Relationship of Late Lactation Milk Somatic Cell Count and Cathelicidin with Intramammary Infection in Small Ruminants
Abstract
:1. Introduction
2. Results
2.1. Prevalence of Intramammary Infection According to Milk Bacteriological Culture Results
2.2. Relationship of Somatic Cell Counts and Cathelicidin Levels with Bacteriological Culture Results
2.2.1. Sheep
2.2.2. Goats
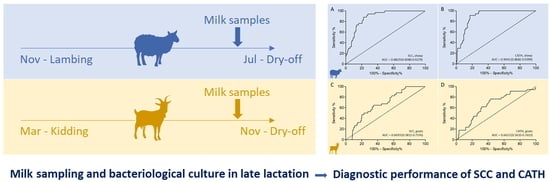
2.3. Test Characteristics of Somatic Cell Count and Cathelicidin Levels Based on Bacteriological Culture Results
2.3.1. Sheep
2.3.2. Goats
2.4. Relationship of Cathelicidin ELISA Results with the Somatic Cell Count
2.4.1. Sheep
2.4.2. Goats
3. Discussion
4. Materials and Methods
4.1. Animals and Milk Samples
4.2. Bacteriological Culture (BC) and Somatic Cell Count (SCC)
4.3. Pan-Cathelicidin ELISA
4.4. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Lopez-Benavides, M.G.; Dohoo, I.; Scholl, D.; Middleton, J.R.; Perez, R. Interpreting Bacteriological Culture Results to Diagnose Bovine Intramammary Infections; NMC Research Committee Report; National Mastitis Council Inc.: Madison, WI, USA, 2012. [Google Scholar]
- Moroni, P.; Welcome, F.; Addis, M.F. Aetiology, diagnosis and control of mastitis in dairy herds. In Achieving Sustainable Production of Milk. 3: Dairy Herd Management and Welfare; Webster, J., Burleigh, D., Eds.; Science Publishing: Cambridge, UK, 2017; ISBN 9781786760524. [Google Scholar]
- Gelasakis, A.I.; Mavrogianni, V.S.; Petridis, I.G.; Vasileiou, N.G.C.; Fthenakis, G.C. Mastitis in sheep—The last 10 years and the future of research. Vet. Microbiol. 2015, 181, 136–146. [Google Scholar] [CrossRef] [PubMed]
- Bergonier, D.; De Crémoux, R.; Rupp, R.; Lagriffoul, G.; Berthelot, X. Mastitis of dairy small ruminants. Vet. Res. 2003, 34, 689–716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Contreras, A.; Sierra, D.; Sanchez, A.; Corrales, J.C.C.; Marco, J.C.C.; Paape, M.J.J.; Gonzalo, C.; Sánchez, A.; Corrales, J.C.C.; Marco, J.C.C.; et al. Mastitis in small ruminants. Small Rumin. Res. 2007, 68, 145–153. [Google Scholar] [CrossRef]
- Berthelot, X.; Lagriffoul, G.; Concordet, D.; Barillet, F.; Bergonier, D. Physiological and pathological thresholds of somatic cell counts in ewe milk. In Proceedings of the 6th International Sheep Veterinary Congress, Hersonissos, Greece, 17–21 June 2005; pp. 40–43. [Google Scholar]
- Stuhr, T.; Aulrich, K. Intramammary infections in dairy goats: Recent knowledge and indicators for detection of subclinical mastitis. Landbauforschung 2010, 60, 267–280. [Google Scholar]
- Bergonier, D.; Berthelot, X. New advances in epizootiology and control of ewe mastitis. Livest. Prod. Sci. 2003, 79, 1–16. [Google Scholar] [CrossRef]
- McDougall, S.; Supré, K.; De Vliegher, S.; Haesebrouck, F.; Hussein, H.; Clausen, L.; Prosser, C. Diagnosis and treatment of subclinical mastitis in early lactation in dairy goats. J. Dairy Sci. 2010, 93, 4710–4721. [Google Scholar] [CrossRef] [Green Version]
- Harmon, R.J. Physiology of mastitis and factors affecting somatic cell counts. J. Dairy Sci. 1994, 77, 2103–2112. [Google Scholar] [CrossRef]
- Souza, F.N.; Blagitz, M.G.; Penna, C.F.A.M.; Della Libera, A.M.M.P.; Heinemann, M.B.; Cerqueira, M.M.O.P. Somatic cell count in small ruminants: Friend or foe? Small Rumin. Res. 2012, 107, 65–75. [Google Scholar] [CrossRef]
- Tedde, V.; Bronzo, V.; Puggioni, G.M.G.; Pollera, C.; Casula, A.; Curone, G.; Moroni, P.; Uzzau, S.; Addis, M.F. Milk cathelicidin and somatic cell counts in dairy goats along the course of lactation. J. Dairy Res. 2019, 86, 217–221. [Google Scholar] [CrossRef]
- Perrin, G.G.; Mallereau, M.P.; Lenfant, D.; Baudry, C. Relationships between California mastitis test (CMT) and somatic cell counts in dairy goats. Small Rumin. Res. 1997, 26, 167–170. [Google Scholar] [CrossRef]
- Jimenez-Granado, R.; Sanchez-Rodriguez, M.; Arce, C.; Rodriguez-Estevez, V. Factors affecting somatic cell count in dairy goats: A review. Span. J. Agric. Res. 2014, 12, 133–150. [Google Scholar] [CrossRef] [Green Version]
- Paape, M.J.; Poutrel, B.; Contreras, A.; Marco, J.C.; Capuco, A.V. Milk Somatic Cells and Lactation in Small Ruminants. J. Dairy Sci. 2001, 84, E237–E244. [Google Scholar] [CrossRef]
- Fragkou, I.A.; Boscos, C.M.; Fthenakis, G.C. Diagnosis of clinical or subclinical mastitis in ewes. Small Rumin. Res. 2014, 118, 86–92. [Google Scholar] [CrossRef]
- Dohoo, I.R.; Smith, J.; Andersen, S.; Kelton, D.F.; Godden, S. Diagnosing intramammary infections: Evaluation of definitions based on a single milk sample. J. Dairy Sci. 2011, 94, 250–261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walker, J.B.; Rajala-Schultz, P.J.; Walker, W.L.; Mathews, J.L.; Gebreyes, W.A.; DeGraves, F.J. Variation in daily shedding patterns of Staphylococcus aureus in naturally occurring intramammary infections. J. Vet. Diagn. Investig. 2011, 23, 1114–1122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koop, G.; Nielen, M.; van Werven, T. Diagnostic tools to monitor udder health in dairy goats. Vet. Q. 2012, 32, 37–44. [Google Scholar] [CrossRef] [Green Version]
- Viguier, C.; Arora, S.; Gilmartin, N.; Welbeck, K.; O’Kennedy, R. Mastitis detection: Current trends and future perspectives. Trends Biotechnol. 2009, 27, 486–493. [Google Scholar] [CrossRef]
- Wheeler, T.T.; Ledgard, A.; Smolenski, G.A.; Backmann, E.; McDonald, R.; Lee, R.S.F. Innate immune proteins as biomarkers for mastitis and endometritis. In Proceedings of the Australasian Dairy Science Symposium 2012, The 5th Australasian Dairy Science Symposium, Melbourne, Australia, 13–15 November 2012; pp. 294–297. [Google Scholar]
- Addis, M.F.; Pisanu, S.; Marogna, G.; Cubeddu, T.; Pagnozzi, D.; Cacciotto, C.; Campesi, F.; Schianchi, G.; Rocca, S.; Uzzau, S. Production and release of antimicrobial and immune defense proteins by mammary epithelial cells following Streptococcus uberis infection of sheep. Infect. Immun. 2013, 81, 3182–3197. [Google Scholar] [CrossRef] [Green Version]
- Pisanu, S.; Cubeddu, T.; Pagnozzi, D.; Rocca, S.; Cacciotto, C.; Alberti, A.; Marogna, G.; Uzzau, S.; Addis, M.F. Neutrophil extracellular traps in sheep mastitis. Vet. Res. 2015, 46, 59. [Google Scholar] [CrossRef] [Green Version]
- Katsafadou, A.I.; Tsangaris, G.T.; Anagnostopoulos, A.K.; Billinis, C.; Barbagianni, M.S.; Vasileiou, N.G.C.; Spanos, S.A.; Mavrogianni, V.S.; Fthenakis, G.C. Differential quantitative proteomics study of experimental Mannheimia haemolytica mastitis in sheep. J. Proteom. 2019, 205, 103393. [Google Scholar] [CrossRef]
- Olumee-Shabon, Z.; Swain, T.; Smith, E.A.; Tall, E.; Boehmer, J.L. Proteomic analysis of differentially expressed proteins in caprine milk during experimentally induced endotoxin mastitis. J. Dairy Sci. 2013, 96, 2903–2912. [Google Scholar] [CrossRef] [PubMed]
- Cubeddu, T.; Cacciotto, C.; Pisanu, S.; Tedde, V.; Alberti, A.; Pittau, M.; Dore, S.; Cannas, A.; Uzzau, S.; Rocca, S.; et al. Cathelicidin production and release by mammary epithelial cells during infectious mastitis. Vet. Immunol. Immunopathol. 2017, 189, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Zanetti, M. Cathelicidins, multifunctional peptides of the innate immunity. J. Leukoc. Biol. 2004, 75, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Addis, M.F.; Tedde, V.; Dore, S.; Pisanu, S.; Puggioni, G.M.G.; Roggio, A.M.; Pagnozzi, D.; Lollai, S.; Cannas, E.A.; Uzzau, S. Evaluation of milk cathelicidin for detection of dairy sheep mastitis. J. Dairy Sci. 2016, 99, 6446–6456. [Google Scholar] [CrossRef] [PubMed]
- Addis, M.F.; Tedde, V.; Puggioni, G.M.G.; Pisanu, S.; Casula, A.; Locatelli, C.; Rota, N.; Bronzo, V.; Moroni, P.; Uzzau, S. Evaluation of milk cathelicidin for detection of bovine mastitis. J. Dairy Sci. 2016, 99, 8250–8258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boscos, C.; Stefanakis, A.; Alexopoulos, C.; Samartzi, F. Prevalence of subclinical mastitis and influence of breed, parity, stage of lactation and mammary bacteriological status on Coulter Counter Counts and California Mastitis Test in the milk of Saanen and autochthonous Greek goats. Small Rumin. Res. 1996, 21, 139–147. [Google Scholar] [CrossRef]
- Borregaard, N.; Sørensen, O.E.; Theilgaard-Mönch, K. Neutrophil granules: A library of innate immunity proteins. Trends Immunol. 2007, 28, 340–345. [Google Scholar] [CrossRef]
- Cuccuru, C.; Moroni, P.; Zecconi, A.; Casu, S.; Caria, A.; Contini, A. Milk differential cell counts in relation to total counts in Sardinian ewes. Small Rumin. Res. 1997, 25, 169–173. [Google Scholar] [CrossRef]
- UNI EN ISO 13366-2:2007(IDF 148-2:2006)—Milk: Enumeration of Somatic Cells—Part 2: Guidance on the Operation of Fluoro-opto-electronic Counters. Available online: https://www.iso.org/standard/40260.html (accessed on 1 January 2020).
- Addis, M.F.; Bronzo, V.; Puggioni, G.M.G.; Cacciotto, C.; Tedde, V.; Pagnozzi, D.; Locatelli, C.; Casula, A.; Curone, G.; Uzzau, S.; et al. Relationship between milk cathelicidin abundance and microbiologic culture in clinical mastitis. J. Dairy Sci. 2017, 100, 2944–2953. [Google Scholar] [CrossRef]



| Class | N | Median (IQR) a Cells × 103/mL | Median (IQR) CATH AOD450 |
|---|---|---|---|
| Sheep, BC negative | 281 | 235.0 (122.5–554.5) | 0.0861 (0.0701–0.1071) |
| Sheep, BC positive | 34 | 1637.0 (842.8–14,422.0) | 0.2261 (0.1352–2.275) |
| Goats, BC negative | 189 | 303.0 (104.0–772.5) | 0.1121 (0.0886–0.1501) |
| Goats, BC positive | 34 | 812.5 (232.3–2397.0) | 0.1148 (0.1152–0.2449) |
| Test. | AUC a (95% CI) b | c* c | Jd | Se% (95% CI) e | Sp% (95% CI) f |
|---|---|---|---|---|---|
| Sheep | |||||
| SCC g | 0.8829 (0.8380–0.9279) | 488.5 | 66.36 | 94.12 (80.32–99.28) | 72.24 (66.61–77.40) |
| CATH h | 0.9041 (0.8682–0.9399) | 0.1206 | 74.10 | 91.18 (76.32–98.14) | 82.92 (78.00–87.13) |
| Goats | |||||
| SCC | 0.6693 (0.5811–0.7576) | 422.0 | 29.79 | 64.71 (46.49–80.25) | 65.08 (57.82–71.85) |
| CATH | 0.6623 (0.5633–0.7613) | 0.1183 | 33.61 | 76.47 (55.83–89.25) | 57.14 (49.76–64.30) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puggioni, G.M.G.; Tedde, V.; Uzzau, S.; Dore, S.; Liciardi, M.; Cannas, E.A.; Pollera, C.; Moroni, P.; Bronzo, V.; Addis, M.F. Relationship of Late Lactation Milk Somatic Cell Count and Cathelicidin with Intramammary Infection in Small Ruminants. Pathogens 2020, 9, 37. https://doi.org/10.3390/pathogens9010037
Puggioni GMG, Tedde V, Uzzau S, Dore S, Liciardi M, Cannas EA, Pollera C, Moroni P, Bronzo V, Addis MF. Relationship of Late Lactation Milk Somatic Cell Count and Cathelicidin with Intramammary Infection in Small Ruminants. Pathogens. 2020; 9(1):37. https://doi.org/10.3390/pathogens9010037
Chicago/Turabian StylePuggioni, Giulia Maria Grazia, Vittorio Tedde, Sergio Uzzau, Simone Dore, Manuele Liciardi, Eugenia Agnese Cannas, Claudia Pollera, Paolo Moroni, Valerio Bronzo, and Maria Filippa Addis. 2020. "Relationship of Late Lactation Milk Somatic Cell Count and Cathelicidin with Intramammary Infection in Small Ruminants" Pathogens 9, no. 1: 37. https://doi.org/10.3390/pathogens9010037
APA StylePuggioni, G. M. G., Tedde, V., Uzzau, S., Dore, S., Liciardi, M., Cannas, E. A., Pollera, C., Moroni, P., Bronzo, V., & Addis, M. F. (2020). Relationship of Late Lactation Milk Somatic Cell Count and Cathelicidin with Intramammary Infection in Small Ruminants. Pathogens, 9(1), 37. https://doi.org/10.3390/pathogens9010037