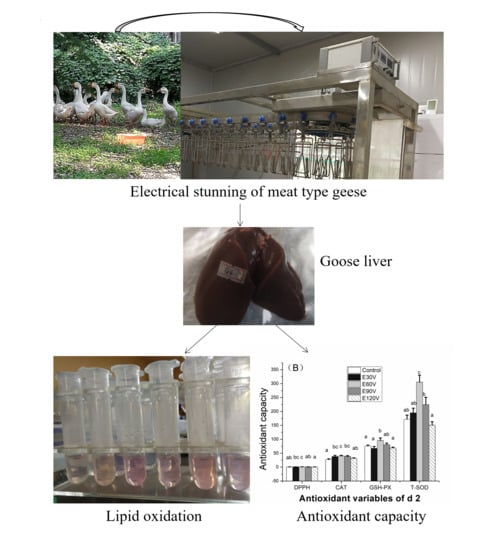
Evaluation of the Effects of Pre-Slaughter High-Frequency Electrical Stunning Current Intensities on Lipid Oxidative Stability and Antioxidant Capacity in the Liver of Yangzhou Goose (Anser cygnoides domesticus)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Birds and Management
2.2. Electrical Stunning System
2.3. Experimental Design
2.4. Slaughter and Sampling
2.5. Lipid Oxidation and Antioxidant Capacity
2.6. Liver Color
2.7. Statistical Analysis
3. Results and Discussion
3.1. Lipid Oxidative Stability
3.2. Antioxidant Capacity Stability
3.3. Relationship between Liver Color and Bleed-out Efficiency or Lipid Oxidation
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Qin, X.; Xiao, B.M.; Liu, L.Z. Forecast of China’s meat goose industry economy from 2018 to 2020. China Poultry 2018, 11, 58–60. [Google Scholar]
- Chen, F.; Zhang, H.; Li, J.; Tian, Y.; Xu, J.; Chen, L. Identification of differentially expressed mirnas in the fatty liver of landes goose (anser anser). Sci. Rep.-UK 2017, 7, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Junwang, T.; Qianqian, F.; Rongyi, S.; Junda, S.; Jun, H.; Dong, N. Digital gene-expression profiling analysis of the fatty liver of landes geese fed different supplemental oils. Gene 2018, 673, 32–45. [Google Scholar]
- Abu-Salem, F.M.; Abou Arab, E.A. Chemical properties, microbiological quality and sensory evaluation of chicken and duck liver paste (foie gras). Grasas Aceites. 2010, 61, 126–135. [Google Scholar] [CrossRef]
- Chen, C.; Yu, Q.; Han, L.; Zhang, J.; Guo, Z. Effects of aldehyde products of lipid oxidation on the color stability and metmyoglobin reducing ability of bovine longissimus muscle. J. Anim. Sci. 2018, 89, 810–816. [Google Scholar] [CrossRef]
- Falowo, A.B.; Fayemi, P.O.; Muchenje, V. Natural antioxidants against lipid-protein oxidative deterioration in meat and meat products: A review. Food Res. Int. 2014, 64, 171–181. [Google Scholar] [CrossRef]
- Hcini, E.; Slima, A.B.; Kallel, I.; Zormati, S.; Traore, A.I.; Gdoura, R. Does supplemental zeolite (clinoptilolite) affect growth performance, meat texture, oxidative stress and production of polyunsaturated fatty acid of turkey poults? Lipids Health Dis. 2018, 17, 177. [Google Scholar] [CrossRef] [Green Version]
- Orkusz, A.; Haraf, G.; Okruszek, A.; Wereńska-Sudnik, M. Lipid oxidation and color changes of goose meat stored under vacuum and modified atmosphere conditions. Poult. Sci. 2016, 96, 731–737. [Google Scholar] [CrossRef]
- Gasparino, E.; Vesco, A.P.D.; Khatlab, A.S.; Zancanela, V.; Grieser, D.O.; Silva, S.C.C. Effects of methionine hydroxy analogue supplementation on the expression of antioxidant-related genes of acute heat stress-exposed broilers. Animal 2017, 12, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Xu, L.; Zhang, H.J.; Yue, H.Y.; Wu, S.G.; Yang, H.M.; Qi, G.H.; Wang, Z.Y. Low-current & high-frequency ES increased oxidative stress, lipid peroxidation, and gene transcription of the mitogen-activated protein kinase/nuclear factor-erythroid 2-related factor 2/antioxidant responsive element (MAPK/Nrf2/ARE) signaling pathway in breast muscle of broilers. Food Chem. 242, 491–496.
- Chen, Y.; Cheng, Y.; Wen, C.; Wang, W.; Kang, Y.; Wang, A. The protective effects of modified palygorskite on the broilers fed a purified zearalenone-contaminated diet. Poult. Sci. 2019, 89, 3802–3810. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, H.J.; Wan, X.L.; Yang, H.M.; Wang, Z.Y.; Qi, G.H.; Farnell, M. Evaluation of pre-slaughter low-current/high-frequency ES on lipid oxidative stability and antioxidant status in thigh muscle of broilers. Int. J. Food Sci. Tech. 2019. [CrossRef]
- Sabow, A.B.; Zulkifli, I.; Goh, Y.M.; Ab Kadir, M.Z.A.; Kaka, U.; Imlan, J.C. Bleeding efficiency, microbiological quality and oxidative stability of meat from goats subjected to slaughter without stunning in comparison with different methods of pre-slaughter ES. PLoS ONE 2016, 11, e0152661. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.C. Effects and Mechanism of Electrical Stunning on Meat Quality of Broilers; Nanjing Agricultural University: Nanjing, China, 2015; Available online: http://cdmd.cnki.com.cn/Article/CDMD-10307-1017044285.htm (accessed on 10 November 2019).
- Fernandez, X.; Leprettre, S.; Dubois, J.P.; Auvergne, A.; Babile, R. The influence of current parameters during the water-bath stunning of overfed geese (anser anser) on blood loss and on fatty liver and meat downgrading. Anim. Res. 2003, 52, 383–397. [Google Scholar] [CrossRef]
- Turcsán, Z.; Varga, L.; Szigeti, J.; Turcsán, J.; Csurák, I.; Szalai, M. Effects of electrical stunning frequency and voltage combinations on the presence of engorged blood vessels in goose liver. Poult. Sci. 2003, 82, 1816–1819. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, L.; Yue, H.Y.; Wu, S.G.; Zhang, H.J.; Ji, F. Effect of electrical stunning current and frequency on meat quality, plasma parameters, and glycolytic potential in broilers. Poult. Sci. 2011, 90, 1823–1830. [Google Scholar] [CrossRef]
- Favre, L.C.; Cristina, S.; María, P.L.F.; María, F.M.; María, P.B. Optimization of β-cyclodextrin-based extraction of antioxidant and anti-browning activities from thyme leaves by response surface methodology. Food Chem. 2018, 265, 86–95. [Google Scholar] [CrossRef]
- Terevinto, A.; Ramos, A.; Castroman, G.; Cabrera, M.C.; Saadoun, A. Oxidative status, in vitro iron-induced lipid oxidation and superoxide dismutase, catalase and glutathione peroxidase activities in rhea meat. Meat Sci. 2010, 84, 706–710. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, H.J.; Yue, H.Y.; Wu, S.G.; Yang, H.M.; Wang, Z.Y.; Qi, G.H. Gas stunning with CO2 affected meat colour, lipid peroxidation, oxidative stress, and gene expression of mitogen-activated protein kinases, glutathione s-transferases, and cu/zn-superoxide dismutase in the skeletal muscles of broilers. J. Anim. Sci. Biotechnol. 2018, 9, 37. [Google Scholar] [CrossRef]
- Karen A., M.; Nicolaou, A. Lipidomics of polyunsaturated-fatty-acid-derived oxygenated metabolites. Biochem. Soc. T. 2011, 39, 1240–1246. [Google Scholar]
- Pizzimenti, S.; Ciamporcero, E.; Daga, M.; Pettazzoni, P.; Arcaro, A.; Cetrangolo, G.; Minelli, R.; Dianzani, C.; Lepore, A.; Gentile, F.; et al. Interaction of aldehydes derived from lipid peroxidation and membrane proteins. Front. Physiol. 2013, 242, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Alía, M.; Horcajo, C.; Bravo, L.; Goya, L. Effect of grape antioxidant dietary fiber on the total antioxidant capacity and the activity of liver antioxidant enzymes in rats. Nutr. Res. 2003, 23, 1251–1267. [Google Scholar] [CrossRef] [Green Version]
- Xiong, Y.L.; Blanchard, S.P.; Ooizumi, T.; Ma, Y. Hydroxyl radical and ferryl-generating systems promote gel network formation of myofibrillar protein. J. Food Sci. 2010, 75, C215–C221. [Google Scholar] [CrossRef]
- Turcsán, Z.; Szigeti, J.; Varga, L.; Farkas, L.; Birkás, E.; Turcsán, J. The effects of electrical and controlled atmosphere stunning methods on meat and liver quality of geese. Poult. Sci. 2001, 80, 1647–1651. [Google Scholar] [CrossRef]
- Fernandez, X.; Lahirigoyen, E.; Bouillier-Oudot, M.; Vitezica, Z.; Auvergne, A. The effects of stunning methods on product qualities in force-fed ducks and geese. 2. fatty liver quality. Animal 2010, 4, 139–146. [Google Scholar] [CrossRef] [Green Version]
- Gregory, N.G.; Wilkins, L.J. Effect of slaughter method on bleeding efficiency in chickens. J. Sci. Food Agr. 2010, 47, 13–20. [Google Scholar] [CrossRef]
- Alvarado, C.Z.; Richards, M.P.; O’Keefe, S.F.; Wang, H. The effect of blood removal on oxidation and shelf life of broiler breast meat. Poult. Sci. 2007, 86, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, S. Reducing lipid peroxidation for improving colour stability of beef and lamb: on-farm considerations. J. Sci. Food Agric. 2012, 92, 719–729. [Google Scholar] [CrossRef] [PubMed]
- Vieira, S.A.; Zhang, G.; Decker, E.A. Biological Implications of Lipid Oxidation Products. J. Am. Oil Chem. Soc. 2017, 94, 339–351. [Google Scholar] [CrossRef]


| Ingredients, % | Content |
|---|---|
| Corn | 61.00 |
| Soybean meal | 25.00 |
| Rice husk | 10.40 |
| Dicalcium phosphate | 1.00 |
| Salt | 0.30 |
| Limestone | 1.20 |
| DL-Methionine | 0.10 |
| Premix 1 | 1.00 |
| Nutrient level (calculated) | |
| 2 ME (MJ/kg) | 10.89 |
| 2 CP, % | 16.60 |
| 2 CF, % | 6.63 |
| Calcium, % | 0.87 |
| Available phosphorus, % | 0.51 |
| Lysine, % | 0.81 |
| Methionine, % | 0.34 |
| Time | Stunning Methods 1 | PooledSEM | p-Value | ||||
|---|---|---|---|---|---|---|---|
| Control | E30V | E60V | E90V | E120V | |||
| 2 d 0 | 6.72 ± 0.63 a y | 6.29 ± 0.44 a | 6.87 ± 0.53 a xy | 8.18 ± 0.62 a y | 10.57 ± 0.34 b y | 0.41 | <0.01 |
| d 2 | 6.98 ± 0.62 y | 7.00 ± 0.86 | 7.60 ± 0.81 y | 6.00 ± 1.07 x | 4.34 ± 0.54 x | 0.38 | 0.06 |
| d 4 | 5.09 ± 0.04 x | 5.41 ± 0.14 | 5.34 ± 0.16 x | 5.36 ± 0.13 x | 5.54 ± 0.12 x | 0.06 | 0.16 |
| Pooled SEM | 0.33 | 1.66 | 1.81 | 0.40 | 0.73 | ||
| p-Value | 0.04 | 0.16 | 0.03 | 0.03 | <0.01 | ||
| Variables | Stunning Methods 2 | PooledSEM | p-Value | ||||
|---|---|---|---|---|---|---|---|
| Control | E30V | E60V | E90V | E120V | |||
| Blood loss 1 (%) | 5.85 ± 0.25 | 5.39 ± 0.09 | 5.41 ± 0.19 | 5.51 ± 0.21 | 5.30 ± 0.22 | 0.09 | 0.36 |
| Lightness (L *) | 27.78 ± 0.44 a | 29.80 ± 0.60 bc | 31.56 ± 0.68 c | 28.08 ± 0.80 ab | 29.95 ± 0.61 bc | 0.35 | < 0.01 |
| Redness (a *) | 9.22 ± 0.37 | 10.35 ± 0.55 | 9.43 ± 0.28 | 10.40 ± 0.69 | 9.82 ± 0.45 | 0.22 | 0.34 |
| Yellowness (b *) | 7.37 ± 0.38 | 8.74 ± 0.35 | 9.20 ± 0.51 | 8.24 ± 0.47 | 9.05 ± 0.64 | 0.23 | 0.07 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Farnell, M.B.; Lu, Q.; Zhou, X.; Farnell, Y.Z.; Yang, H.; Wan, X.; Xu, L.; Wang, Z. Evaluation of the Effects of Pre-Slaughter High-Frequency Electrical Stunning Current Intensities on Lipid Oxidative Stability and Antioxidant Capacity in the Liver of Yangzhou Goose (Anser cygnoides domesticus). Animals 2020, 10, 311. https://doi.org/10.3390/ani10020311
Zhang X, Farnell MB, Lu Q, Zhou X, Farnell YZ, Yang H, Wan X, Xu L, Wang Z. Evaluation of the Effects of Pre-Slaughter High-Frequency Electrical Stunning Current Intensities on Lipid Oxidative Stability and Antioxidant Capacity in the Liver of Yangzhou Goose (Anser cygnoides domesticus). Animals. 2020; 10(2):311. https://doi.org/10.3390/ani10020311
Chicago/Turabian StyleZhang, Xin, Morgan B. Farnell, Qian Lu, Xiaoyi Zhou, Yuhua Z. Farnell, Haiming Yang, Xiaoli Wan, Lei Xu, and Zhiyue Wang. 2020. "Evaluation of the Effects of Pre-Slaughter High-Frequency Electrical Stunning Current Intensities on Lipid Oxidative Stability and Antioxidant Capacity in the Liver of Yangzhou Goose (Anser cygnoides domesticus)" Animals 10, no. 2: 311. https://doi.org/10.3390/ani10020311
APA StyleZhang, X., Farnell, M. B., Lu, Q., Zhou, X., Farnell, Y. Z., Yang, H., Wan, X., Xu, L., & Wang, Z. (2020). Evaluation of the Effects of Pre-Slaughter High-Frequency Electrical Stunning Current Intensities on Lipid Oxidative Stability and Antioxidant Capacity in the Liver of Yangzhou Goose (Anser cygnoides domesticus). Animals, 10(2), 311. https://doi.org/10.3390/ani10020311