Temperature-Dependent Helium Ion-Beam Mixing in an Amorphous SiOC/Crystalline Fe Composite
Abstract
:1. Introduction
2. Materials and Methods
2.1. Marker Specimen Preparation
2.2. Ion Irradiation and Rutherford Backscattering Spectrometry (RBS) Experiment
2.3. Stopping and Range of Ions in Matter (SRIM) Simulation and Microstructure Characterization
3. Results
3.1. Stopping and Range of Ions in Matter (SRIM) Simulation
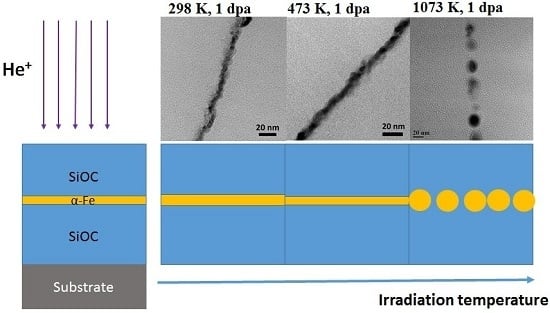
3.2. Analysis of Microstructure before and after Irradiation
3.3. RBS Results
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Matteson, S.; Nicolet, M.A. Ion Mixing. Annu Rev. Mater. Sci. 1983, 13, 339–362. [Google Scholar] [CrossRef]
- Perez, A.; Abonneau, E.; Fuchs, G.; Treilleux, M.; Mchargue, C.J.; Joslin, D.L. Ion-Beam Mixing of Metal Ceramic Interfaces. Nucl. Instrum. Methods Phys. Res. B 1992, 65, 129–138. [Google Scholar] [CrossRef]
- Nordlund, K.; Ghaly, M.; Averback, R.S. Mechanisms of ion beam mixing in metals and semiconductors. J. Appl. Phys. 1998, 83, 1238–1246. [Google Scholar] [CrossRef]
- Nastasi, M.; Hung, L.S.; Mayer, J.W. Phase Formation by Ion-Beam Mixing in Ni/Al, Pd/Al, and Pt/Al Bilayers. Appl. Phys. Lett. 1983, 43, 831–833. [Google Scholar] [CrossRef]
- Averback, R.S. Fundamental-Aspects of Ion-Beam Mixing. Nucl. Instrum. Methods Phys. Res. B 1986, 15, 675–687. [Google Scholar] [CrossRef]
- Paine, B.M.; Averback, R.S. Ion-Beam Mixing—Basic Experiments. Nucl. Instrum. Methods Phys. Res. B 1985, 7–8, 666–675. [Google Scholar] [CrossRef]
- Nastasi, M.; Mayer, J.W. Ion-Beam Mixing in Metallic and Semiconductor-Materials. Mater. Sci. Eng. R Rep. 1994, 12, 1–52. [Google Scholar] [CrossRef]
- Rehn, L.E.; Okamoto, P.R. Recent Progress in Understanding Ion-Beam Mixing of Metals. Nucl. Instrum. Methods Phys. Res. B 1989, 39, 104–113. [Google Scholar] [CrossRef]
- Dereus, R.; Vredenberg, A.M.; Voorrips, A.C.; Tissink, H.C.; Saris, F.W. Critical-Temperatures for Radiation Enhanced Diffusion and Metastable Alloy Formation in Ion-Beam Mixing. Nucl. Instrum. Methods Phys. Res. B 1991, 53, 24–34. [Google Scholar] [CrossRef]
- Fu, C.C.; Torre, J.D.; Willaime, F.; Bocquet, J.L.; Barbu, A. Multiscale modelling of defect kinetics in irradiated iron. Nat. Mater. 2005, 4, 68–74. [Google Scholar] [CrossRef]
- Cheng, Y.T.; Vanrossum, M.; Nicolet, M.A.; Johnson, W.L. Influence of Chemical Driving Forces in Ion Mixing of Metallic Bilayers. Appl. Phys. Lett. 1984, 45, 185–187. [Google Scholar] [CrossRef]
- Wang, Z.L.; Westendorp, J.F.M.; Saris, F.W. Laser and Ion-Beam Mixing of Cu-Au-Cu and Cu-W-Cu Thin-Films. Nucl. Instrum. Methods Phys. Res. 1983, 209, 115–124. [Google Scholar] [CrossRef]
- Westendorp, H.; Wang, Z.L.; Saris, F.W. Ion-Beam Mixing of Cu-Au and Cu-W Systems. Nucl. Instrum. Methods Phys. Res. 1982, 194, 453–456. [Google Scholar] [CrossRef]
- Bolse, W. Mechanisms of ion beam induced atomic mixing in solids. Mater. Sci. Eng. A 1998, 253, 194–201. [Google Scholar] [CrossRef]
- Soraru, G.D.; Suttor, D. High temperature stability of sol-gel-derived SiOC glasses. J. Sol-Gel Sci. Technol. 1999, 14, 69–74. [Google Scholar] [CrossRef]
- Soraru, G.D.; Dallapiccola, E.; DAndrea, G. Mechanical characterization of sol-gel-derived silicon oxycarbide glasses. J. Am. Ceram. Soc. 1996, 79, 2074–2080. [Google Scholar] [CrossRef]
- Harshe, R.; Balan, C.; Riedel, R. Amorphous Si(Al)OC ceramic from polysiloxanes: Bulk ceramic processing, crystallization behavior and applications. J. Eur. Ceram. Soc. 2004, 24, 3471–3482. [Google Scholar] [CrossRef]
- Rouxel, T.; Massouras, G.; Soraru, G.D. High temperature behavior of a gel-derived SiOC glass: Elasticity and viscosity. J. Sol-Gel Sci. Technol. 1999, 14, 87–94. [Google Scholar] [CrossRef]
- Rouxel, T.; Soraru, G.D.; Vicens, J. Creep viscosity and stress relaxation of gel-derived silicon oxycarbide glasses. J. Am. Ceram. Soc. 2001, 84, 1052–1058. [Google Scholar] [CrossRef]
- Nastasi, M.; Su, Q.; Price, L.; Santana, J.A.; Chen, T.; Balerio, R.; Shao, L. Superior radiation tolerant materials: Amorphous silicon oxycarbide. J. Nucl. Mater. 2015, 461, 200–205. [Google Scholar] [CrossRef]
- Su, Q.; Cui, B.; Kirk, M.A.; Nastasi, M. Cascade effects on the irradiation stability of amorphous SiOC. Philos. Mag. Lett. 2016, 96, 60–66. [Google Scholar] [CrossRef]
- Su, Q.; Price, L.; Shao, L.; Nastasi, M. Dose dependence of radiation damage in nano-structured amorphous SiOC/crystalline Fe composite. Mater. Res. Lett. 2016, 4, 48–54. [Google Scholar] [CrossRef]
- Su, Q.; Price, L.; Santana, J.A.C.; Shao, L.; Nastasi, M. Irradiation tolerance of amorphous SiOC/crystalline Fe composite. Mater. Lett. 2015, 155, 138–141. [Google Scholar] [CrossRef]
- Odette, G.R.; Alinger, M.J.; Wirth, B.D. Recent developments in irradiation-resistant steels. Annu. Rev. Mater. Res. 2008, 38, 471–503. [Google Scholar] [CrossRef]
- Su, Q.; Jian, J.; Wang, H.; Nastasi, M. Thermal Stability of Amorphous SiOC/Crystalline Fe Composite. Philos. Mag. 2015, 34, 3876–3887. [Google Scholar] [CrossRef]
- Su, Q.; Cui, B.; Kirk, M.A.; Nastasi, M. In situ observation of radiation damage in nano-structured amorphous SiOC/crystalline Fe composite. Scr. Mater. 2016, 113, 79–83. [Google Scholar] [CrossRef]
- Su, Q.; Wang, F.; Cui, B.; Nastasi, M. Temperature-dependent ion-beam mixing in amorphous SiOC/crystalline Fe composite. Mater. Res. Lett. 2016, 4, 198–203. [Google Scholar] [CrossRef]
- Zinkle, S.J.; Was, G.S. Materials challenges in nuclear energy. Acta Mater. 2013, 61, 735–758. [Google Scholar] [CrossRef]
- Zinkle, S.J.; Busby, J.T. Structural materials for fission & fusion energy. Mater. Today 2009, 12, 12–19. [Google Scholar]
- Ziegler, J.F.; Biersack, J.P. Littmark, the Stopping and Range of Ions in Solids; Pergamon Press: New York, NY, USA, 1985. [Google Scholar]
- Lewis, A.C.; Josell, D.; Weihs, T.P. Stability in thin film multilayers and microlaminates: The role of free energy, structure, and orientation at interfaces and grain boundaries. Scr. Mater. 2003, 48, 1079–1085. [Google Scholar] [CrossRef]
- Josell, D.; Carter, W.C.; Bonevich, J.E. Stability of multilayer structures: Capillary effects. Nanostruct. Mater. 1999, 12, 387–390. [Google Scholar] [CrossRef]






| Specimens | Layer Spreading after Thermal Annealing (nm) | Layer Spreading after 1 dpa Irradiation (nm) |
|---|---|---|
| 298 K | 0 | 2.5 ± 0.4 |
| 473 K | 1.0 ± 0.3 | −2.0 ± 0.3 |
| 673 K | 1.0 ± 0.3 | −2.6 ± 0.6 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, Q.; Price, L.; Shao, L.; Nastasi, M. Temperature-Dependent Helium Ion-Beam Mixing in an Amorphous SiOC/Crystalline Fe Composite. Metals 2016, 6, 261. https://doi.org/10.3390/met6110261
Su Q, Price L, Shao L, Nastasi M. Temperature-Dependent Helium Ion-Beam Mixing in an Amorphous SiOC/Crystalline Fe Composite. Metals. 2016; 6(11):261. https://doi.org/10.3390/met6110261
Chicago/Turabian StyleSu, Qing, Lloyd Price, Lin Shao, and Michael Nastasi. 2016. "Temperature-Dependent Helium Ion-Beam Mixing in an Amorphous SiOC/Crystalline Fe Composite" Metals 6, no. 11: 261. https://doi.org/10.3390/met6110261
APA StyleSu, Q., Price, L., Shao, L., & Nastasi, M. (2016). Temperature-Dependent Helium Ion-Beam Mixing in an Amorphous SiOC/Crystalline Fe Composite. Metals, 6(11), 261. https://doi.org/10.3390/met6110261