.; Vanoosthuyse, F.; Joannin, R. An interactive model to predict codling moth development and insecticide application effectiveness. IOBC-WPRS Bull. 2016, 112, 65–70. [Google Scholar]
- Jones, V.P. Using phenology models to estimate insecticide effects on population dynamics: Examples from codling moth and obliquebanded leafroller. Pest Manag. Sci. 2021, 77, 1081–1093. [Google Scholar] [CrossRef]
- Roltsch, W.J.; Zalom, A.G.; Strand, J.F.; Pitcairn, M.J.; Frank, J.R.; Ann, G.Z.; Strand, J.F.; Pitcairn, M.J. Evaluation of several degree-day estimation methods in California climates. Int. J. Biometeorol. 1999, 42, 169–176. [Google Scholar] [CrossRef]
- Takeuchi, Y.; Tripodi, A.; Montgomery, K. SAFARIS: A spatial analytic framework for pest forecast systems. Front. Insect Sci. 2023, 3, 1198355. [Google Scholar] [CrossRef]
- Barker, B.S.; Coop, L.; Wepprich, T.; Grevstad, F.S.; Cook, G. DDRP: Real-time phenology and climatic suitability modeling of invasive insects. PLoS ONE 2020, 15, e0244005. [Google Scholar] [CrossRef] [PubMed]
- Coop, L.B.; Barker, B.S. Advances in understanding species ecology: Phenological and life cycle modeling of insect pests. In Integrated Management of Insect Pests: Current and Future Developments; Kogan, M., Heinrichs, E., Eds.; Burleigh Dodds Science Publishing: Sawston, UK, 2020; pp. 43–96. [Google Scholar]
- Welch, S.M.; Croft, B.A.; Brunner, J.F.; Michels, M.; Welch, B.; Croft, J.; Brunner, M.; Michels, S.M. PETE: An extension phenology modeling system for management of multi-species pest complex. Environ. Entomol. 1978, 7, 482–494. [Google Scholar] [CrossRef]
- Nietschke, B.S.; Magarey, R.D.; Borchert, D.M.; Calvin, D.D.; Jones, E. A developmental database to support insect phenology models. Crop Prot. 2007, 26, 1444–1448. [Google Scholar] [CrossRef]
- Orlandini, S.; Magarey, R.D.; Woo Park, E.; Sporleder, M.; Kroschel, J. Methods of agroclimatology: Modeling approaches for pests and diseases. In Agroclimatology: Linking Agriculture to Climate, Agronomy Monograph 60; Hatfield, J.H., Sivakuma, M.V.K., Prueger, J.H., Eds.; Wiley: Hoboken, NJ, USA, 2018; pp. 453–488. Available online: https://acsess.onlinelibrary.wiley.com/doi/book/10.2134/agronmonogr60 (accessed on 18 December 2023).
- Arnold, C.Y.Y. Maximum-minimum temperatures as a basis for computing heat units. Proc. Soc. Hortic. Sci. 1960, 76, 682–692. [Google Scholar]
- Wang, J.Y. A critique of the heat unit approach to plant response studies. Ecology 1960, 41, 785–790. [Google Scholar] [CrossRef]
- Chuine, I.; Régnière, J. Process-based models of phenology for plants and animals. Annu. Rev. Ecol. Evol. Syst. 2017, 48, 159–182. [Google Scholar] [CrossRef]
- Mirhosseini, M.A.; Fathipour, Y.; Reddy, G.V.P.R. Arthropod development’s response to temperature: A review and new software for modeling. Ann. Entomol. Soc. Am. 2017, 110, 507–520. [Google Scholar] [CrossRef]
- Knight, A.L. Adjusting the phenology model of codling moth (Lepidoptera: Tortricidae) in Washington State apple orchards. Environ. Entomol. 2007, 36, 1485–1493. [Google Scholar] [CrossRef]
- Brunner, J.F.; Hoyt, S.C.; Wright, M.A. Codling Moth Control—A New Tool for Timing Sprays; Cooperative Extension Bulletin, 1072; Washington State University: Pullman, WA, USA, 1987. [Google Scholar]
- Barros-Parada, W.; Knight, A.L.; Fuentes-Contreras, E. Modeling codling moth (Lepidoptera: Tortricidae) phenology and predicting egg hatch in apple orchards of the Maule Region, Chile. Chil. J. Agric. Res. 2015, 75, 57–62. [Google Scholar] [CrossRef]
- Jorgensen, C.D.; Martinsen, M.E.; Westover, L.J. Validating Michigan State University’s codling moth model (MOTHMDL) in an arid environment. Gt. Lakes Entomol. 1979, 12, 203–212. [Google Scholar]
- Song, Y.H.; Coop, L.B.; Omeg, M.; Rield, H. Development of a phenology model for predicting Western cherry fruit fly, Rhagoletis indifferens Curran (Diptera: Tephritidae), emergence in the mid Columbia area of the western United States. J. Asia. Pac. Entomol. 2003, 6, 187–192. [Google Scholar] [CrossRef]
- Régnière, J.; Sharov, A. Phenology of Lymantria dispar (Lepidoptera: Lymantriidae), male flight and the effect of moth dispersal in heterogeneous landscapes. Int. J. Biometeorol. 1998, 41, 161–168. [Google Scholar] [CrossRef]
- Régnière, J.; Sharov, A. Simulating temperature-dependent ecological processes at the sub-continental scale: Male gypsy moth flight phenology as an example. Int. J. Biometeorol. 1999, 42, 146–152. [Google Scholar] [CrossRef]
- Schaub, L.P.; Ravlin, F.W.; Gray, D.R.; Logan, J.A. Landscape framework to predict phenological events for gypsy moth (Lepidoptera: Lymantriidae) management programs. Environ. Entomol. 1995, 24, 10–18. [Google Scholar] [CrossRef]
- Russo, J.M.; Liebhold, A.M.; Kelley, J.G.W. Mesoscale weather data as input to a gypsy moth (Lepidoptera: Lymantriidae) phenology model. J. Econ. Entomol. 1993, 86, 838–844. [Google Scholar] [CrossRef]
- Foster, J.R.; Townsend, P.A.; Mladenoff, D.J. Mapping asynchrony between gypsy moth egg-hatch and forest leaf-out: Putting the phenological window hypothesis in a spatial context. For. Ecol. Manag. 2013, 287, 67–76. [Google Scholar] [CrossRef]
- Samietz, J.; Graf, B.; Höhn, H.; Schaub, L.P.; Höpli, H.U. SOPRA: Forecasting tool for fruit tree pest insects. Rev. Suisse Vitic. Arboric. Hortic. 2007, 39, 187–194. [Google Scholar]
- Caprio, J.M.; Hopp, R.J.; Williams, J.S. Computer mapping in phenological analysis. In Phenology and Seasonality Modeling; Lieth, H., Ed.; Springer: Berlin/Heidelberg, Germany, 1974; pp. 77–82. [Google Scholar]
- Lieth, H.; Radford, J.S. Phenology, resource management, and synagraphic computer mapping. Bioscience 1971, 21, 62–70. [Google Scholar] [CrossRef]
- Croft, B.A.; Howes, J.L.; Welch, S.M. A computer-based, extension pest management delivery system. Environ. Entomol. 1976, 5, 20–34. [Google Scholar] [CrossRef]
- Régnière, J.; Cooke, B.; Bergeron, V. BioSIM: A Computer-Based Decision Support Tool for Seasonal Planning of Pest Management Activities, User’s Manual; Information Report LAU-X-116; Canadian Forest Service: Quebec, QC, Canada, 1995. [Google Scholar]
- Régnière, J. Generalized approach to landscape-wide seasonal forecasting with temperature-driven simulation models. Environ. Entomol. 1996, 25, 869–881. [Google Scholar] [CrossRef]
- Jarvis, C.H. GEO_BUG: A geographical modelling environment for assessing the likelihood of pest development. Environ. Model. Softw. 2001, 16, 753–765. [Google Scholar] [CrossRef]
- Fand, B.B.; Choudhary, J.S.; Kumar, M.; Bal, S.K. Phenology modelling and GIS applications in pest management: A tool for studying and understanding insect-pest dynamics in the context of global climate change. In Approaches to Plant Stress and Their Management; Gaur, R.K., Sharma, P., Eds.; Springer: New Delhi, India, 2014; pp. 107–124. [Google Scholar]
- Daly, C.; Neilson, R.P.; Phillips, D.L. A statistical-topographic model for mapping climatological precipitation over mountainous terrain. J. Appl. Meteorol. 1994, 33, 140–158. [Google Scholar] [CrossRef]
- Daly, C.; Halbleib, H.; Smith, J.I.; Gibson, W.P.; Doggett, M.K.; Taylor, G.H.; Curtis, J.; Pasteris, P.P. Physiographically sensitive mapping of climatological temperature and precipitation across the conterminous United States. Int. J. Climatol. 2008, 28, 2031–2064. [Google Scholar] [CrossRef]
- Daly, C. Guidelines for assessing the suitability of spatial climate data sets. Int. J. Climatol. 2006, 26, 707–721. [Google Scholar] [CrossRef]
- Barker, B.S.; Coop, L.; Duan, J.J.; Petrice, T.R. An integrative phenology and climatic suitability model for emerald ash borer. Front. Insect Sci. 2023, 3, 1239173. [Google Scholar] [CrossRef]
- Crimmins, T.M.; Gerst, K.L.; Huerta, D.G.; Marsh, R.L.; Posthumus, E.E.; Rosemartin, A.H.; Switzer, J.; Weltzin, J.F.; Coop, L.B.; Dietschler, N.; et al. Short-term forecasts of insect phenology inform pest management. Ann. Entomol. Soc. Am. 2020, 113, 139–148. [Google Scholar] [CrossRef]
- Delahaut, K. Insects. In Phenology: An Integrative Environmental Science; Schwartz, M., Ed.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2003; pp. 405–419. [Google Scholar]
- Damos, P.T.; Savopoulou-Soultani, M. Temperature-driven models for insect development and vital thermal requirements. Psyche 2012, 2012, 123405. [Google Scholar] [CrossRef]
- Pruess, K.P. Degree-day methods for pest management. Environ. Entomol. 1983, 12, 613–619. [Google Scholar] [CrossRef]
- Campbell, A.; Frazer, B.D.; Gilbert, N.; Gutierrez, A.P.; Mackauer, M. Temperature requirements of some aphids and their parasites. J. Appl. Ecol. 1974, 11, 431–438. [Google Scholar] [CrossRef]
- Rebaudo, F.; Rabhi, V.B. Modeling temperature-dependent development rate and phenology in insects: Review of major developments, challenges, and future directions. Entomol. Exp. Appl. 2018, 166, 607–617. [Google Scholar] [CrossRef]
- Baskerville, G.L.; Emin, P. Rapid estimation of heat accumulation from maximum and minimum temperatures. Ecology 1969, 50, 514–517. [Google Scholar] [CrossRef]
- Riedl, H.; Croft, B.A.; Howitt, A.J. Forecasting codling moth phenology based on pheromone trap catches and physiological-time models. Can. Entomol. 1976, 108, 449–460. [Google Scholar] [CrossRef]
- Welch, S.M.; Croft, B.A.; Michels, M.F. Validation of pest management models. Environ. Entomol. 1981, 10, 425–432. [Google Scholar] [CrossRef]
- Coop, L. What’s New—Online IPM Weather Data, Degree-Day, and Plant Disease Risk Models 2000–2003. Available online: https://uspest.org/wea/weanew03.html (accessed on 22 November 2023).
- Coop, L. What’s New—IPM Pest and Plant Disease Models and Forecasting—For Agricultural, Pest Management, and Plant Biosecurity Decision Support in the US. Available online: https://uspest.org/wea/weanew0409.html (accessed on 22 November 2023).
- Willmott, C.J.; Robeson, S.M. Climatologically aided interpolation (CAI) of terrestrial air temperature. Int. J. Climatol. 1995, 15, 221–229. [Google Scholar] [CrossRef]
- SAFARIS Brown Marmorated Stink Bug (Halymorpha halys) Phenological Stages. In Spatial Analytic Framework for Advanced Risk Information Systems (SAFARIS); U.S. Dept. of Agriculture (USDA): Washington, DC, USA; North Carolina State University: Raleigh, NC, USA, 2022; Available online: https://safaris.cipm.info (accessed on 23 May 2022).
- Sheehan, K.A. User’s Guide for GMPHEN: Gypsy Moth Phenology Model (General Technical Report NE-158); United States Department of Agriculture, Forest Service, Northeastern Experiment Station: Newtown Sqaure, PA, USA, 1992. [Google Scholar]
- Grevstad, F.S.; Coop, L.B. The consequences of photoperiodism for organisms in new climates. Ecol. Appl. 2015, 25, 1506–1517. [Google Scholar] [CrossRef]
- Kogan, M. Areawide Management of the Codling Moth: Implementation of a Comprehensive IPM Program for Pome Fruit Crops in the Western U.S.; Integrated Plant Protection Center, Oregon State University: Corvallis, OR, USA, 1994. [Google Scholar]
- USA National Phenology Network Emerald Ash Borer Adult Emergence Forecast; USA National Phenology Network: Tucson, AZ, USA, 2023. Available online: https://www.usanpn.org/files/npn/maps/eab_adult.png (accessed on 13 April 2023).
- CAPS Resource and Collaboration Site. Animal and Plant Health Inspection Service (APHIS), Plant Protection and Quarantine (PPQ). Available online: https://caps.ceris.purdue.edu (accessed on 18 December 2023).
- Jarvis, C.H.; Baker, R.H.A. Risk assessment for nonindigenous pests: 2. Accounting for interyear climate variability. Divers. Distrib. 2001, 7, 237–248. [Google Scholar] [CrossRef]
- Jarvis, C.H.; Baker, R.H.A. Risk assessment for nonindigenous pests: I. Mapping the outputs of phenology models to assess the likelihood of establishment. Divers. Distrib. 2001, 7, 223–235. [Google Scholar] [CrossRef]
- Sporleder, M.; Juarez, H.; Simon, R.; Kroschel, J. ILCYM-Insect life cycle modeling: Software for developing temperature-based insect phenology models with applications for regional and global pest risk assessments and mapping. In Proceedings of the 15th Triennial ISTRC Symposium of the International Society for Tropical Root Crops (ISTRC), Lima, Peru, 2–6 November 2009; pp. 216–223. [Google Scholar]
- Stoeckli, S.C.; Hirschi, M.; Spirig, C.; Calanca, P.; Rotach, M.W.; Samietz, J. Impact of climate change on voltinism and prospective diapause induction of a global pest insect—Cydia pomonella (L.). PLoS ONE 2012, 7, e35723. [Google Scholar] [CrossRef]
- Jakoby, O.; Lischke, H.; Wermelinger, B. Climate change alters elevational phenology patterns of the European spruce bark beetle (Ips typographus). Glob. Chang. Biol. 2019, 25, 4048–4063. [Google Scholar] [CrossRef]
- Jönsson, A.M.; Pulatov, B.; Linderson, M.L.; Hall, K. Modelling as a tool for analysing the temperature-dependent future of the Colorado potato beetle in Europe. Glob. Chang. Biol. 2013, 19, 1043–1055. [Google Scholar] [CrossRef] [PubMed]
- Kroschel, J.; Sporleder, M.; Tonnang, H.E.Z.; Juarez, H.; Carhuapoma, P.; Gonzales, J.C.; Simon, R. Predicting climate-change-caused changes in global temperature on potato tuber moth Phthorimaea operculella (Zeller) distribution and abundance using phenology modeling and GIS mapping. Agric. For. Meteorol. 2013, 170, 228–241. [Google Scholar] [CrossRef]
- Crossley, M.S.; Lagos-Kutz, D.; Davis, T.S.; Eigenbrode, S.D.; Hartman, G.L.; Voegtlin, D.J.; Snyder, W.E. Precipitation change accentuates or reverses temperature effects on aphid dispersal. Ecol. Appl. 2022, 32, e2593. [Google Scholar] [CrossRef] [PubMed]
- Ward, S.F.; Moon, R.D.; Herms, D.A.; Aukema, B.H. Determinants and consequences of plant–insect phenological synchrony for a non-native herbivore on a deciduous conifer: Implications for invasion success. Oecologia 2019, 190, 867–878. [Google Scholar] [CrossRef]
- van Asch, M.; Visser, M.E. Phenology of forest caterpillars and their host trees: The importance of synchrony. Annu. Rev. Entomol. 2007, 52, 37–55. [Google Scholar] [CrossRef]
- Forkner, R.E.; Marquis, R.J.; Lill, J.T.; Corff, J.L. Timing is everything? Phenological synchrony and population variability in leaf-chewing herbivores of Quercus. Ecol. Entomol. 2008, 33, 276–285. [Google Scholar] [CrossRef]
- Westbrook, J.; Fleischer, S.; Jairam, S.; Meagher, R.; Nagoshi, R. Multigenerational migration of fall armyworm, a pest insect. Ecosphere 2019, 10, e02919. [Google Scholar] [CrossRef]
- Westbrook, J.K.; Nagoshi, R.N.; Meagher, R.L.; Fleischer, S.J.; Jairam, S. Modeling seasonal migration of fall armyworm moths. Int. J. Biometeorol. 2016, 60, 255–267. [Google Scholar] [CrossRef]
- Ali, S.; Bhutta, Z.A.; Reboita, M.S.; Goheer, M.A.; Ebrahimi, S.; Rozante, J.R.; Kiani, R.S.; Muhammad, S.; Khan, F.; Rahman, M.M.; et al. A 5-km gridded product development of daily temperature and precipitation for Bangladesh, Nepal, and Pakistan from 1981 to 2016. Geosci. Data J. 2023; in press. [Google Scholar] [CrossRef]
- Xavier, A.C.; Scanlon, B.R.; King, C.W.; Alves, A.I. New and improved Brazilian daily weather gridded data (1961–2020). Int. J. Climatol. 2022, 42, 8390–8404. [Google Scholar] [CrossRef]
- Fang, S.; Mao, K.; Xia, X.; Wang, P.; Shi, J.; Bateni, S.M.; Xu, T.; Cao, M.; Heggy, E.; Qin, Z. Dataset of daily near-surface air temperature in China from 1979 to 2018. Earth Syst. Sci. Data 2022, 14, 1413–1432. [Google Scholar] [CrossRef]
- Qin, R.; Zhao, Z.; Xu, J.; Ye, J.S.; Li, F.M.; Zhang, F. HRLT: A high-resolution (1 d, 1 km) and long-term (1961–2019) gridded dataset for surface temperature and precipitation across China. Earth Syst. Sci. Data 2022, 14, 4793–4810. [Google Scholar] [CrossRef]
- Nengzouzam, G.; Hodam, S.; Bandyopadhyay, A.; Bhadra, A. Spatial and temporal trends in high resolution gridded temperature data over India. Asia-Pac. J. Atmos. Sci. 2019, 55, 761–772. [Google Scholar] [CrossRef]
- Cornes, R.C.; van der Schrier, G.; van den Besselaar, E.; Jones, P.D. An ensemble version of the E-OBS temperature and precipitation data sets. J. Geophys. Res. 2018, 123, 9391–9409. [Google Scholar] [CrossRef]
- Thornton, M.M.; Strestha, R.; Wei, Y.; Thornton, P.E.; Kao, S.-C.; Wilson, B.E. Daymet: Daily Surface Weather Data on a 1-Km Grid for North America, Version 4; Oak Ridge National Laboratory Distributed Active Archive Center: Oak Ridge, TN, USA, 2020. [Google Scholar]
- Thornton, P.E.; Shrestha, R.; Thornton, M.; Kao, S.C.; Wei, Y.; Wilson, B.E. Gridded daily weather data for North America with comprehensive uncertainty quantification. Sci. Data 2021, 8, 190. [Google Scholar] [CrossRef] [PubMed]
- Xavier, A.C.; King, C.W.; Scanlon, B.R. Daily gridded meteorological variables in Brazil (1980–2013). Int. J. Climatol. 2016, 36, 2644–2659. [Google Scholar] [CrossRef]
- Jarvis, C.H.; Stuart, N. Accounting for error when modelling with time series data: Estimating the development of crop pests throughout the year. Trans. GIS 2001, 5, 327–343. [Google Scholar] [CrossRef]
- Jarvis, C.H.; Baker, R.H.A.; Morgan, D. The impact of interpolated daily temperature data on landscape-wide predictions of invertebrate pest phenology. Agric. Ecosyst. Environ. 2003, 94, 169–181. [Google Scholar] [CrossRef]
- Jarvis, C.H.; Collier, R.H. Evaluating an interpolation approach for modelling spatial variability in pest development. Bull. Entomol. Res. 2002, 92, 219–231. [Google Scholar] [CrossRef]
- Racca, P.; Zeuner, T.; Jung, J.; Kleinhenz, B. Model validation and use of geographic information systems in crop protection warning service. In Precision Crop Protection: The Challenge and Use of Heterogeneity; Oerke, E.C., Gerhards, R., Menz, G., Sikora, R., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 259–276. [Google Scholar]
- Jarvis, C.H.; Stuart, N. A comparison among strategies for interpolating maximum and minimum daily air temperatures. Part I: The selection of “guiding” topographic and land cover variables. J. Appl. Meteorol. 2001, 40, 1060–1074. [Google Scholar] [CrossRef]
- Suppo, C.; Bras, A.; Robinet, C. A temperature- and photoperiod-driven model reveals complex temporal population dynamics of the invasive box tree moth in Europe. Ecol. Modell. 2020, 432, 109229. [Google Scholar] [CrossRef]
- Primack, R.B.; Gallinat, A.S.; Ellwood, E.R.; Crimmins, T.M.; Schwartz, M.D.; Staudinger, M.D.; Miller-Rushing, A.J. Ten best practices for effective phenological research. Int. J. Biometeorol. 2023, 67, 1509–1522. [Google Scholar] [CrossRef] [PubMed]
- Probert, A.F.; Wegmann, D.; Volery, L.; Adriaens, T.; Bakiu, R.; Bertolino, S.; Essl, F.; Gervasini, E.; Groom, Q.; Latombe, G.; et al. Identifying, reducing, and communicating uncertainty in community science: A focus on alien species. Biol. Invasions 2022, 24, 3395–3421. [Google Scholar] [CrossRef] [PubMed]
- Nijhout, H.F. Development and evolution of adaptive polyphenisms. Evol. Dev. 2003, 5, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Neslon, R.J.; Denlinger, D.L.; Somers, D.E. Photoperiodism. The Biological Calendar; Oxford University Press: New York, NY, USA, 2010. [Google Scholar]
- Régnier, B.; Legrand, J.; Rebaudo, F. Modeling temperature-dependent development rate in insects and implications of experimental design. Environ. Entomol. 2022, 51, 132–144. [Google Scholar] [CrossRef] [PubMed]
- Shaffer, P.L. Prediction of variation in development period of insects and mites reared at constant temperature. Environ. Entomol. 1983, 12, 1012–1019. [Google Scholar] [CrossRef]
- Wagner, T.L.; Wu, H.-I.; Feldman, R.M.; Sharpe, P.J.H.; Coulson, R.N. Multiple-cohort approach for simulating development of insect populations under variable temperatures. Ann. Entomol. Soc. Am. 1985, 78, 691–704. [Google Scholar] [CrossRef]
- Sharpe, P.J.H.; Curry, G.L.; DeMichele, D.W.; Cole, C.L. Distribution model of organism development times. J. Theor. Biol. 1977, 66, 21–38. [Google Scholar] [CrossRef]
- Yonow, T.; Zalucki, M.P.; Sutherst, R.W.; Dominiak, B.C.; Maywald, G.F.; Maelzer, D.A.; Kriticos, D.J. Modelling the population dynamics of the Queensland fruit fly, Bactrocera (Dacus) tryoni: A cohort-based approach incorporating the effects of weather. Ecol. Modell. 2004, 173, 9–30. [Google Scholar] [CrossRef]
- Howe, R.W. Temperature effects on embryonic development in insects. Annu. Rev. Entomol. 1967, 10, 15–42. [Google Scholar] [CrossRef]
- Curry, G.U.Y.L.; Feldman, R.M.; Smith, C. A stochastic model of a temperature-dependent population. Theor. Popul. Biol. 1978, 13, 197–213. [Google Scholar] [CrossRef] [PubMed]
- Rowley, C.; Cherrill, A.; Leather, S.R.; Pope, T.W. Degree-day based phenological forecasting model of saddle gall midge (Haplodiplosis marginata) (Diptera: Cecidomyiidae) emergence. Crop Prot. 2017, 102, 154–160. [Google Scholar] [CrossRef]
- Grevstad, F.S.; Wepprich, T.; Barker, B.S.; Coop, L.B.; Shaw, R.; Bourchier, R.S. Combining photoperiod and thermal responses to predict phenological mismatch for introduced insects. Ecol. Appl. 2022, 32, e2557. [Google Scholar] [CrossRef] [PubMed]
- Ogburn, E.C.; Ohmen, T.M.; Huseth, A.S.; Reisig, D.D.; Kennedy, G.G.; Walgenbach, J.F. Temperature-driven differences in phenology and habitat suitability for brown marmorated stink bug, Halyomorpha halys, in two ecoregions of North Carolina. J. Pest Sci. 2023, 96, 373–387. [Google Scholar] [CrossRef]
- Nielsen, A.L.; Chen, S.; Fleischer, S.J. Coupling developmental physiology, photoperiod, and temperature to model phenology and dynamics of an invasive heteropteran, Halyomorpha halys. Front. Physiol. 2016, 7, 165. [Google Scholar] [CrossRef] [PubMed]
- Quesada-Moraga, E.; Valverde-García, P.; Garrido-Jurado, I. The effect of temperature and soil moisture on the development of the preimaginal Mediterranean fruit fly (Diptera: Tephritidae). Environ. Entomol. 2012, 41, 966–970. [Google Scholar] [CrossRef]
- Ma, G.; Tian, B.-L.; Zhao, F.; Wei, G.-S.; Hoffmann, A.A.; Ma, C.-S. Soil moisture conditions determine phenology and success of larval escape in the peach fruit moth, Carposina sasakii (Lepidoptera, Carposinidae): Implications for predicting drought effects on a diapausing insect. Appl. Soil Ecol. 2017, 110, 65–72. [Google Scholar] [CrossRef]
- Tauber, M.J.; Tauber, C.A. Insect seasonality: Diapause maintenance, termination, and postdiapause development. Annu. Rev. Entomol. 1976, 21, 81–107. [Google Scholar] [CrossRef]
- Beck, S.D. Insect Photoperiodism; Academic Press: London, UK, 1968. [Google Scholar]
- Wolda, H. Insect seasonality: Why? Annu. Rev. Ecol. Syst. 1988, 19, 1–18. [Google Scholar] [CrossRef]
- Tauber, M.J.; Tauber, C.A.; Nyrop, J.P.; Villani, M.G. Moisture, a vital but neglected factor in the seasonal ecology of insects: Hypotheses and tests of mechanisms. Environ. Entomol. 1998, 27, 523–530. [Google Scholar] [CrossRef]
- McDougall, R.N.; Ogburn, E.C.; Walgenbach, J.F.; Nielsen, A.L. Diapause termination in invasive populations of the brown marmorated stink bug (Hemiptera: Pentatomidae) in response to photoperiod. Environ. Entomol. 2021, 50, 1400–1406. [Google Scholar] [CrossRef] [PubMed]
- Moraiti, C.A.; Köppler, K.; Vogt, H.; Papadopoulos, N.T. Effects of photoperiod and relative humidity on diapause termination and post-winter development of Rhagoletis cerasi pupae. Bull. Entomol. Res. 2020, 110, 588–596. [Google Scholar] [CrossRef] [PubMed]
- Kamiyama, M.T.; Bradford, B.Z.; Groves, R.L.; Guédot, C. Degree day models to forecast the seasonal phenology of Drosophila suzukii in tart cherry orchards in the Midwest U.S. PLoS ONE 2020, 15, e0227726. [Google Scholar] [CrossRef] [PubMed]
- Yee, D.A.; Ezeakacha, N.F.; Abbott, K.C. The interactive effects of photoperiod and future climate change may have negative consequences for a wide-spread invasive insect. Oikos 2017, 126, 40–51. [Google Scholar] [CrossRef]
- Forsythe, W.C.; Rykiel, E.J.; Stahl, R.S.; Wu, H.; Schoolfield, R.M. A model comparison for daylength as a function of latitude and day of year. Ecol. Modell. 1995, 80, 87–95. [Google Scholar] [CrossRef]
- Bean, D.W.; Dalin, P.; Dudley, T.L. Evolution of critical day length for diapause induction enables range expansion of Diorhabda carinulata, a biological control agent against tamarisk (Tamarix spp.). Evol. Appl. 2012, 5, 511–523. [Google Scholar] [CrossRef]
- Efron, B.; Gong, G. A leisurely look at the bootstrap, the jackknife, and cross-validation. Am. Stat. 1983, 37, 36–48. [Google Scholar]
- Hevesi, J.A.; Istok, J.D.; Flint, A.L. Precipitation estimation in mountainous terrain using multivariate geostatistics. Part I: Structural analysis. J. Appl. Meteorol. Climatol. 1992, 31, 661–676. [Google Scholar] [CrossRef]
- Morris, M.T.; Carley, J.R.; Colón, E.; Gibbs, A.; de Pondeca, M.S.F.V.; Levine, S. A quality assessment of the real-time mesoscale analysis (RTMA) for aviation. Weather Forecast. 2020, 35, 977–996. [Google Scholar] [CrossRef]
- Muñoz-Sabater, J.; Dutra, E.; Agustí-Panareda, A.; Albergel, C.; Arduini, G.; Balsamo, G.; Boussetta, S.; Choulga, M.; Harrigan, S.; Hersbach, H.; et al. ERA5-Land: A state-of-the-art global reanalysis dataset for land applications. Earth Syst. Sci. Data 2021, 13, 4349–4383. [Google Scholar] [CrossRef]
- Work, T.T.; McCullough, D.G.; Cavey, J.F.; Komsa, R. Arrival rate of nonindigenous insect species into the United States through foreign trade. Biol. Invasions 2005, 7, 323–332. [Google Scholar] [CrossRef]
- Turner, R.M.; Brockerhoff, E.G.; Bertelsmeier, C.; Blake, R.E.; Caton, B.; James, A.; MacLeod, A.; Nahrung, H.F.; Pawson, S.M.; Plank, M.J.; et al. Worldwide border interceptions provide a window into human-mediated global insect movement. Ecol. Appl. 2021, 31, e02412. [Google Scholar] [CrossRef] [PubMed]
- Seebens, H.; Meyerson, L.A.; Rahlao, S.J.; Lenzner, B.; Tricarico, E.; Aleksanyan, A.; Courchamp, F.; Keskin, E.; Saeedi, H.; Tawake, A.; et al. Chapter 2. Trends and status of alien and invasive alien species. In Thematic Assessment Report on Invasive Alien Species and Their Control of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services; Roy, H.E., Pauchard, A., Stoett, P., Renard Truong, T., Bacher, S., Galil, B.S., Hulme, P.E., Ikeda, T., Sankaran, K.V., McGeoch, M.A., et al., Eds.; IPBES Secretariat: Bonn, Germany, 2023; pp. 1–264. [Google Scholar]
- Yamanaka, T.; Morimoto, N.; Nishida, G.M.; Kiritani, K.; Moriya, S.; Liebhold, A.M. Comparison of insect invasions in North America, Japan and their Islands. Biol. Invasions 2015, 17, 3049–3061. [Google Scholar] [CrossRef]
- Nowatzki, T.M.; Tollefson, J.J.; Calvin, D.D. Development and validation of models for predicting the seasonal emergence of corn rootworm (Coleoptera: Chrysomelidae) beetles in Iowa. Environ. Entomol. 2002, 31, 864–873. [Google Scholar] [CrossRef]
- Rhodes, M.W.; Bennie, J.J.; Spalding, A.; Ffrench-Constant, R.H.; Maclean, I.M.D. Recent advances in the remote sensing of insects. Biol. Rev. 2022, 97, 343–360. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Zhang, X. Mapping crop phenology in near real-time using satellite remote sensing: Challenges and opportunities. J. Remote Sens. 2021, 2021, 8379391. [Google Scholar] [CrossRef]
- Stein, A.F.; Draxler, R.R.; Rolph, G.D.; Stunder, B.J.B.; Cohen, M.D.; Ngan, F. NOAA’s HYSPLIT atmospheric transport and dispersion modeling system. Bull. Am. Meteorol. Soc. 2015, 96, 2059–2077. [Google Scholar] [CrossRef]
- Jamieson, M.A.; Trowbridge, A.M.; Raffa, K.F.; Lindroth, R.L. Consequences of climate warming and altered precipitation patterns for plant-insect and multitrophic interactions. Plant Physiol. 2012, 160, 1719–1727. [Google Scholar] [CrossRef]
- Iler, A.M.; Caradonna, P.J.; Forrest, J.R.K.; Post, E. Demographic consequences of phenological shifts in response to climate change. Annu. Rev. Ecol. Evol. Syst. 2021, 52, 221–245. [Google Scholar] [CrossRef]
- Skendžić, S.; Zovko, M.; Živković, I.P.; Lešić, V.; Lemić, D. The impact of climate change on agricultural insect pests. Insects 2021, 12, 440. [Google Scholar] [CrossRef]
- Zeng, J.; Liu, Y.; Zhang, H.; Liu, J.; Jiang, Y.; Wyckhuys, K.A.G.; Wu, K. Global warming modifies long-distance migration of an agricultural insect pest. J. Pest Sci. 2020, 93, 569–581. [Google Scholar] [CrossRef]




| Map Types | Platform | Organization | Website |
|---|---|---|---|
| Generic DD | AgWeather Vegetable Disease & Insect Forecasting Network | UW | https://agweather.cals.wisc.edu/vdifn?p=insect (accessed on 19 December 2023). |
| Generic DD | Enviroweather | MSU | https://www.enviroweather.msu.edu/ (accessed on 19 December 2023). |
| Generic DD | USPest degree-day mapping | OSU | https://uspest.org/cgi-bin/usmapmaker.pl (accessed on 19 December 2023). |
| DD lookup table, phenological events | DDRP | OSU | http://uspest.org/CAPS/ (accessed on 19 December 2023). |
| DD lookup table | PestCAST and FOWeekly | SAFARIS | https://safaris.cipm.info (accessed on 19 December 2023). |
| Phenological events | Pheno Forecasts | USA NPN | https://www.usanpn.org/news/forecasts (accessed on 19 December 2023). |
| Species | Established | Series |
|---|---|---|
| Alfalfa weevil [Hypera postica (Gyllenhal)] | Yes | FO Weekly |
| Asian longhorn beetle [Anoplophora glabripennis Motschulsky)] | Yes | DDRP, FO Weekly, PestCAST, Pheno Forecast |
| Asiatic rice borer (Chilo suppressalis Walker) | No | DDRP |
| Black spruce beetle [Tetropium castaneum (L.)] | No | FO Weekly |
| Brown marmorated stinkbug [Halymorpha halys (Stål)] | Yes | FO Weekly |
| Brown spruce longhorn beetle [Tetropium fuscum (F.)] | No | FO Weekly |
| Box tree moth [Cydalima perspectalis (Walker)] | Yes | FO Weekly, PestCAST |
| Cereal leaf beetle [Oulema melanopus (L.)] | Yes | FO Weekly |
| Cotton cutworm [Spodoptera litura (F.)] | No | DDRP, FO Weekly |
| Egyptian cottonworm [Spodoptera littoralis (Boisduval)] | No | DDRP |
| Emerald ash borer (Agrilus planipennis Fairmaire) | Yes | DDRP, FO Weekly, Pheno Forecast |
| European cherry fruit fly [Rhagoletis cerasi (L.)] | Yes | PestCAST |
| European grapevine moth [Lobesia botrana (Denis & Schiffermüller) | No | FO Weekly |
| False codling moth [Thaumatotibia leucotreta (Meyrick)] | No | DDRP |
| Hemlock woolly adelgid [Adelges tsugae (Annand)] | Yes | Pheno Forecast |
| Honeydew moth (Cryptoblabes gnidiella Millière) | No | FO Weekly, DDRP |
| Japanese beetle (Popillia japonica Newman) | Yes | FO Weekly, PestCAST |
| Japanese flower thrips (Thrips setosus Moulton) | No | FO Weekly |
| Japanese pine sawyer beetle (Monochamis alternatus Hope) | No | DDRP |
| Light brown apple moth [Epiphyas postvittana (Walker)] | Yes | FO Weekly, DDRP |
| Oak ambrosia beetle [Platypus quercivorus (Murayama)] | No | DDRP |
| Old world bollworm [Helicoverpa armigera (Hübner)] | No | DDRP, FO Weekly, PestCAST |
| Pine tree lappet moth [Dendrolimus pini (L.)] | No | DDRP |
| Pink bollworm [Pectinophora gossypiella (Saunders)] | Yes | FO Weekly |
| Silver Y moth [Autographa gamma (L.)] | No | FO Weekly, DDRP |
| Sirex woodwasp [Sirex noctilio (F.)] | Yes | FO Weekly |
| Small tomato borer [Neoleucinodes elegantalis (Guenée)] | No | DDRP |
| Spongy moth [Lymantria dispar (L.)] | Yes | FO Weekly, PestCAST, Pheno Forecast |
| Spotted lanternfly [Lycorma delicatula (White)] | Yes | FO Weekly, PestCAST |
| Summer fruit tortrix [Adoxophyes orana (Fischer von Rösslerstamm)] | No | FO Weekly |
| Sunn pest (Eurygaster integriceps Puton) | No | DDRP |
| Tomato leafminer [Tuta absoluta (Meyrick)] | No | DDRP |
| Winter moth [Operophtera brumata (L.)] | Yes | Pheno Forecast |
| Region | Product | Temporal Coverage | Spatial Resolution | Developer | URL |
|---|---|---|---|---|---|
| Global | NMME | Forecasts up to 12 months | 0.1° | National Oceanic and Atmospheric Administration | https://www.cpc.ncep.noaa.gov/products/NMME (accessed on 19 December 2023). |
| CONUS | PRISM | 1981 to present | 4 km, 800 m | PRISM Climate Group, Oregon State University | http://prism.oregonstate.edu (accessed on 19 December 2023). |
| CONUS, HI, GU, PR, AK | RTMA | 2019 to present | 2.5 km (CONUS, HI, GU), 3 km (AK), 1.25 km (PR) | National Oceanic and Atmospheric Administration | https://www.nco.ncep.noaa.gov/pmb/products/rtma (accessed on 19 December 2023). |
| CONUS | TopoWX | 1948 to 2016 | 800 m | University of Montana | http://www.ntsg.umt.edu/project/topowx.php (accessed on 19 December 2023). |
| CONUS | METDATA | 1979 to present | 4 km | University of Idaho | https://www.sciencebase.gov/catalog/item/54dd5df2e4b08de9379b38d8 (accessed on 19 December 2023). |
| CONUS, HI, GU, PR, VI, AK, NPOI | NDFD | Forecasts up to 7 days | 5 km (CONUS), 2.5 km (HI, GU), 1.25 km (PR, VI), 6 km (AK), 10 km (NPOI) | National Oceanic and Atmospheric Administration | https://vlab.noaa.gov/web/mdl/ndfd (accessed on 19 December 2023). |
| Europe | E-OBS | 1950 to previous month | 0.1° | EU-FP6 project UERRA (Uncertainties in Ensembles of Regional ReAnalyses) | https://surfobs.climate.copernicus.eu (accessed on 19 December 2023). |
| North America | Daymet | 1980 to previous calendar year | 1 km | Oak Ridge National Laboratory, University of Montana | http://www.ntsg.umt.edu/project/daymet.php (accessed on 19 December 2023). |
| Bangladesh, Nepal, and Pakistan | Unknown | 1981 to 2016 | 5 km | Ali et al. [78] | https://doi.org/10.6084/m9.figshare.21565149.v1 (accessed on 19 December 2023). |
| Brazil | BR-DWGD | 1980 to 2016 | 0.1° | Xavier et al. [79] | https://sites.google.com/site/alexandrecandidoxavierufes/brazilian-daily-weather-gridded-data (accessed on 19 December 2023). |
| China | CDAT | 1999 to 2018 | 0.1° | Fang et al. [80] | https://zenodo.org/record/5513811#.YtnLNXbMIuU (accessed on 19 December 2023). |
| China | HRLT | 1961 to 2019 | 0.1° | Qin et al. [81] | https://doi.org/10.1594/PANGAEA.941329 (accessed on 19 December 2023). |
| India | HRDGT | 1951–2016 | 0.1° | Nengzouzam et al. [82], India Meteorological Department | https://searchworks.stanford.edu/view/13160050 (accessed on 19 December 2023). |
| UK | HadUK-Grid | 1884 to present | 1 km | Met Office | https://www.metoffice.gov.uk/research/climate/maps-and-data/data/haduk-grid/haduk-grid (accessed on 19 December 2023). |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barker, B.S.; Coop, L. Phenological Mapping of Invasive Insects: Decision Support for Surveillance and Management. Insects 2024, 15, 6. https://doi.org/10.3390/insects15010006
Barker BS, Coop L. Phenological Mapping of Invasive Insects: Decision Support for Surveillance and Management. Insects. 2024; 15(1):6. https://doi.org/10.3390/insects15010006
Chicago/Turabian StyleBarker, Brittany S., and Leonard Coop. 2024. "Phenological Mapping of Invasive Insects: Decision Support for Surveillance and Management" Insects 15, no. 1: 6. https://doi.org/10.3390/insects15010006
APA StyleBarker, B. S., & Coop, L. (2024). Phenological Mapping of Invasive Insects: Decision Support for Surveillance and Management. Insects, 15(1), 6. https://doi.org/10.3390/insects15010006