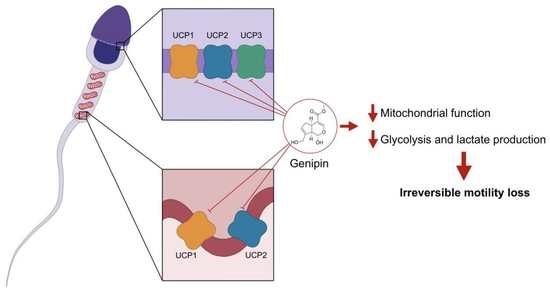
Inhibition of Mitochondrial Uncoupling Proteins Arrests Human Spermatozoa Motility without Compromising Viability
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Chemicals
2.2. Patient Recruitment and Seminal Sample Preparation
2.3. Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR)
2.4. Western Blot
2.5. Immunofluorescence Staining
2.6. Experimental Groups
2.7. Spermatozoa Viability and Motility Analysis
2.8. Genipin Effects on Spermatozoa Motility
2.9. JC-1 Assay for Mitochondrial Membrane Potential Analysis
2.10. Detection of Intracellular Reactive Oxygen Species (ROS)
2.11. Proton Nuclear Magnetic Resonance (1H-NMR)
2.12. Ferric-Reducing Antioxidant Power Assay (FRAP)
2.13. Statistical Analysis
3. Results
3.1. UCP1-6 mRNAs and UCP1, UCP2, and UCP3 Proteins Are Present in Human Spermatozoa
3.2. UCP1, UCP2, and UCP3 Are Mainly Located at the Equatorial and Acrosome Region of the Head of Human Spermatozoa
3.3. UCPs Inhibition Induces Motility Loss without Compromising Viability of Human Spermatozoa
3.4. Human Spermatozoa Motility Loss Due to UCPs Inhibition Is Not Recovered by Incubation with Albumin or Theophylline
3.5. UCP3 Relative Expression in Spermatozoa Does Not Differ between Normozoospermic and Asthenozoospermic Men
3.6. UCPs Inhibition Decreases Mitochondrial Membrane Potential and Lactate Production in Human Spermatozoa
3.7. UCPs Inhibition Does Not Increase Total ROS Production by Human Spermatozoa
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Monteiro, B.S.; Freire-Brito, L.; Carrageta, D.F.; Oliveira, P.F.; Alves, M.G. Mitochondrial Uncoupling Proteins (UCPs) as Key Modulators of ROS Homeostasis: A Crosstalk between Diabesity and Male Infertility? Antioxidants 2021, 10, 1746. [Google Scholar] [CrossRef] [PubMed]
- Hirschenson, J.; Melgar-Bermudez, E.; Mailloux, R.J. The Uncoupling Proteins: A Systematic Review on the Mechanism Used in the Prevention of Oxidative Stress. Antioxidants 2022, 11, 322. [Google Scholar] [CrossRef] [PubMed]
- Ardalan, A.; Smith, M.D.; Jelokhani-Niaraki, M. Uncoupling Proteins and Regulated Proton Leak in Mitochondria. Int. J. Mol. Sci. 2022, 23, 1528. [Google Scholar] [CrossRef] [PubMed]
- Nicholls, D.G. Mitochondrial proton leaks and uncoupling proteins. Biochim. Biophys. Acta Bioenerg. 2021, 1862, 148428. [Google Scholar] [CrossRef] [PubMed]
- Levine, H.; Jorgensen, N.; Martino-Andrade, A.; Mendiola, J.; Weksler-Derri, D.; Jolles, M.; Pinotti, R.; Swan, S.H. Temporal trends in sperm count: A systematic review and meta-regression analysis of samples collected globally in the 20th and 21st centuries. Hum. Reprod. Update 2022, 1–20. [Google Scholar] [CrossRef]
- Agarwal, A.; Parekh, N.; Panner Selvam, M.K.; Henkel, R.; Shah, R.; Homa, S.T.; Ramasamy, R.; Ko, E.; Tremellen, K.; Esteves, S.; et al. Male Oxidative Stress Infertility (MOSI): Proposed Terminology and Clinical Practice Guidelines for Management of Idiopathic Male Infertility. World J. Mens. Health 2019, 37, 296–312. [Google Scholar] [CrossRef]
- Martin-Hidalgo, D.; Bragado, M.J.; Batista, A.R.; Oliveira, P.F.; Alves, M.G. Antioxidants and Male Fertility: From Molecular Studies to Clinical Evidence. Antioxidants 2019, 8, 89. [Google Scholar] [CrossRef]
- Forrester, S.J.; Kikuchi, D.S.; Hernandes, M.S.; Xu, Q.; Griendling, K.K. Reactive Oxygen Species in Metabolic and Inflammatory Signaling. Circ. Res. 2018, 122, 877–902. [Google Scholar] [CrossRef]
- Montano, L.; Pironti, C.; Pinto, G.; Ricciardi, M.; Buono, A.; Brogna, C.; Venier, M.; Piscopo, M.; Amoresano, A.; Motta, O. Polychlorinated Biphenyls (PCBs) in the Environment: Occupational and Exposure Events, Effects on Human Health and Fertility. Toxics 2022, 10, 365. [Google Scholar] [CrossRef]
- Moreira, S.; Silva, R.; Carrageta, D.F.; Alves, M.G.; Seco-Rovira, V.; Oliveira, P.F.; Pereira, M.D. Carbamate Pesticides: Shedding Light on Their Impact on the Male Reproductive System. Int. J. Mol. Sci. 2022, 23, 8206. [Google Scholar] [CrossRef]
- Athayde, K.S.; Cocuzza, M.; Agarwal, A.; Krajcir, N.; Lucon, A.M.; Srougi, M.; Hallak, J. Development of normal reference values for seminal reactive oxygen species and their correlation with leukocytes and semen parameters in a fertile population. J. Androl. 2007, 28, 613–620. [Google Scholar] [CrossRef]
- Hosseinzadeh Colagar, A.; Karimi, F.; Jorsaraei, S.G. Correlation of sperm parameters with semen lipid peroxidation and total antioxidants levels in astheno- and oligoasheno- teratospermic men. Iran. Red Crescent Med. J. 2013, 15, 780–785. [Google Scholar] [CrossRef]
- Kurkowska, W.; Bogacz, A.; Janiszewska, M.; Gabrys, E.; Tiszler, M.; Bellanti, F.; Kasperczyk, S.; Machon-Grecka, A.; Dobrakowski, M.; Kasperczyk, A. Oxidative Stress is Associated with Reduced Sperm Motility in Normal Semen. Am. J. Mens Health 2020, 14, 1557988320939731. [Google Scholar] [CrossRef]
- Aitken, R.J.; De Iuliis, G.N.; Finnie, J.M.; Hedges, A.; McLachlan, R.I. Analysis of the relationships between oxidative stress, DNA damage and sperm vitality in a patient population: Development of diagnostic criteria. Hum. Reprod. 2010, 25, 2415–2426. [Google Scholar] [CrossRef]
- Tarozzi, N.; Bizzaro, D.; Flamigni, C.; Borini, A. Clinical relevance of sperm DNA damage in assisted reproduction. Reprod. Biomed. Online 2007, 14, 746–757. [Google Scholar] [CrossRef]
- Cissen, M.; Wely, M.V.; Scholten, I.; Mansell, S.; Bruin, J.P.; Mol, B.W.; Braat, D.; Repping, S.; Hamer, G. Measuring Sperm DNA Fragmentation and Clinical Outcomes of Medically Assisted Reproduction: A Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0165125. [Google Scholar] [CrossRef]
- McQueen, D.B.; Zhang, J.; Robins, J.C. Sperm DNA fragmentation and recurrent pregnancy loss: A systematic review and meta-analysis. Fertil. Steril. 2019, 112, 54–60.e53. [Google Scholar] [CrossRef]
- Kreiter, J.; Rupprecht, A.; Zimmermann, L.; Moschinger, M.; Rokitskaya, T.I.; Antonenko, Y.N.; Gille, L.; Fedorova, M.; Pohl, E.E. Molecular Mechanisms Responsible for Pharmacological Effects of Genipin on Mitochondrial Proteins. Biophys. J. 2019, 117, 1845–1857. [Google Scholar] [CrossRef]
- WHO. Laboratory Manual for the Examination and Processing of Human Semen, 6th ed.; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Bernardino, R.L.; Costa, A.R.; Martins, A.D.; Silva, J.; Barros, A.; Sousa, M.; Sa, R.; Alves, M.G.; Oliveira, P.F. Estradiol modulates Na+-dependent HCO3− transporters altering intracellular pH and ion transport in human Sertoli cells: A role on male fertility? Biol. Cell 2016, 108, 179–188. [Google Scholar] [CrossRef]
- Pereira, S.C.; Martins, A.C.; Moreira, B.P.; Bernardino, R.L.; Barros, A.; Monteiro, M.P.; Oliveira, P.F.; Alves, M.G. Obesity-related genes are expressed in human Sertoli cells and modulated by energy homeostasis regulating hormones. J. Cell Physiol. 2021, 236, 5265–5277. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, J.; Li, B.; Li, D.; Meng, Z.; Sun, S.K. Biocompatible therapeutic albumin/genipin bioglue for postoperative wound adhesion and residual tumor ablation. Biomaterials 2021, 279, 121179. [Google Scholar] [CrossRef] [PubMed]
- Carrageta, D.F.; Guerra-Carvalho, B.; Sousa, M.; Barros, A.; Oliveira, P.F.; Monteiro, M.P.; Alves, M.G. Mitochondrial Activation and Reactive Oxygen-Species Overproduction during Sperm Capacitation are Independent of Glucose Stimuli. Antioxidants 2020, 9, 750. [Google Scholar] [CrossRef] [PubMed]
- Carrageta, D.F.; Freire-Brito, L.; Oliveira, P.F.; Alves, M.G. Evaluation of Human Spermatozoa Mitochondrial Membrane Potential Using the JC-1 Dye. Curr. Protoc. 2022, 2, e531. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Ledesma, A.; de Lacoba, M.G.; Rial, E. The mitochondrial uncoupling proteins. Genome Biol. 2002, 3, REVIEWS3015. [Google Scholar] [CrossRef]
- Wang, X.; Qian, H.; Huang, X.; Li, J.; Zhang, J.; Zhu, N.; Chen, H.; Zhu, C.; Wang, J.; Zhang, P.; et al. UCP2 Mitigates the Loss of Human Spermatozoa Motility by Promoting mROS Elimination. Cell Physiol. Biochem. 2018, 50, 952–962. [Google Scholar] [CrossRef]
- Karlsson, M.; Zhang, C.; Mear, L.; Zhong, W.; Digre, A.; Katona, B.; Sjostedt, E.; Butler, L.; Odeberg, J.; Dusart, P.; et al. A single-cell type transcriptomics map of human tissues. Sci. Adv. 2021, 7, eabh2169. [Google Scholar] [CrossRef]
- Du Plessis, S.S.; Agarwal, A.; Halabi, J.; Tvrda, E. Contemporary evidence on the physiological role of reactive oxygen species in human sperm function. J. Assist. Reprod. Genet. 2015, 32, 509–520. [Google Scholar] [CrossRef]
- Jones, R.; James, P.S.; Oxley, D.; Coadwell, J.; Suzuki-Toyota, F.; Howes, E.A. The equatorial subsegment in mammalian spermatozoa is enriched in tyrosine phosphorylated proteins. Biol. Reprod. 2008, 79, 421–431. [Google Scholar] [CrossRef]
- Anifandis, G.; Messini, C.; Dafopoulos, K.; Sotiriou, S.; Messinis, I. Molecular and cellular mechanisms of sperm-oocyte interactions opinions relative to in vitro fertilization (IVF). Int. J. Mol. Sci. 2014, 15, 12972–12997. [Google Scholar] [CrossRef] [Green Version]
- Wolkowicz, M.J.; Digilio, L.; Klotz, K.; Shetty, J.; Flickinger, C.J.; Herr, J.C. Equatorial segment protein (ESP) is a human alloantigen involved in sperm-egg binding and fusion. J. Androl. 2008, 29, 272–282. [Google Scholar] [CrossRef]
- Klinovska, K.; Sebkova, N.; Dvorakova-Hortova, K. Sperm-egg fusion: A molecular enigma of mammalian reproduction. Int. J. Mol. Sci. 2014, 15, 10652–10668. [Google Scholar] [CrossRef]
- Ren, M.; Xu, Y.; Phoon, C.K.L.; Erdjument-Bromage, H.; Neubert, T.A.; Rajan, S.; Hussain, M.M.; Schlame, M. Condensed Mitochondria Assemble Into the Acrosomal Matrix During Spermiogenesis. Front. Cell Dev. Biol. 2022, 10, 867175. [Google Scholar] [CrossRef]
- Ichikawa, T.; Oeda, T.; Ohmori, H.; Schill, W.B. Reactive oxygen species influence the acrosome reaction but not acrosin activity in human spermatozoa. Int. J. Androl. 1999, 22, 37–42. [Google Scholar] [CrossRef]
- Aitken, R.J.; Paterson, M.; Fisher, H.; Buckingham, D.W.; van Duin, M. Redox regulation of tyrosine phosphorylation in human spermatozoa and its role in the control of human sperm function. J. Cell Sci. 1995, 108 Pt 5, 2017–2025. [Google Scholar] [CrossRef]
- Griveau, J.F.; Renard, P.; Le Lannou, D. Superoxide anion production by human spermatozoa as a part of the ionophore-induced acrosome reaction process. Int. J. Androl. 1995, 18, 67–74. [Google Scholar] [CrossRef]
- Zhang, C.Y.; Parton, L.E.; Ye, C.P.; Krauss, S.; Shen, R.; Lin, C.T.; Porco, J.A., Jr.; Lowell, B.B. Genipin inhibits UCP2-mediated proton leak and acutely reverses obesity- and high glucose-induced beta cell dysfunction in isolated pancreatic islets. Cell Metab. 2006, 3, 417–427. [Google Scholar] [CrossRef]
- Carrageta, D.F.; Dias, T.R.; Alves, M.G.; Oliveira, P.F.; Monteiro, M.P.; Silva, B.M. Anti-obesity potential of natural methylxanthines. J. Funct. Foods 2018, 43, 84–94. [Google Scholar] [CrossRef]
- Buffone, M.G.; Wertheimer, E.V.; Visconti, P.E.; Krapf, D. Central role of soluble adenylyl cyclase and cAMP in sperm physiology. Biochim. Biophys. Acta 2014, 1842, 2610–2620. [Google Scholar] [CrossRef]
- Guerra-Carvalho, B.; Carrageta, D.F.; Crisostomo, L.; Carvalho, R.A.; Alves, M.G.; Oliveira, P.F. Molecular mechanisms regulating spermatogenesis in vertebrates: Environmental, metabolic, and epigenetic factor effects. Anim. Reprod. Sci. 2022, 246, 106896. [Google Scholar] [CrossRef]
- Jo, M.J.; Jeong, S.; Yun, H.K.; Kim, D.Y.; Kim, B.R.; Kim, J.L.; Na, Y.J.; Park, S.H.; Jeong, Y.A.; Kim, B.G.; et al. Genipin induces mitochondrial dysfunction and apoptosis via downregulation of Stat3/mcl-1 pathway in gastric cancer. BMC Cancer 2019, 19, 739. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, T.B.; Jia, D.H.; Sun, W.Q.; Wang, C.L.; Gu, A.Z.; Yang, X.M. Genipin inhibits the growth of human bladder cancer cells via inactivation of PI3K/Akt signaling. Oncol. Lett. 2018, 15, 2619–2624. [Google Scholar] [CrossRef] [PubMed]
- Hereng, T.H.; Elgstoen, K.B.; Cederkvist, F.H.; Eide, L.; Jahnsen, T.; Skalhegg, B.S.; Rosendal, K.R. Exogenous pyruvate accelerates glycolysis and promotes capacitation in human spermatozoa. Hum. Reprod. 2011, 26, 3249–3263. [Google Scholar] [CrossRef] [PubMed]
- Vozza, A.; Parisi, G.; De Leonardis, F.; Lasorsa, F.M.; Castegna, A.; Amorese, D.; Marmo, R.; Calcagnile, V.M.; Palmieri, L.; Ricquier, D.; et al. UCP2 transports C4 metabolites out of mitochondria, regulating glucose and glutamine oxidation. Proc. Natl. Acad. Sci. USA 2014, 111, 960–965. [Google Scholar] [CrossRef] [PubMed]
- Kutsche, H.S.; Schreckenberg, R.; Weber, M.; Hirschhauser, C.; Rohrbach, S.; Li, L.; Niemann, B.; Schulz, R.; Schluter, K.D. Alterations in Glucose Metabolism During the Transition to Heart Failure: The Contribution of UCP-2. Cells 2020, 9, 552. [Google Scholar] [CrossRef]
- Anderson, E.J.; Yamazaki, H.; Neufer, P.D. Induction of endogenous uncoupling protein 3 suppresses mitochondrial oxidant emission during fatty acid-supported respiration. J. Biol. Chem. 2007, 282, 31257–31266. [Google Scholar] [CrossRef]
- Teshima, Y.; Akao, M.; Jones, S.P.; Marban, E. Uncoupling protein-2 overexpression inhibits mitochondrial death pathway in cardiomyocytes. Circ. Res. 2003, 93, 192–200. [Google Scholar] [CrossRef]
- Lee, K.U.; Lee, I.K.; Han, J.; Song, D.K.; Kim, Y.M.; Song, H.S.; Kim, H.S.; Lee, W.J.; Koh, E.H.; Song, K.H.; et al. Effects of recombinant adenovirus-mediated uncoupling protein 2 overexpression on endothelial function and apoptosis. Circ. Res. 2005, 96, 1200–1207. [Google Scholar] [CrossRef]
- Brand, M.D.; Pamplona, R.; Portero-Otin, M.; Requena, J.R.; Roebuck, S.J.; Buckingham, J.A.; Clapham, J.C.; Cadenas, S. Oxidative damage and phospholipid fatty acyl composition in skeletal muscle mitochondria from mice underexpressing or overexpressing uncoupling protein 3. Biochem. J. 2002, 368, 597–603. [Google Scholar] [CrossRef]
- Horimoto, M.; Fulop, P.; Derdak, Z.; Wands, J.R.; Baffy, G. Uncoupling protein-2 deficiency promotes oxidant stress and delays liver regeneration in mice. Hepatology 2004, 39, 386–392. [Google Scholar] [CrossRef]
- Vidal-Puig, A.J.; Grujic, D.; Zhang, C.Y.; Hagen, T.; Boss, O.; Ido, Y.; Szczepanik, A.; Wade, J.; Mootha, V.; Cortright, R.; et al. Energy metabolism in uncoupling protein 3 gene knockout mice. J. Biol. Chem. 2000, 275, 16258–16266. [Google Scholar] [CrossRef]
- Chianese, R.; Pierantoni, R. Mitochondrial Reactive Oxygen Species (ROS) Production Alters Sperm Quality. Antioxidants 2021, 10, 92. [Google Scholar] [CrossRef]
- Cho, Y.S. Genipin, an Inhibitor of UCP2 as a Promising New Anticancer Agent: A Review of the Literature. Int. J. Mol. Sci. 2022, 23, 5637. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, R.; Ye, M.; Zhang, L. Genipin protects against H2O2-induced oxidative damage in retinal pigment epithelial cells by promoting Nrf2 signaling. Int. J. Mol. Med. 2019, 43, 936–944. [Google Scholar] [CrossRef]
- Travis, A.J.; Jorgez, C.J.; Merdiushev, T.; Jones, B.H.; Dess, D.M.; Diaz-Cueto, L.; Storey, B.T.; Kopf, G.S.; Moss, S.B. Functional relationships between capacitation-dependent cell signaling and compartmentalized metabolic pathways in murine spermatozoa. J. Biol. Chem. 2001, 276, 7630–7636. [Google Scholar] [CrossRef]
- Plaza Davila, M.; Martin Munoz, P.; Tapia, J.A.; Ortega Ferrusola, C.; Balao da Silva, C.C.; Pena, F.J. Inhibition of Mitochondrial Complex I Leads to Decreased Motility and Membrane Integrity Related to Increased Hydrogen Peroxide and Reduced ATP Production, while the Inhibition of Glycolysis Has Less Impact on Sperm Motility. PLoS ONE 2015, 10, e0138777. [Google Scholar] [CrossRef] [Green Version]







| Gene | Sequence 5′-3′ | Annealing Temperature | Number of Cycles |
|---|---|---|---|
| UCP1 (NM_021833.5) | FWD: GCTCCAGGTCCAAGGTGAAT RVS: TTGCTTCCTAAACTAGGTGCTG | 59 °C | 40 |
| UCP2 (NM_001381943.1) | FWD: GAAGCCTCTACAATGGGCTGG RVS: CAGAGCCCTTGGTGTAGAACTG | 63 °C | 30 |
| UCP3S (NM_022803.3) | FWD: CAACCTGGGATGTAGCGGTG RVS: GAGCGTGTGGAGACAGTGAG | 60 °C | 25 |
| UCP3L (NM_003356.4) | FWD: TGGAACGTGGTGATGTTCGTA RVS: AGACCAGAATCCCTCCTCCT | 60 °C | 33 |
| UCP4 (NM_004277.5) | FWD: CGCACAGCTCTAGGGATCAT RVS: CGTGTCTGTAAATGGCGGGT | 60 °C | 33 |
| UCP5 (NM_001282195.2) | FWD: GACTTTCCCTGTGGACCTTACC RVS: GCGCATGGAACATCCCTCTAT | 60 °C | 33 |
| UCP6 (NM_001010875.4) | FWD: ACCCTGTTGATGTTGTGAGG RVS: TAGCCAGAACATCTGCCATCTC | 63 °C | 33 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carrageta, D.F.; Freire-Brito, L.; Guerra-Carvalho, B.; Ribeiro, J.C.; Monteiro, B.S.; Barros, A.; Oliveira, P.F.; Monteiro, M.P.; Alves, M.G. Inhibition of Mitochondrial Uncoupling Proteins Arrests Human Spermatozoa Motility without Compromising Viability. Antioxidants 2023, 12, 409. https://doi.org/10.3390/antiox12020409
Carrageta DF, Freire-Brito L, Guerra-Carvalho B, Ribeiro JC, Monteiro BS, Barros A, Oliveira PF, Monteiro MP, Alves MG. Inhibition of Mitochondrial Uncoupling Proteins Arrests Human Spermatozoa Motility without Compromising Viability. Antioxidants. 2023; 12(2):409. https://doi.org/10.3390/antiox12020409
Chicago/Turabian StyleCarrageta, David F., Laís Freire-Brito, Bárbara Guerra-Carvalho, João C. Ribeiro, Bruno S. Monteiro, Alberto Barros, Pedro F. Oliveira, Mariana P. Monteiro, and Marco G. Alves. 2023. "Inhibition of Mitochondrial Uncoupling Proteins Arrests Human Spermatozoa Motility without Compromising Viability" Antioxidants 12, no. 2: 409. https://doi.org/10.3390/antiox12020409
APA StyleCarrageta, D. F., Freire-Brito, L., Guerra-Carvalho, B., Ribeiro, J. C., Monteiro, B. S., Barros, A., Oliveira, P. F., Monteiro, M. P., & Alves, M. G. (2023). Inhibition of Mitochondrial Uncoupling Proteins Arrests Human Spermatozoa Motility without Compromising Viability. Antioxidants, 12(2), 409. https://doi.org/10.3390/antiox12020409