Influence of Hurdle Technology on Foodborne Pathogen Survival in the Human Gastrointestinal Tract
Abstract
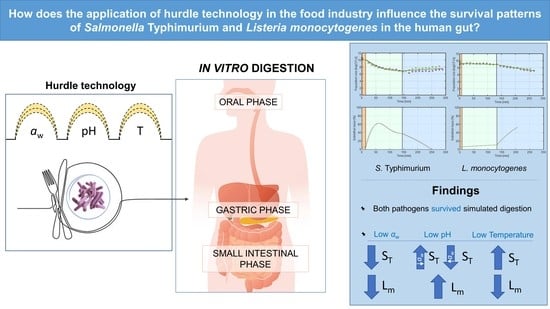
:1. Introduction
2. Materials and Methods
2.1. Microorganisms and Preculture Conditions
2.2. Inoculum Preparation
2.3. Food Model System Development and Preparation
2.4. Assessment and Modelling of Boundaries of Growth
2.5. Hurdle Technology Application on the Developed FMS
2.6. Enzymes and Bile Acids
2.7. Simulated Digestion Fluids
2.8. In Vitro Digestion Model System
2.9. Hurdle Technology Application and Microbiological Analysis
2.10. Modelling Microbial Kinetics
2.11. Statistical Analysis
3. Results and Discussion
3.1. Assessment and Modelling of Growth Boundaries
3.2. Effect of the Hurdle Technology Application on the Survival of Salmonella Typhimurium and Listeria Monocytogenes
3.2.1. Salmonella Typhimurium
- General remarks
- 2.
- Hurdles during in vitro digestion
Hurdle of aw
Hurdle of pH
Hurdle of Storage Temperature
3.2.2. Listeria monocytogenes
- General remarks
- 2.
- Hurdles during in vitro digestion
Hurdle of aw
Hurdle of pH
Hurdle of Storage Temperature
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- European Food Safety Authority; European Centre for Disease Prevention and Control. The European Union One Health 2020 Zoonoses Report. EFSA J. 2021, 19, e06971. [Google Scholar]
- Khan, I.; Tango, C.N.; Miskeen, S.; Lee, B.H.; Oh, D.H. Hurdle Technology: A Novel Approach for Enhanced Food Quality and Safety-A Review. Food Control 2017, 73, 1426–1444. [Google Scholar] [CrossRef]
- Beristaín-Bauza, S.; Martínez-Niño, A.; Ramírez-González, A.P.; Ávila-Sosa, R.; Ruíz-Espinosa, H.; Ruiz-López, I.I.; Ochoa-Velasco, C.E. Inhibition of Salmonella Typhimurium Growth in Coconut (Cocos nucifera L.) Water by Hurdle Technology. Food Control 2018, 92, 312–318. [Google Scholar] [CrossRef]
- Leistner, L. Introduction to Hurdle Technology. In Food Preservation by Combined Processes; FLAIR Report; European Commission: Brussels, Belgium, 1994. [Google Scholar]
- Singh, S.; Shalini, R. Effect of Hurdle Technology in Food Preservation: A Review. Crit. Rev. Food Sci. Nutr. 2016, 56, 641–649. [Google Scholar] [CrossRef] [PubMed]
- Annous, B.; Gurtler, J. Salmonella: Distribution, Adaptation, Control Measures and Molecular Technologies. In Adaptation of Salmonella to Antimicrobials in Food-Processing Environments; In Tech: Rijeka, Croatia, 2012; pp. 124–146. [Google Scholar]
- Yousef, A.; Courtney, P. Basics of Stress Adaptation and Implications in New-Generation Foods. In Microbial Stress Adaptation and Food Safety; CRC Press: Boca Raton, FL, USA, 2002. [Google Scholar]
- Ye, B.; He, S.; Zhou, X.; Cui, Y.; Zhou, M.; Shi, X. Response to acid adaptation in Salmonella enterica serovar Enteritidis. J. Food Sci. 2019, 84, 599–605. [Google Scholar] [CrossRef]
- Mutz, Y.S.; Rosario, D.K.A.; Castro, V.S.; Bernardes, P.C.; Paschoalin, V.M.F.; Conte-Junior, C.A. Prior Exposure to Dry-Cured Meat Promotes Resistance to Simulated Gastric Fluid in Salmonella Typhimurium. Foods 2019, 8, 603. [Google Scholar] [CrossRef] [PubMed]
- Rychlik, I.; Barrow, P.A. Salmonella Stress Management and Its Relevance to Behaviour during Intestinal Colonisation and Infection. FEMS Microbiol. Rev. 2005, 29, 1021–1040. [Google Scholar] [CrossRef] [PubMed]
- Ahmer, B.M.M.; Gunn, J.S. Interaction of Salmonella spp. With the Intestinal Microbiota. Front. Microbiol. 2011, 2, 101. [Google Scholar] [CrossRef]
- Koziolek, M.; Grimm, M.; Becker, D.; Iordanov, V.; Zou, H.; Shimizu, J.; Wanke, C.; Garbacz, G.; Weitschies, W. Investigation of pH and Temperature Profiles in the GI Tract of Fasted Human Subjects Using the Intellicap® System. J. Pharm. Sci. 2015, 104, 2855–2863. [Google Scholar] [CrossRef]
- Just, J.R.; Daeschel, M.A. Antimicrobial Effects of Wine on Escherichia Coli O157: H7 and Salmonella Typhimurium in a Model Stomach System. J. Food Sci. 2003, 68, 285–290. [Google Scholar] [CrossRef]
- Wijnands, L.M.; Teunis, P.F.M.; Kuijpers, A.F.A.; Delfgou-Van Asch, E.H.M.; Pielaat, A. Quantification of Salmonella Survival and Infection in an in Vitro Model of the Human Intestinal Tract as Proxy for Foodborne Pathogens. Front. Microbiol. 2017, 8, 1139. [Google Scholar] [CrossRef] [PubMed]
- Verheyen, D.; Bolívar, A.; Pérez-Rodríguez, F.; Baka, M.; Skåra, T.; Van Impe, J.F. Effect of Food Microstructure on Growth Dynamics of Listeria Monocytogenes in Fish-Based Model Systems. Int. J. Food Microbiol. 2018, 283, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Akritidou, T.; Akkermans, S.; Gaspari, S.; Azraini, N.D.; Smet, C.; Van de Wiele, T.; Van Impe, J.F.M. Effect of Gastric PH and Bile Acids on the Survival of Listeria Monocytogenes and Salmonella Typhimurium during Simulated Gastrointestinal Digestion. Innov. Food Sci. Emerg. Technol. 2022, 82, 103161. [Google Scholar] [CrossRef]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST Static in Vitro Simulation of Gastrointestinal Food Digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, R.S.; Weimer, P.J.; Kunz, D.A. Use of Photocatalytic Reduction to Hasten Preparation of Culture Media for Saccharolytic Clostridium Species. Braz. J. Microbiol. 2003, 34, 22–26. [Google Scholar] [CrossRef]
- Uchino, Y.; Ken-Ichiro, S. A Simple Preparation of Liquid Media for the Cultivation of Strict Anaerobes. J. Pet. Environ. Biotechnol. S 2012, 3, 1–3. [Google Scholar] [CrossRef]
- Geeraerd, A.H.; Herremans, C.H.; Van Impe, J.F. Structural Model Requirements to Describe Microbial Inactivation during a Mild Heat Treatment. Int. J. Food Microbiol. 2000, 59, 185–209. [Google Scholar] [CrossRef] [PubMed]
- Akkermans, S.; Logist, F.; Van Impe, J.F. An Interaction Model for the Combined Effect of Temperature, PH and Water Activity on the Growth Rate of E. Coli K12. Food Res. Int. 2018, 106, 1123–1131. [Google Scholar] [CrossRef] [PubMed]
- Koutsoumanis, K.P.; Kendall, P.A.; Sofos, J.N. Effect of Food Processing-Related Stresses on Acid Tolerance of Listeria Monocytogenes. Appl. Environ. Microbiol. 2003, 69, 7514–7516. [Google Scholar] [CrossRef] [PubMed]
- Koutsoumanis, K.P.; Kendall, P.A.; Sofos, J.N. Modeling the Boundaries of Growth of Salmonella Typhimurium in Broth as a Function of Temperature, Water Activity, and PH. J. Food Prot. 2004, 67, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.C.; Wiedmann, M. Physiology and Genetics of Listeria Monocytogenes Survival and Growth at Cold Temperatures. Crit. Rev. Food Sci. Nutr. 2009, 49, 237–253. [Google Scholar] [CrossRef] [PubMed]
- Mckellar, R.C.; Lu, X. A Probability of Growth Model for Escherichia Coli O157: H7 as a Function of Temperature, PH, Acetic Acid, and Salt. J. Food Prot. 2001, 64, 1922–1928. [Google Scholar] [CrossRef]
- Wilson, P.D.G.; Brocklehurst, T.F.; Arino, S.; Thuault, D.; Jakobsen, M.; Lange, M.; Farkas, J.; Wimpenny, J.W.T.; Van Impe, J.F. Modelling Microbial Growth in Structured Foods: Towards a Unified Approach. Int. J. Food Microbiol. 2002, 73, 275–289. [Google Scholar] [CrossRef] [PubMed]
- Meldrum, R.J.; Brocklehurst, T.F.; Wilson, D.R.; Wilson, P.D.G. The Effects of Cell Immobilization, PH and Sucrose on the Growth of Listeria Monocytogenes Scott A at 10 °C. Food Microbiol. 2003, 20, 97–103. [Google Scholar] [CrossRef]
- Koseki, S.; Mizuno, Y.; Sotome, I. Modeling of Pathogen Survival during Simulated Gastric Digestion. Appl. Environ. Microbiol. 2011, 77, 1021–1032. [Google Scholar] [CrossRef] [PubMed]
- Akritidou, T.; Akkermans, S.; Smet, C.; Delens, V.; Van Impe, J.F.M. Effect of Food Structure and Buffering Capacity on Pathogen Survival during in Vitro Digestion. Food Res. Int. 2023, 164, 112305. [Google Scholar] [CrossRef]
- International Commission on Microbiological Specifications for Foods. Microorganisms in Foods 5: Characteristics of Microbial Pathogens; Springer Science & Business Media: Berlin/Heidelberg, Germany, 1996; Volume 5. [Google Scholar]
- Wei, X.; Lau, S.K.; Chaves, B.D.; Danao, M.G.C.; Agarwal, S.; Subbiah, J. Effect of water activity on the thermal inactivation kinetics of Salmonella in milk powders. J. Dairy Sci. 2020, 103, 6904–6917. [Google Scholar] [CrossRef] [PubMed]
- Beuchat, L.R.; Komitopoulou, E.; Beckers, H.; Betts, R.P.; Bourdichon, F.; Fanning, S.; Joosten, H.M.; Ter Kuile, B.H. Low-Water Activity Foods: Increased Concern as Vehicles of Foodborne Pathogens. J. Food Prot. 2013, 76, 150–172. [Google Scholar] [CrossRef] [PubMed]
- Dhaliwal, H.K.; Gänzle, M.; Roopesh, M.S. Influence of Drying Conditions, Food Composition, and Water Activity on the Thermal Resistance of Salmonella Enterica. Food Res. Int. 2021, 147, 110548. [Google Scholar] [CrossRef]
- D’Aoust, J.Y. Psychrotrophy and Foodborne Salmonella. Int. J. Food Microbiol. 1991, 13, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.A.; Yuk, H.G.; Liu, D.; Ding, T. Recent advances in understanding the effect of acid-adaptation on the cross-protection to food-related stress of common foodborne pathogens. Crit. Rev. Food Sci. Nutr. 2022, 62, 7336–7353. [Google Scholar] [CrossRef] [PubMed]
- Koutsoumanis, K.P.; Sofos, J.N. Comparative Acid Stress Response of Listeria Monocytogenes, Escherichia Coli O157: H7 and Salmonella Typhimurium after Habituation at Different PH Conditions. Lett. Appl. Microbiol. 2004, 38, 321–326. [Google Scholar] [CrossRef]
- Zhang, Y.; Gross, C.A. Cold shock response in bacteria. Annu. Rev. Genet. 2021, 55, 377–400. [Google Scholar] [CrossRef] [PubMed]
- Craig, J.E.; Boyle, D.; Francis, K.P.; Gallagher, M.P. Expression of the Cold-Shock Gene CspB in Salmonella Typhimurium Occurs below a Threshold Temperature. Microbiology 1998, 144 Pt 3, 697–704. [Google Scholar] [CrossRef] [PubMed]
- Keto-Timonen, R.; Hietala, N.; Palonen, E.; Hakakorpi, A.; Lindström, M.; Korkeala, H. Cold Shock Proteins: A Minireview with Special Emphasis on Csp-Family of Enteropathogenic Yersinia. Front. Microbiol. 2016, 7, 1151. [Google Scholar] [CrossRef] [PubMed]
- White, S.J.; McClung, D.M.; Wilson, J.G.; Roberts, B.N.; Donaldson, J.R. Influence of PH on Bile Sensitivity amongst Various Strains of Listeria Monocytogenes under Aerobic and Anaerobic Conditions. J. Med. Microbiol. 2015, 64, 1287–1296. [Google Scholar] [CrossRef] [PubMed]
- O’Driscoll, B.; Gahan, C.G.; Hill, C. Adaptive Acid Tolerance Response in Listeria Monocytogenes: Isolation of an Acid-Tolerant Mutant Which Demonstrates Increased Virulence. Appl. Environ. Microbiol. 1996, 62, 1693–1698. [Google Scholar] [CrossRef] [PubMed]
- Gahan, C.G.M.; Hill, C. Gastrointestinal Phase of Listeria Monocytogenes Infection. J. Appl. Microbiol. 2005, 98, 1345–1353. [Google Scholar] [CrossRef]
- Csonka, L.N. Physiological and Genetic Responses of Bacteria to Osmotic Stress. Microbiol. Rev. 1989, 53, 121–147. [Google Scholar] [CrossRef]
- Shabala, L.; Lee, S.H.; Cannesson, P.; Ross, T. Acid and NaCl Limits to Growth of Listeria Monocytogenes and Influence of Sequence of Inimical Acid and NaCl Levels on Inactivation Kinetics. J. Food Prot. 2008, 71, 1169–1177. [Google Scholar] [CrossRef] [PubMed]
- Lou, Y.; Yousef, A.E. Adaptation to Sublethal Environmental Stresses Protects Listeria Monocytogenes against Lethal Preservation Factors. Appl. Environ. Microbiol. 1997, 63, 1252–1255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Makariti, I.P.; Grivokostopoulos, N.C.; Skandamis, P.N. Effect Of Oxygen Availability and PH On Adaptive Acid Tolerance Response of Immobilized Listeria Monocytogenes in Structured Growth Media. Food Microbiol. 2021, 99, 103826. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhu, L.; Dong, P.; Liang, R.; Mao, Y.; Yang, X.; Zhang, Y.; Luo, X. Acid Tolerance Response of Listeria Monocytogenes in Various External PHs with Different Concentrations of Lactic Acid. Foodborne Pathog. Dis. 2020, 17, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Barria, C.; Malecki, M.; Arraiano, C.M. Bacterial Adaptation to cold. Microbiology 2013, 159 Pt 12, 2437–2443. [Google Scholar] [CrossRef] [PubMed]
- Aldsworth, T.G.; Sharman, R.L.; Dodd, C.E. Bacterial Suicide through Stress. Cell. Mol. Life Sci. 1999, 56, 378–383. [Google Scholar] [CrossRef] [PubMed]





| Gastric Phase | Intestinal Phase | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Experimental Condition | rmax (1/min) | Nres [log(CFU)] | MSE | rmax (1/min) | Final Log Reduction [log(CFU) ± SD] | ||||
| 95% Confidence Interval | 95% Confidence Interval | ||||||||
| A | aw 0.994–pH 6.6–25 °C | +0.012 a | [+0.009, +0.015] | - | - | 0.107 | +0.003 a | [+0.001, +0.006] | 2.023 ± 0.167 a |
| B | aw 0.994–pH 6.6–10 °C | +0.052 b | [+0.041, +0.062] | 6.931 a | [6.716, 7.147] | 0.128 | −0.004 b | [−0.003, −0.001] | 2.497 ± 0.199 b |
| C | aw 0.994–pH 5.0–25 °C | +0.009 c | [+0.007, +0.011] | - | - | 0.070 | +0.007 c | [+0.005, +0.009] | 2.067 ± 0.132 a |
| D | aw 0.994–pH 5.0–10 °C | +0.095 d | [+0.072, +0.019] | 6.202 a | [5.933, 6.467] | 0.285 | −0.012 d | [−0.018, −0.007] | 1.303 ± 0.265 c |
| E | aw 0.931–pH 6.6–25 °C | +0.026 g | [+0.015, +0.037] | 3.288 b | [0.236, 6.341] | 0.495 | −0.004 b | [−0.012, +0.004] | 2.251 ± 0.287 b |
| F | aw 0.931–pH 6.6–10 °C | +0.012 a | [+0.010, +0.013] | - | - | 0.045 | +0.004 e | [+0.002, +0.005] | 1.962 ± 0.103 a |
| G | aw 0.931–pH 5.0–25 °C | +0.031 f | [+0.024, +0.038] | - | - | 0.754 | +0.021 f | [+0.014, +0.028] | 6.629 ± 0.423 d |
| H | aw 0.931–pH 5.0–10 °C | +0.015 e | [+0.006, +0.023] | - | - | 0.988 | +0.004 e | [+0.003, +0.011] | 2.408 ± 0.492 b |
| Experimental Condition | Gastric Phase | Intestinal Phase | Final Log Reduction [log(CFU) ± SD] | ||||||
|---|---|---|---|---|---|---|---|---|---|
| rmax (1/min) | rmax (1/min) | Nres [log(CFU)] | MSE | ||||||
| 95% Confidence Interval | 95% Confidence Interval | 95% Confidence Interval | |||||||
| A | aw 0.994–pH 6.6–25 °C | +0.003 a | [+0.002, +0.003] | +0.018 a | [+0.013, +0.024] | 6.698 a | [3.388, 10.009] | 0.066 | 2.728 ± 0.146 a |
| B | aw 0.994–pH 6.6–10 °C | +0.012 b | [+0.009, +0.015] | +0.016 b | [+0.005, +0.027] | 7.000 a | [4.550, 9.450] | 0.120 | 3.303 ± 0.225 b |
| C | aw 0.994–pH 5.0–25 °C | +0.003 a | [+0.002, +0.004] | +0.010 c | [+0.005, +0.001] | - | 0.167 | 1.657 ± 0.177 c | |
| D | aw 0.994–pH 5.0–10 °C | +0.006 c | [+0.006, +0.007] | +0.010 c | [+0.009, +0.011] | - | 0.030 | 1.939 ± 0.077 d | |
| E | aw 0.909–pH 6.6–25 °C | +0.004 d | [+0.001, +0.007] | +0.055 g | [+0.045, +0.065] | 5.115 b | [4.783, 5.448] | 0.150 | 4.032 ± 0.189 g |
| F | aw 0.909–pH 6.6–10 °C | +0.002 e | [+0.001,+0.004] | +0.041 f | [+0.028, +0.053] | 6.584 a | [6.238, 6.931] | 0.123 | 2.573 ± 0.158 f |
| G | aw 0.909–pH 5.0–25 °C | +0.004 d | [+0.002,+0.006] | +0.033 e | [+0.017, +0.049] | 6.374 a | [6.459, 7.009] | 0.070 | 2.216 ± 0.131 e |
| H | aw 0.909–pH 5.0–10 °C | +0.003 a | [+0.002,+0.004] | +0.013 d | [+0.012, +0.015] | - | 0.030 | 2.147 ± 0.075 e | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akritidou, T.; Akkermans, S.; Smet, C.; de Mey, F.; Van Impe, J.F.M. Influence of Hurdle Technology on Foodborne Pathogen Survival in the Human Gastrointestinal Tract. Microorganisms 2023, 11, 405. https://doi.org/10.3390/microorganisms11020405
Akritidou T, Akkermans S, Smet C, de Mey F, Van Impe JFM. Influence of Hurdle Technology on Foodborne Pathogen Survival in the Human Gastrointestinal Tract. Microorganisms. 2023; 11(2):405. https://doi.org/10.3390/microorganisms11020405
Chicago/Turabian StyleAkritidou, Theodora, Simen Akkermans, Cindy Smet, Fien de Mey, and Jan F. M. Van Impe. 2023. "Influence of Hurdle Technology on Foodborne Pathogen Survival in the Human Gastrointestinal Tract" Microorganisms 11, no. 2: 405. https://doi.org/10.3390/microorganisms11020405
APA StyleAkritidou, T., Akkermans, S., Smet, C., de Mey, F., & Van Impe, J. F. M. (2023). Influence of Hurdle Technology on Foodborne Pathogen Survival in the Human Gastrointestinal Tract. Microorganisms, 11(2), 405. https://doi.org/10.3390/microorganisms11020405