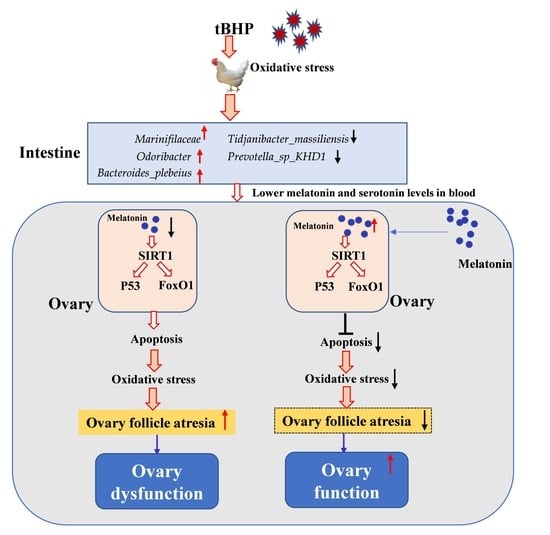
The Effect of Oxidative Stress on the Chicken Ovary: Involvement of Microbiota and Melatonin Interventions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals, Diets and Design
2.2. Sample Collection and Measurements
2.3. Culture of Layer Ovaries In Vitro
2.4. Reproduction Performance and Blood Hormone Assay
2.5. Tissue Antioxidant Capacity
2.6. Histology and Follicle Counts
2.7. TUNEL Assay
2.8. Ovary Function Related mRNA Expression by Real-Time PCR
2.9. Western Blotting Assay
2.10. Gut Microbiota and Short-Chain Fatty Acids (SCFA) Analysis
2.11. Metabolic Profiling Analysis
2.12. Statistical Analysis
3. Results
3.1. Oxidative Stress Reduced Reproductive Performance, Ovary Indices and Serum Hormone Concentration
3.2. tBHP Induced Oxidative Stress Decreased Ovarian Function
3.3. Oxidative Stress Reduced Cecal Short Chain Fatty Acids and Induced Gut Microbiota Dysbiosis
3.4. Oxidative Stress Changed Amino Acid Biosynthesis and Ovarian Serotonin and Melatonin Concentration
3.5. Melatonin Ameliorates tBHP Induced Oxidative Stress Ovarian Dysfunction In Vitro
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shkolnik, K.; Tadmor, A.; Ben-Dor, S.; Nevo, N.; Galiani, D.; Dekel, N. Reactive oxygen species are indispensable in ovulation. Proc. Natl. Acad. Sci. USA 2011, 108, 1462–1467. [Google Scholar] [CrossRef] [Green Version]
- Talukder, S.; Kerrisk, K.L.; Gabai, G.; Celi, P. Role of oxidant–antioxidant balance in reproduction of domestic animals. Anim. Prod. Sci. 2017, 57, 1588–1597. [Google Scholar] [CrossRef]
- Martínez-Álvarez, R.M.; Morales, A.E.; Sanz, A. Antioxidant defenses in fish: Biotic and abiotic factors. Rev. Fish. Biol. Fish. 2005, 15, 75–88. [Google Scholar] [CrossRef]
- Ma, Y.; Zhu, M.; Miao, L.; Zhang, X.; Dong, X.; Zou, X. Mercuric chloride induced ovarian oxidative stress by suppressing Nrf2-Keap1 signal pathway and its downstream genes in laying hens. Biol. Trace Elem. Res. 2018, 185, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Yeh, J.; Bowman, M.J.; Browne, R.W.; Chen, N. Reproductive aging results in a reconfigured ovarian antioxidant defense profile in rats. Fertil. Steril. 2005, 84, 1109–1113. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.Z.; Cheung, H.H.; Zhang, C.; Wu, J.; Chan, W.Y. Melatonin as potential targets for delaying ovarian aging. Curr. Drug Targets 2019, 20, 16–28. [Google Scholar] [CrossRef] [PubMed]
- Celi, P.; Gabai, G. Oxidant/antioxidant balance in animal nutrition and health: The role of protein oxidation. Front. Vet. Sci. 2015, 2, 48. [Google Scholar] [CrossRef] [Green Version]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Yang, Z.Q.; Celi, P.; Yan, L.; Ding, X.M.; Bai, S.P.; Zeng, Q.F.; Mao, X.B.; Feng, B.; Xu, S.Y.; et al. Alteration of the antioxidant capacity and gut microbiota under high levels of molybdenum and green tea polyphenols in laying hens. Antioxidants 2019, 8, 503. [Google Scholar] [CrossRef] [Green Version]
- Paithankar, J.G.; Saini, S.; Dwivedi, S.; Sharma, A.; Chowdhuri, D.K. Heavy metal associated health hazards: An interplay of oxidative stress and signal transduction. Chemosphere 2021, 262, 128350. [Google Scholar] [CrossRef]
- Gupta, R.K.; Miller, K.P.; Babus, J.K.; Flaws, J.A. Methoxychlor inhibits growth and induces atresia of antral follicles through an oxidative stress pathway. Toxicol. Sci. 2006, 93, 382–392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Devine, P.J.; Perreault, S.D.; Luderer, U. Roles of reactive oxygen species and antioxidants in ovarian toxicity. Biol. Reprod. 2012, 86, 27. [Google Scholar] [CrossRef]
- Shen, M.; Lin, F.; Zhang, J.; Tang, Y.; Chen, W.K.; Liu, H. Involvement of the up-regulated FoxO1 expression in follicular granulosa cell apoptosis induced by oxidative stress. J. Biol. Chem. 2012, 287, 25727–25740. [Google Scholar] [CrossRef] [Green Version]
- Shen, M.; Jiang, Y.; Guan, Z.Q.; Cao, Y.; Li, L.C.; Liu, H.L.; Sun, S.C. Protective mechanism of FSH against oxidative damage in mouse ovarian granulosa cells by repressing autophagy. Autophagy 2017, 13, 1364–1385. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zheng, C.; Lanza, I.R.; Nair, K.S.; Raftery, D.; Vitek, O. Interdependence of signal processing and analysis of urine 1H NMR spectra for metabolic profiling. Anal. Chem. 2009, 81, 6080–6088. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Wang, P.; Yin, J.; Jin, S.; Su, W.; Tian, J.; Li, T.; Yao, K. Effects of ornithine α-ketoglutarate on growth performance and gut microbiota in a chronic oxidative stress pig model induced by D-galactose. Food Funct. 2020, 11, 472–482. [Google Scholar] [CrossRef]
- Hu, Y.; Chen, D.; Zheng, P.; Yu, J.; He, J.; Mao, X.; Yu, B. The bidirectional interactions between resveratrol and gut microbiota: An insight into oxidative stress and inflammatory bowel disease therapy. BioMed Res. Int. 2019, 2019, 5403761. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Ma, Y.; Ding, S.; Jiang, H.; Fang, J. Effects of melatonin on intestinal microbiota and oxidative stress in colitis mice. BioMed Res. Int. 2018, 2018, 2607679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Yan, A.; Liu, X.; Ma, Y.; Zhao, F.; Wang, M.; Loor, J.J.; Wang, H. Melatonin ameliorates ochratoxin A induced liver inflammation, oxidative stress and mitophagy in mice involving in intestinal microbiota and restoring the intestinal barrier function. J. Hazard. Mater. 2021, 407, 124489. [Google Scholar] [CrossRef] [PubMed]
- Tamura, H.; Nakamura, Y.; Korkmaz, A.; Manchester, L.C.; Tan, D.X.; Sugino, N.; Reiter, R.J. Melatonin and the ovary: Physiological and pathophysiological implications. Fertil. Steril. 2009, 92, 328–343. [Google Scholar] [CrossRef]
- Galano, A.; Tan, D.X.; Reiter, R.J. Melatonin as a natural ally against oxidative stress: A physicochemical examination. J. Pineal Res. 2011, 51, 1–16. [Google Scholar] [CrossRef]
- Hao, E.Y.; Chen, H.; Wang, D.H.; Huang, C.X.; Tong, Y.G.; Chen, Y.F.; Zhou, R.Y.; Huang, R.L. Melatonin regulates the ovarian function and enhances follicle growth in aging laying hens via activating the mammalian target of rapamycin pathway. Poult. Sci. 2020, 99, 2185–2195. [Google Scholar] [CrossRef]
- Jia, Y.; Yang, M.; Zhu, K.; Wang, L.; Song, Y.; Wang, J.; Qin, W.; Xu, Z.; Chen, Y.; Liu, G. Melatonin implantation improved the egg-laying rate and quality in hens past their peak egg-laying age. Sci. Rep. 2016, 6, 39799. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bordone, L.; Guarente, L. Calorie restriction, SIRT1 and metabolism: Understanding longevity. Nat. Rev. Mol. Cell Biol. 2005, 6, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Alam, F.; Syed, H.; Amjad, S.; Baig, M.; Khan, T.A.; Rehman, R. Interplay between oxidative stress, SIRT1, reproductive and metabolism functions. Curr. Res. Physiol. 2021, 4, 119–124. [Google Scholar] [CrossRef]
- Singh, V.; Ubaid, S. Role of silent information regulator 1 (SRIT1) in regulation oxidative stress and inflammation. Inflammation 2020, 43, 1589–1598. [Google Scholar] [CrossRef]
- Tao, X.; Zhang, X.; Ge, S.Q.; Zhang, E.H.; Zhang, B. Expression of SIRT1 in the ovaries of rats with polycystic ovary syndrome before and after therapeutic intervention with exenatide. Int. J. Clin. Exp. Pathol. 2015, 8, 8276–8283. [Google Scholar]
- Ding, M.; Lei, J.; Han, H.; Li, W.; Qu, Y.; Fu, E.; Fu, F.; Wang, X. SIRT1 protects against myocardial ischemia-reperfusion injury via activating eNOS in diabetic rats. Cardiovasc. Diabetol. 2015, 14, 143. [Google Scholar] [CrossRef] [Green Version]
- Salminen, A.; Kaarniranta, K.; Kauppinen, A. Crosstalk between oxidative stress and SIRT1: Impact on the aging process. Int. J. Mol. Sci. 2013, 14, 3834–3859. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, Y.L.; Jiang, X.T.; Ma, F.F.; Han, J.; Tang, X.L. Resveratrol prevents osteoporosis by upregulating FoxO1 transcriptional activity. Int. J. Mol. Med. 2018, 41, 202–212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, Z.; Sun, W.; Liu, Y.; Li, Z.; Han, D.; Cheng, X.; Yan, J.; Yang, X. Dynamics of serum phosphorus, calcium, and hormones during egg laying cycle in Hy-Line Brown laying hens. Poult. Sci. 2019, 98, 2193–2200. [Google Scholar] [CrossRef] [PubMed]
- Luan, Y.; Edmonds, W.E.; Woodruff, T.K.; Kim, S.Y. Inhibitors of apoptosis protect the ovarian reserve from cyclophosphamide. J. Endocrinol. 2019, 240, 243–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, S.; Wu, X.; Dong, Y.; Li, Z.; Lin, Y.; Che, L.; Li, J.; Feng, B.; Fang, Z.; Zhuo, Y.; et al. Glucose activates the primordial follicle through the AMPK/mTOR signaling pathway. Clin. Transl. Med. 2020, 10, e122. [Google Scholar] [CrossRef]
- Johnson, A.L. Ovarian follicle selection and granulosa cell differentiation. Poult. Sci. 2015, 94, 2193–2200. [Google Scholar] [CrossRef]
- Myers, M.; Britt, K.L.; Wreford, N.G.M.; Ebling, F.J.P.; Kerr, J.B. Methods for quantifying follicular number within the mouse ovary. Reproduction 2004, 127, 569–580. [Google Scholar] [CrossRef]
- Zhuo, Y.; Hua, L.; Feng, B.; Jiang, X.M.; Jiang, L.; Jiang, D.D.; Huang, X.H.; Zhu, Y.G.; Li, Z.; Yan, L.J.; et al. Fibroblast growth factor 21 coordinates adiponectin to mediate the beneficial effect of low-protein diet on primordial follicle reserve. EbioMedicine 2019, 41, 623–635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Wan, C.; Zhao, S.; Yang, Z.; Celi, P.; Ding, X.; Bai, S.; Zeng, Q.; Mao, X.; Xu, S.; et al. Differential analysis of gut microbiota and the effect of dietary Enterococcus feacium supplementation in broiler breeders with high or low laying performance. Poult. Sci. 2021, 100, 1109–1119. [Google Scholar] [CrossRef]
- Zhuo, Y.; Feng, B.; Xuan, Y.; Che, L.; Feng, Z.; Lin, Y.; Xu, S.; Li, J.; Wu, D. Inclusion of purified dietary fiber during gestation improved the reproductive performance of sows. J. Anim. Sci. Biotechnol. 2020, 11, 47. [Google Scholar] [CrossRef] [PubMed]
- Van den Hurk, R.; Zhao, J. Formation of mammalian oocytes and their growth, differentiation and maturation within ovarian follicles. Theriogenology 2005, 63, 1717–1751. [Google Scholar] [CrossRef] [PubMed]
- Findlay, J.K.; Hutt, K.J.; Hickey, M.; Anderson, R.A. How is the number of primordial follicles in the ovarian reserve established? Biol. Reprod. 2015, 93, 111–117. [Google Scholar] [CrossRef]
- Agarwal, A.; Aponte-Mellado, A.; Premkumar, B.J.; Shaman, A.; Gupta, S. The effects of oxidative stress on female reproduction: A review. Reprod. Biol. Endocrinol. 2012, 10, 49. [Google Scholar] [CrossRef] [Green Version]
- Cao, Y.; Shen, M.; Jiang, Y.; Sun, S.C.; Liu, H. Melatonin reduces oxidative damage in mouse granulosa cells via restraining JNK-dependent autophagy. Reproduction 2018, 155, 307–319. [Google Scholar] [CrossRef]
- Fu, Y.; He, C.J.; Ji, P.Y.; Zhuo, Z.Y.; Tian, X.Z.; Wang, F.; Tan, D.X.; Liu, G.S. Effects of melatonin on the proliferation and apoptosis of sheep granulosa cells under thermal stress. Int. J. Mol. Sci. 2014, 15, 21090–21104. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Ji, Y.; Yin, C.; Deng, M.; Tang, T.; Deng, B.; Ren, W.; Deng, J.; Yin, Y.; Tan, C. Differential analysis of gut microbiota correlated with oxidative stress in sows with high or low litter performance during lactation. Front. Microbiol. 2018, 9, 1665. [Google Scholar] [CrossRef]
- He, Y.; Deng, H.; Jiang, Z.; Li, Q.; Shi, M.; Chen, H.; Han, Z. Effects of melatonin on follicular atresia and granulosa cell apoptosis in the porcine. Mol. Reprod. Dev. 2016, 83, 692–700. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhao, X.; Jiang, Y.; Zhao, W.; Guo, T.; Cao, Y.; Teng, J.; Hao, X.; Zhao, J.; Yang, Z. Antioxidant status and gut microbiota change in an aging mouse model as influenced by exopolysaccharide produced by Lactobacillus plantarum YW11 isolated from Tibetan kefir. J. Dairy Sci. 2017, 100, 6025–6041. [Google Scholar] [CrossRef]
- Yuan, Z.H.; Wang, J.P.; Zhang, K.Y.; Ding, X.M.; Bai, S.P.; Zeng, Q.F.; Xuan, Y.; Su, Z.W. Effect of vanadium and tea polyphenols on intestinal morphology, microflora and short-chain fatty acid profile of laying hens. Poult. Sci. 2017, 174, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Abou-Seif, M.A.; Youssef, A.A. Oxidative stress and male IGF-1, gonadotropin and related hormones in diabetic patients. Clin. Chem. Lab. Med. 2001, 39, 618–623. [Google Scholar] [CrossRef]
- Tsai-Turton, M.; Luderer, U. Opposing effects of glutathione depletion and follicle-stimulating hormone on reactive oxygen species and apoptosis in cultured prevulatory rat follicles. Endocrinology 2006, 147, 1224–1236. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, K.; Sato, F.E.; Kasahara, E.; Jikumaru, M.; Hiramoto, K.; Tabata, H.; Katsuragi, M.; Odo, S.; Utsumi, K.; Indoue, M. Effect of oxidative stress during repeated ovulation on the structure and functions of the ovary, oocytes, and their mitochondria. Free Radic. Biol. Med. 2010, 49, 674–681. [Google Scholar] [CrossRef]
- Ley, R.E.; Turnbaugh, P.J.; Klein, S.; Gordon, J.I. Microbial ecology: Human gut microbes associated with obesity. Nature 2006, 444, 1022–1023. [Google Scholar] [CrossRef]
- Brannian, J.D.; Hansen, K.A. Leptin and ovarian folliculogenesis: Implications for ovulation induction and ART outcomes. Semin. Reprod. Med. 2002, 20, 103–112. [Google Scholar] [CrossRef]
- Yu, H.; Kuang, M.; Wang, Y.; Rodeni, S.; Wei, Q.; Wang, W.; Mao, D. Sodium arsenite injection induces ovarian oxidative stress and affects steroidogenesis in rats. Biol. Trace Elem. Res. 2019, 189, 186–193. [Google Scholar] [CrossRef]
- Higashi, Y.; Sukhanov, S.; Anwar, A.; Shai, S.Y.; Delafontaine, P. IGF-1, oxidative stress and atheroprotection. Trends Endocrinol. Metab. 2010, 21, 245–254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, X.; Jiang, X.; Li, J.; Bai, Y.; Li, Z.; Wei, P.; Sun, S.; Liang, Y.; Han, S.; Li, X.; et al. Insulin-like growth factor-1 attenuates oxidative stress-induced hepatocyte premature senescence in liver fibrogenesis via regulating nuclear p53-progerin interaction. Cell Death Dis. 2019, 10, 451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, M.; Zhang, X.; Miao, Y.J.; Cao, J.X.; Wu, Z.F.; Weng, P.F. The modulatory effect of (−)-epigallocatechin 3-O-(3-O-methyl) gallate (EGCG3”Me) on intestinal microbiota of high fat diet-induced obesity mice model. Food Res. Int. 2017, 92, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Dam, B.; Misra, A.; Banerjee, S. Role of gut microbiota in combating oxidative stress. In Oxidative Stress in Microbial Diseases; Chakraborti, S., Chakraborti, T., Chattopadhyay, D., Shaha, C., Eds.; Springer: Singapore, 2019; pp. 43–82. [Google Scholar] [CrossRef]
- Pereira, T.M.C.; Pimenta, F.S.; Porto, M.L.; Baldo, M.P.; Campagnaro, B.P.; Gava, A.L.; Meyrelles, S.S.; Vasquez, E.C. Coadjuvants in the diabetic complications: Nutraceuticals and drugs with pleiotropic effects. Int. J. Mol. Sci. 2016, 17, 1273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, L.; Xiao, X.; Zhang, Q.; Zheng, J.; Deng, M.Q. Maternal genistein intake mitigates the deleterious effects of high-fat diet on glucose and lipid metabolism and modulates gut microbiota in adult life of male mice. Front. Physiol. 2019, 10, 985. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corrigan, A.; de Leeuw, M.; Penaud-Frezet, S.; Dimova, D.; Murphy, R.A. Phylogenetic and functional alterations in bacterial community compositions in broiler ceca as a result of mannan oligosaccharide supplementation. Appl. Environ. Microbiol. 2015, 81, 3460–3470. [Google Scholar] [CrossRef] [Green Version]
- Tong, L.C.; Wang, Y.; Wang, Z.B.; Liu, W.Y.; Sun, S.; Li, L.; Su, D.F.; Zhang, L.C. Propionate ameliorates dextran sodium sulfate-induced colitis by improving intestinal barrier function and reducing inflammation and oxidative stress. Front. Pharmacol. 2016, 7, 253. [Google Scholar] [CrossRef]
- Wang, Y.Z.; Zeng, S.M. Melatonin promotes ubiquitination of phosphorylated pro-apoptotic protein Bcl-2-interacting mediator of cell death-extra long (BimEL) in porcine granulosa cells. Int. J. Mol. Sci. 2018, 19, 3431. [Google Scholar] [CrossRef] [Green Version]
- Allison, A.; Julien, P.; Harry, S. Gut microbiota regulation of tryptophan metabolism in health and disease. Cell Host Microbe 2018, 23, 716–724. [Google Scholar]
- Lv, M.; Yu, B.; Mao, X.B.; Zheng, P.; He, J.; Chen, D.W. Responses of growth performance and tryptophan metabolism to oxidative stress induced by diquat in weaned pigs. Animal 2012, 6, 928–934. [Google Scholar] [CrossRef] [PubMed]
- O’Mahony, S.M.; Clarke, G.; Borre, Y.E.; Dinan, T.G.; Cryan, J.F. Serotonin, tryptophan metabolism and the brain-gut-microbiome axis. Behav. Brain Res. 2015, 15, 32–48. [Google Scholar] [CrossRef]
- Hegde, K.R.; Varma, S.D. Prevention of oxidative stress to the retina by pyruvate. A preliminary report. Ophthalmologica 2008, 222, 194–198. [Google Scholar] [CrossRef] [PubMed]
- Varma, S.D.; Hegde, K.; Henein, M. Oxidative damage to mouse lens in culture. Protective effect of pyruvate. Biochim. Biophys. Acta 2003, 1621, 246–252. [Google Scholar] [CrossRef]
- Tavsan, Z.; Kayali, H.A. Influence of the oxidative stress induced by the organophosphate pesticide bromopropylate on the mitochondrial respiratory chain in Trichoderma harzianum. Process. Biochem. 2014, 49, 745–750. [Google Scholar] [CrossRef]
- Hinder, L.M.; Vivekanandan-Giri, A.; Mclean, L.L.; Pennathur, S.; Feldman, E.L. Decreased glycolytic and tricarboxylic acid cycle intermediates coincide with peripheral nervous system oxidative stress in a murine model of type 2 diabetes. J. Endocrinol. 2013, 216, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Ding, M.; Feng, N.; Tang, D.; Feng, J.; Li, Z.; Jia, M.; Liu, Z.; Gu, X.; Wang, Y.; Fu, F.; et al. Melatonin prevents Drp1-mediated mitochondrial fission in diabetic hearts through SIRT1-PGC1α pathway. J. Pineal Res. 2018, 65, e12491. [Google Scholar] [CrossRef] [Green Version]
- Hsu, Y.J.; Hsu, S.C.; Hsu, C.P.; Chen, Y.H.; Chang, Y.L.; Sadoshima, J.C.; Huang, S.M.; Tsai, C.S.; Lin, C.Y. Sirtuin 1 protects the aging heart from contractile dysfundcion mediated through the inhibition of endoplasmic reticulum stress-mediated apoptosis in cardiac-specific Sirtuin 1 knockout mouse model. Int. J. Cardiol. 2017, 228, 543–552. [Google Scholar] [CrossRef]
- Shen, M.; Cao, Y.; Jiang, Y.; Wei, Y.H.; Liu, H.L. Melatonin protects mouse granulosa cells against oxidative damage by inhibiting FOXO1-mediated autophagy: Implication of an antioxidation-independent mechanism. Redox Biol. 2018, 18, 138–157. [Google Scholar] [CrossRef] [PubMed]
- Hao, E.Y.; Wang, D.H.; Chang, L.Y.; Huang, C.X.; Chen, H.; Yue, Q.X.; Zhou, R.Y.; Huang, R.L. Melatonin regulates chicken granulosa cell proliferation and apoptosis by activating the mTOR signaling pathway via its receptors. Poult. Sci. 2020, 99, 6147–6162. [Google Scholar] [CrossRef] [PubMed]









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Jia, R.; Gong, H.; Celi, P.; Zhuo, Y.; Ding, X.; Bai, S.; Zeng, Q.; Yin, H.; Xu, S.; et al. The Effect of Oxidative Stress on the Chicken Ovary: Involvement of Microbiota and Melatonin Interventions. Antioxidants 2021, 10, 1422. https://doi.org/10.3390/antiox10091422
Wang J, Jia R, Gong H, Celi P, Zhuo Y, Ding X, Bai S, Zeng Q, Yin H, Xu S, et al. The Effect of Oxidative Stress on the Chicken Ovary: Involvement of Microbiota and Melatonin Interventions. Antioxidants. 2021; 10(9):1422. https://doi.org/10.3390/antiox10091422
Chicago/Turabian StyleWang, Jianping, Ru Jia, Haojie Gong, Pietro Celi, Yong Zhuo, Xuemei Ding, Shiping Bai, Qiufeng Zeng, Huadong Yin, Shengyu Xu, and et al. 2021. "The Effect of Oxidative Stress on the Chicken Ovary: Involvement of Microbiota and Melatonin Interventions" Antioxidants 10, no. 9: 1422. https://doi.org/10.3390/antiox10091422
APA StyleWang, J., Jia, R., Gong, H., Celi, P., Zhuo, Y., Ding, X., Bai, S., Zeng, Q., Yin, H., Xu, S., Liu, J., Mao, X., & Zhang, K. (2021). The Effect of Oxidative Stress on the Chicken Ovary: Involvement of Microbiota and Melatonin Interventions. Antioxidants, 10(9), 1422. https://doi.org/10.3390/antiox10091422