Influence of Molasses Residue on Treatment of Cow Manure in an Anaerobic Filter with Perforated Weed Membrane and a Conventional Reactor: Variations of Organic Loading and a Machine Learning Application
Abstract
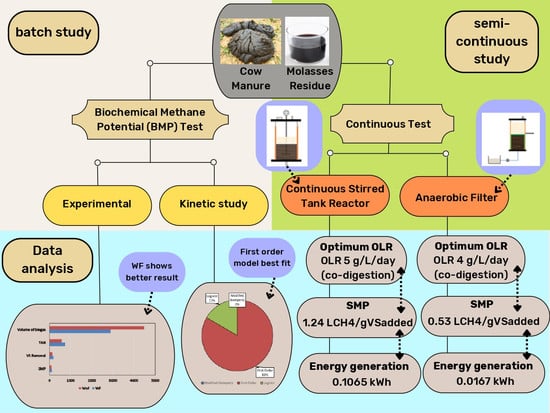
:1. Introduction
2. Materials and Methods
2.1. Physiochemical Characterization of CM, MR, and CM + MR
2.2. Biomethane Potential (BMP) Test without Filter (WoF) and with Filter (WF)
2.3. Continuous Study
2.3.1. Continuous Stirred Tank Reactor (CSTR) Setup
2.3.2. Anaerobic Filter (AF) Setup
2.4. Analytical Method
2.5. Kinetic Analysis
- Rm = maximum biogas generation rate (L/d);
- = lag phase (day);
- S(t) = cumulative biogas production at digestion time “t” days;
- S = biogas potential of the substrate (L);
- K = biogas production rate constant;
- t = time (days).
2.6. Artificial Neural Network (ANN) Analysis
2.7. Projection of Electrical Energy
3. Results and Discussion
3.1. Summary of BMP Test of AD for CM, MR, and CM + MR
3.2. Continuous Study of CM, MR, and CM + MR at increasing OLR
3.2.1. Biogas Production and pH at Different OLRs
3.2.2. VS removal and Specific Methane Production (SMP) at Different OLRs
3.2.3. IA/PA Ratio and Total Ammonia Nitrogen at Different OLRs
3.3. Kinetic Analysis of Biogas Production from BMP Test
3.4. Artificial Neural Network (ANN) Analysis for BMP Test
3.5. Electrical Energy Generated from Laboratory Scale (LS) and on-Farm Scale (OFS) Projection
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Abbreviation | Meaning |
| AD | Anaerobic Digestion |
| ACoD | Anaerobic Co-digestion |
| MR | Molasses Residue |
| CM | Cow Manure |
| AF | Anaerobic Filter with perforated membrane |
| CSTR | Continuous Stirred Tank Reactor |
| ANN | Artificial Neural Network |
| BMP | Biomethane Potential |
| OLR | Organic Loading Rate |
| SMP | Specific Methane Production |
| VS | Volatile Solids |
| MG | Modified Gompertz |
| LG | Logistic |
| FO | First Order |
| RMSE | Root Mean Square Error |
| R2 | Correlation coeeficient |
| OFS | On-Farm Scale |
| VFA | Volatile Fatty Acid |
| C/N | Carbon to Nitrogen |
| UASB | Up-flow Anaerobic Sludge Blanket |
| CH4 | Methane |
| HRT | Hydraulic Retention Time |
| TS | Total Solids |
| TDS | Total Dissolved Solids |
| COD | Chemical Oxygen Demand |
| O&G | Oil and Grease |
| WoF | Without Filter |
| WF | With Filter |
| VSS | Volatile Suspended Solids |
| S/I | Substrate to Inoculum |
| IA/PA | Total alkalinity ratio |
| TAN | Total Ammonia Nitrogen |
| LCFA | Long-chain fatty acids |
| LS | Lab scale |
References
- Yetilmezsoy, K.; Kiyan, E.; Ilhan, F.; Özçimen, D.; Koçer, A.T. Screening plant growth effects of sheep slaughterhouse waste-derived soil amendments in Greenhouse Trials. J. Environ. Manag. 2022, 318, 115586. [Google Scholar] [CrossRef] [PubMed]
- Yetilmezsoy, K.; Dinç-Şengönül, B.; Ilhan, F.; Kıyan, E.; Yüzer, N. Use of sheep slaughterhouse-derived struvite in the production of environmentally sustainable cement and fire-resistant wooden structures. J. Clean. Prod. 2022, 366, 132948. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, J.; Krooneman, J.; Euverink, G. Strategies to boost anaerobic digestion performance of cow manure: Laboratory achievements and their full-scale application potential. Sci. Total Environ. 2021, 755, 142940. [Google Scholar] [CrossRef]
- Abdeshahian, P.; Lim, J.; Ho, W.; Hashim, H.; Lee, C. Potential of biogas production from farm animal waste in Malaysia. Renew. Sustain. Energy Rev. 2016, 60, 714–723. [Google Scholar] [CrossRef]
- Carlin, N.T.; Annamalai, K.; Harman, W.L.; Sweeten, J.M. The economics of reburning with cattle manure-based biomass in existing coal-fired power plants for NOx and CO2 Emissions Control. Biomass Bioenergy 2009, 33, 1139–1157. [Google Scholar] [CrossRef]
- Oliveira, C.; Fuess, L.; Soares, L.; Damianovic, M. Thermophilic biomethanation of sugarcane molasses comparing single and two-stage systems: Process performance and energetic potential. Bioresour. Technol. Rep. 2020, 12, 100590. [Google Scholar] [CrossRef]
- Meng, X.; Yuan, X.; Ren, J.; Wang, X.; Zhu, W.; Cui, Z. Methane production and characteristics of the microbial community in a two-stage fixed-bed anaerobic reactor using molasses. Bioresour. Technol. 2017, 241, 1050–1059. [Google Scholar] [CrossRef]
- Suhartini, S.; Hidayat, N.; Rohma, N.A.; Paul, R.; Pangestuti, M.B.; Utami, R.N.; Nurika, I.; Melville, N. Sustainable strategies for anaerobic digestion of oil palm empty fruit bunches in Indonesia: A Review. Int. J. Sustain. Energy 2022, 41, 2044–2096. [Google Scholar] [CrossRef]
- Assis, T.I.; Gonçalves, R.F. Valorization of food waste by anaerobic digestion: A Bibliometric and systematic review focusing on optimization. J. Environ. Manag. 2022, 320, 115763. [Google Scholar] [CrossRef] [PubMed]
- Szabo-Corbacho, M.A.; Pacheco-Ruiz, S.; Míguez, D.; Hooijmans, C.M.; Brdjanovic, D.; García, H.A.; van Lier, J.B. Influence of the sludge retention time on membrane fouling in an anaerobic membrane bioreactor (anmbr) treating lipid-rich dairy wastewater. Membranes 2022, 12, 262. [Google Scholar] [CrossRef]
- Abid, M.; Wu, J.; Seyedsalehi, M.; Hu, Y.; Tian, G. Novel insights of impacts of solid content on high solid anaerobic digestion of cow manure: Kinetics and microbial community dynamics. Bioresour. Technol. 2021, 333, 125205. [Google Scholar] [CrossRef]
- Chiumenti, A.; da Borso, F.; Limina, S. Dry anaerobic digestion of cow manure and agricultural products in a full-scale plant: Efficiency and comparison with wet fermentation. Waste Manag. 2018, 71, 704–710. [Google Scholar] [CrossRef]
- Abubakar, B.S.U.; Ismail, N. Anaerobic digestion of Cow Dung for Biogas Production. J. Eng. Appl. Sci. 2012, 7, 169–172. [Google Scholar]
- Zhang, J.; Tian, H.; Wang, X.; Tong, Y. Effects of activated carbon on mesophilic and thermophilic anaerobic digestion of food waste: Process performance and life cycle assessment. Chem. Eng. J. 2020, 399, 125757. [Google Scholar] [CrossRef]
- Wang, M.; Wang, J.; Li, Y.; Li, Q.; Li, P.; Luo, L.; Zhen, F.; Zheng, G.; Sun, Y. Low-temperature pretreatment of biomass for enhancing biogas production: A Review. Fermentation 2022, 8, 562. [Google Scholar] [CrossRef]
- Ceron-Vivas, A.; Cáceres, K.T.; Rincón, A.; Cajigas, Á.A. Influence of pH and the C/N ratio on the biogas production of wastewater. Rev. Fac. Ing. Univ. Antioq. 2019, 92, 70–79. [Google Scholar] [CrossRef]
- Tawfik, A.; Hassan, G.K.; Yu, Z.; Salah, H.A.; Hassan, M.; Meng, F. Dynamic Approach for mono- and di-fermentation of black liquor and livestock wastewater for 2-Bio-(H2&CH4) production. Biomass Bioenergy 2021, 145, 105947. [Google Scholar]
- Ning, J.; Zhou, M.; Pan, X.; Li, C.; Lv, N.; Wang, T.; Cai, G.; Wang, R.; Li, J.; Zhu, G. Simultaneous biogas and biogas slurry production from co-digestion of pig manure and corn straw: Performance Optimization and Microbial Community shift. Bioresour. Technol. 2019, 282, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Kainthola, J.; Kalamdhad, A.S.; Goud, V.V. Optimization of methane production during anaerobic co-digestion of rice straw and hydrilla verticillata using response surface methodology. Fuel 2019, 235, 92–99. [Google Scholar] [CrossRef]
- Li, C.; Xie, S.; Wang, Y.; Jiang, R.; Wang, X.; Lv, N.; Pan, X.; Cai, G.; Yu, G.; Wang, Y. Multi-functional biochar preparation and heavy metal immobilization by co-pyrolysis of livestock feces and biomass waste. Waste Manag. 2021, 134, 241–250. [Google Scholar] [CrossRef]
- Kongjan, P.; O-Thong, S.; Angelidaki, I. Biohydrogen production from desugared molasses (DM) using thermophilic mixed cultures immobilized on heat treated anaerobic sludge granules. Int. J. Hydrog. Energy 2011, 36, 14261–14269. [Google Scholar] [CrossRef]
- Li, Q.; Li, Y. Coproduction of hydrogen and methane in a CSTR-IC two-stage anaerobic digestion system from molasses wastewater. Water Sci. Technol. 2019, 79, 270–277. [Google Scholar] [CrossRef]
- Keskin, T.; Hallenbeck, P.C. Hydrogen production from sugar industsry wastes using single-stage photofermentation. Bioresour. Technol. 2012, 112, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Ahmad, R.; Phulpoto, I.; Kashif, M.; Shen, P. Effect of rice winery wastewater as a co-substrate to enhance anaerobic digestion of molasses for methane production. Bioresour. Technol. Rep. 2022, 18, 101062. [Google Scholar] [CrossRef]
- Chen, Y.; Yu, N.; Sun, Z.; Gou, M.; Xia, Z.; Tang, Y.; Kida, K. Acclimation Improves Methane Production from Molasses Wastewater with High Salinity in an Upflow Anaerobic Filter Reactor: Performance and Microbial Community Dynamics. Appl. Biochem. Biotechnol. 2020, 191, 397–411. [Google Scholar] [CrossRef]
- De Vrieze, J.; Coma, M.; Debeuckelaere, M.; Van der Meeren, P.; Rabaey, K. High salinity in molasses wastewaters shifts anaerobic digestion to carboxylate production. Water Res. 2016, 98, 293–301. [Google Scholar] [CrossRef] [Green Version]
- Pratt, S.; Liew, D.; Batstone, D.; Werker, A.; Morgan-Sagastume, F.; Lant, P. Inhibition by fatty acids during fermentation of pre-treated waste activated sludge. J. Biotechnol. 2012, 159, 38–43. [Google Scholar] [CrossRef]
- Bedoić, R.; Špehar, A.; Puljko, J.; Čuček, L.; Ćosić, B.; Pukšec, T.; Duić, N. Opportunities and challenges: Experimental and kinetic analysis of anaerobic co-digestion of food waste and rendering industry streams for biogas production. Renew. Sustain. Energy Rev. 2020, 130, 109951. [Google Scholar] [CrossRef]
- Kainthola, J.; Kalamdhad, A.S.; Goud, V.V. Enhanced methane production from anaerobic co-digestion of rice straw and hydrilla verticillata and its kinetic analysis. Biomass Bioenergy 2019, 125, 8–16. [Google Scholar] [CrossRef]
- Fang, C.; Boe, K.; Angelidaki, I. Anaerobic co-digestion of by-products from sugar production with cow manure. Water Res. 2011, 45, 3473–3480. [Google Scholar] [CrossRef]
- Aboudi, K.; Álvarez-Gallego, C.; Romero-García, L. Evaluation of methane generation and process stability from anaerobic co-digestion of sugar beet by-product and cow manure. J. Biosci. Bioeng. 2016, 121, 566–572. [Google Scholar] [CrossRef]
- Sarker, S.; Møller, H.B. Regulating feeding and increasing methane yield from co-digestion of C5 molasses and cattle manure. Energy Convers. Manag. 2014, 84, 7–12. [Google Scholar] [CrossRef]
- López-Gutiérrez, I.; Montiel-Corona, V.; Calderón-Soto, L.F.; Palomo-Briones, R.; Méndez-Acosta, H.O.; Razo-Flores, E.; Ontiveros-Valencia, A.; Alatriste-Mondragón, F. Evaluation of the continuous methane production from an enzymatic agave bagasse hydrolysate in suspended (CSTR) and granular biomass systems (UASB). Fuel 2021, 304, 121406. [Google Scholar] [CrossRef]
- Vendruscolo, E.C.; Mesa, D.; Rissi, D.V.; Meyer, B.H.; De Oliveira Pedrosa, F.; De Souza, E.M.; Cruz, L.M. Microbial communities network analysis of anaerobic reactors fed with bovine and swine slurry. Sci. Total Environ. 2020, 742, 140314. [Google Scholar] [CrossRef]
- Musa, M.A.; Idrus, S.; Che Man, H.; Nik Daud, N.N. Performance comparison of conventional and modified upflow anaerobic sludge blanket (UASB) reactors treating high-strength cattle slaughterhouse wastewater. Water 2019, 11, 806. [Google Scholar] [CrossRef] [Green Version]
- Marzuki, T.; Idrus, S.; Musa, M.; Wahab, A.; Jamali, N.; Man, H.; Ng, S. Enhancement of Bioreactor Performance Using Acclimatised Seed Sludge in Anaerobic Treatment of Chicken Slaughterhouse Wastewater: Laboratory Achievement, Energy Recovery, and Its Commercial-Scale Potential. Animals 2021, 11, 3313. [Google Scholar] [CrossRef] [PubMed]
- Daud, M.; Rizvi, H.; Akram, M.; Ali, S.; Rizwan, M.; Nafees, M.; Jin, Z. Review of Upflow Anaerobic Sludge Blanket Reactor Technology: Effect of Different Parameters and Developments for Domestic Wastewater Treatment. J. Chem. 2018, 2018, 1596319. [Google Scholar] [CrossRef] [Green Version]
- Gunay, A.; Karadag, D. Recent developments in the anaerobic digestion of olive mill effluents. Process Biochem. 2015, 50, 1893–1903. [Google Scholar] [CrossRef]
- Jaman, K.; Amir, N.; Musa, M.A.; Zainal, A.; Yahya, L.; Wahab, A.M.A.; Suhartini, S.; Marzuki, T.N.T.M.; Harun, R.; Idrus, S. Anaerobic digestion, codigestion of food waste, and chicken dung: Correlation of kinetic parameters with digester performance and on-farm electrical energy generation potential. Fermentation 2022, 8, 28. [Google Scholar] [CrossRef]
- Li, L.; He, Q.; Zhao, X.; Wu, D.; Wang, X.; Peng, X. Anaerobic digestion of food waste: Correlation of kinetic parameters with operational conditions and process performance. Biochem. Eng. J. 2018, 130, 1–9. [Google Scholar] [CrossRef]
- Pečar, D.; Goršek, A. Kinetics of methane production during anaerobic digestion of chicken manure with sawdust and Miscanthus. Biomass Bioenergy 2020, 143, 105820. [Google Scholar] [CrossRef]
- Mougari, N.E.; Largeau, J.F.; Himrane, N.; Hachemi, M.; Tazerout, M. Application of artificial neural network and kinetic modeling for the prediction of biogas and methane production in anaerobic digestion of several organic wastes. Int. J. Green Energy 2021, 18, 1584–1596. [Google Scholar] [CrossRef]
- Mohamed, Z.E. Using the artificial neural networks for prediction and validating solar radiation. J. Egypt. Math. Soc. 2019, 27, 47. [Google Scholar] [CrossRef] [Green Version]
- Gómez-Quiroga, X.; Aboudi, K.; Fernández-Güelfo, L.A.; Álvarez-Gallego, C.J.; Romero-García, L.I. Thermophilic anaerobic co-digestion of exhausted sugar beet pulp with cow manure to boost the performance of the process: The effect of manure proportion. Water 2020, 13, 67. [Google Scholar] [CrossRef]
- Ohuchi, Y.; Ying, C.; Lateef, S.A.; Ihara, I.; Iwasaki, M.; Inoue, R.; Umetsu, K. Anaerobic co-digestion of sugar beet tops silage and dairy cow manure under thermophilic condition. J. Mater. Cycles Waste Manag. 2014, 17, 540–546. [Google Scholar] [CrossRef]
- Dhamodharan, K.; Kumar, V.; Kalamdhad, A.S. Effect of different livestock Dungs as inoculum on food waste anaerobic digestion and its kinetics. Bioresour. Technol. 2015, 180, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, Z.; Peng, Y.; Huang, W.; Liu, J.; Mironov, V.; Zhang, S. Deeper insights into the effects of substrate to inoculum ratio selection on the relationship of kinetic parameters, microbial communities, and key metabolic pathways during the anaerobic digestion of food waste. Water Res. 2022, 217, 118440. [Google Scholar] [CrossRef] [PubMed]
- Idrus, S.; Banks, C.J.; Heaven, S. Assessment of the potential for biogas production from wheat straw leachate in upflow anaerobic sludge blanket digesters. Water Sci. Technol. 2012, 66, 2737–2744. [Google Scholar] [CrossRef]
- Rodger, B.; Bridgewater, L. Standard Methods for the Examination of Water and Wastewater, 23rd ed.; American Public Health Association: Washington, DC, USA, 2017. [Google Scholar]
- Muhamad Ng, S.N.; Idrus, S.; Ahsan, A.; Tuan Mohd Marzuki, T.N.; Mahat, S.B. Treatment of wastewater from a food and beverage industry using conventional wastewater treatment integrated with membrane bioreactor system: A pilot-scale case study. Membranes 2021, 11, 456. [Google Scholar] [CrossRef]
- Li, K.; Liu, R.; Sun, C. Comparison of anaerobic digestion characteristics and kinetics of four livestock manures with different substrate concentrations. Bioresour. Technol. 2015, 198, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Deepanraj, B.; Sivasubramanian, V.; Jayaraj, S. Experimental and kinetic study on anaerobic digestion of food waste: The effect of total solids and pH. J. Renew. Sustain. Energy 2015, 7, 063104. [Google Scholar] [CrossRef]
- Khanal, S.K.; Li, Y. Biogas Production and Application. In Bioenergy: Principles and Applications, 1st ed.; Wiley Blackwell: Hoboken, NJ, USA, 2017; pp. 338–360. [Google Scholar]
- Capson-Tojo, G.; Moscoviz, R.; Ruiz, D.; Santa-Catalina, G.; Trably, E.; Rouez, M.; Crest, M.; Steyer, J.P.; Bernet, N.; Delgenès, J.P.; et al. Addition of granular activated carbon and trace elements to favor volatile fatty acid consumption during anaerobic digestion of food waste. Bioresour. Technol. 2018, 260, 157–168. [Google Scholar] [CrossRef]
- Yang, J.; Wang, D.; Luo, Z.; Zeng, W. Anaerobic mono-digestion of pig manure in a leach bed coupled with a methanogenic reactor: Effects of the filter media. J. Clean. Prod. 2019, 234, 1094–1101. [Google Scholar] [CrossRef]
- Du, Q.; Mu, Q.; Wu, G. Metagenomic and bioanalytical insights into quorum sensing of methanogens in anaerobic digestion systems with or without the addition of conductive filter. Sci. Total Environ. 2021, 763, 144509. [Google Scholar] [CrossRef]
- Wyman, V.; Serrano, A.; Fermoso, F.G.; Villa Gomez, D.K. Trace elements effect on hydrolytic stage towards biogas production of model lignocellulosic substrates. J. Environ. Manag. 2019, 234, 320–325. [Google Scholar] [CrossRef]
- Feng, L.; Ward, A.J.; Moset, V.; Møller, H.B. Methane emission during on-site pre-storage of animal manure prior to anaerobic digestion at biogas plant: Effect of storage temperature and addition of food waste. J. Environ. Manag. 2018, 225, 272–279. [Google Scholar] [CrossRef]
- Dasa, K.T.; Westman, S.Y.; Millati, R.; Cahyanto, M.N.; Taherzadeh, M.J.; Niklasson, C. Inhibitory effect of long-chain fatty acids on biogas production and the protective effect of membrane bioreactor. BioMed Res. Int. 2016, 2016, 7263974. [Google Scholar] [PubMed] [Green Version]
- Zainal, A.; Harun, R.; Idrus, S. Performance monitoring of anaerobic digestion at various organic loading rates of commercial Malaysian food waste. Front. Bioeng. Biotechnol. 2022, 10, 775676. [Google Scholar] [CrossRef]
- Fang, C.; Boe, K.; Angelidaki, I. Anaerobic co-digestion of desugared molasses with cow manure; focusing on sodium and potassium inhibition. Bioresour. Technol. 2011, 102, 1005–1011. [Google Scholar] [CrossRef] [PubMed]
- Morales-Polo, C.; del Mar Cledera-Castro, M.; Moratilla Soria, B.Y. Reviewing the anaerobic digestion of food waste: From waste generation and anaerobic process to its perspectives. Appl. Sci. 2018, 8, 1804. [Google Scholar] [CrossRef] [Green Version]
- Egwu, U.; Onyelowe, K.; Tabraiz, S.; Johnson, E.; Mutshow, A. Investigation of the effect of equal and unequal feeding time intervals on process stability and methane yield during anaerobic digestion grass silage. Renew. Sustain. Energy Rev. 2022, 158, 112092. [Google Scholar] [CrossRef]
- Kamyab, B.; Zilouei, H. Investigating the efficiency of biogas production using modelling anaerobic digestion of baker’s yeast wastewater on two-stage mixed-UASB reactor. Fuel 2021, 285, 119198. [Google Scholar] [CrossRef]
- Yin, D.M.; Uwineza, C.; Sapmaz, T.; Mahboubi, A.; De Wever, H.; Qiao, W.; Taherzadeh, M.J. Volatile fatty acids (VFA) production and recovery from chicken manure using a high-solid anaerobic membrane bioreactor (AnMBR). Membranes 2022, 12, 1133. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Chen, S.; Li, X. Anaerobic Co-digestion of Kitchen Waste and Cattle Manure for Methane Production. Energy Sources Part A Recovery Util. Environ. Eff. 2009, 31, 1848–1856. [Google Scholar] [CrossRef]
- Riaño, B.; Molinuevo, B.; García-González, M. Potential for methane production from anaerobic co-digestion of swine manure with winery wastewater. Bioresour. Technol. 2011, 102, 4131–4136. [Google Scholar] [CrossRef]
- Perin, J.H.; Borth, P.B.; Torrecilhas, A.; da Cunha, L.S.; Kuroda, E.; Fernandes, F. Optimization of methane production parameters during anaerobic co-digestion of food waste and garden waste. J. Clean. Prod. 2020, 272, 123130. [Google Scholar] [CrossRef]
- Kleiner, D. NH4+ transport systems. In Alkali Cation Transport Systems in Prokaryotes; Bakker, E., Ed.; CRC Press: Boca Raton, FL, USA, 1993. [Google Scholar]
- Wiegant, W.M.; Zeeman, G. The mechanism of ammonia inhibition in the thermophilic digestion of livestock wastes. Agric. Wastes 1986, 16, 243–253. [Google Scholar] [CrossRef]
- Fuentes, K.; Torres–Lozada, P.; Chaparro, T. Beverage wastewater treatment by anaerobic digestion in two-stages for organic matter removal and energy production. Biomass Bioenergy 2021, 154, 106260. [Google Scholar] [CrossRef]
- Ripley, L.E.; Boyle, W.C.; Converse, J.C. Improved Alkalimetric Monitoring for Anaerobic Digestion of High-Strength Wastes. J. (Water Pollut. Control. Fed.) 1986, 58, 406–411. [Google Scholar]
- Kafle, G.; Chen, L. Comparison on batch anaerobic digestion of five different livestock manures and prediction of biochemical methane potential (BMP) using different statistical models. Waste Manag. 2016, 48, 492–502. [Google Scholar] [CrossRef] [Green Version]
- Rathaur, R.; Dhawane, S.; Ganguly, A.; Mandal, M.; Halder, G. Methanogenesis of organic wastes and their blend in batch anaerobic digester: Experimental and kinetic study. Process Saf. Environ. Prot. 2018, 113, 413–423. [Google Scholar] [CrossRef]
- Ugwu, S.; Enweremadu, C. Enhancing anaerobic digestion of okra waste with the addition of iron nanocomposite (Ppy/Fe3O4). Biofuels 2019, 11, 503–512. [Google Scholar] [CrossRef]












| Parameters | Unit | CM | MR | CM + MR (50:50) | CM + MR (70:30) | CM + MR (30:70) |
|---|---|---|---|---|---|---|
| COD | mg/L | 128,000 ± 16,000 | 70,400 ± 18,400 | 99,200 ± 17,000 | 110,720 ± 16,720 | 87,680 ± 17,680 |
| TN | mg/L | 14,200 ± 1300 | 2500 ± 180 | 8350 ± 220 | 10,690 ± 870 | 4435 ± 240 |
| VS | mg/L | 399,590 ± 33,440 | 44,645 ± 12,770 | 444,235 ± 32,790 | 293,106 ± 27,230 | 151,128 ± 18,970 |
| TS | mg/L | 470,360 ± 33,690 | 46,250 ± 13,770 | 457,290 ± 23,730 | 329,252 ± 27,700 | 173,480 ± 19,740 |
| TDS | mg/L | 7000 ± 940 | 22,925 ± 2100 | 14,962 ± 1370 | 55,870 ± 7210 | 37,050 ± 4290 |
| O&G | mg/L | 42,300 ± 7000 | 1402 ± 200 | 21,220 ± 1200 | 30,030 ± 4900 | 13,670 ± 2240 |
| Color | Pt-Co | 81,000 ± 11,000 | 42,750 ± 6500 | 61,875 ± 4300 | 69,525 ± 9650 | 54,225 ± 7850 |
| Salinity | mS/cm | 11.6 ± 1.6 | 33.75 ± 12.00 | 22.67 ± 2.00 | 18.24 ± 2.68 | 27.10 ± 4.12 |
| C/N ratio | - | 9.01 | 28.16 | 11.88 | 10.36 | 19.78 |
| Parameters | Unit | CM | MR |
|---|---|---|---|
| Sucrose | mg/L | - | - |
| Glucose | mg/L | - | - |
| Galactose | mg/L | 0.292 | - |
| Mannose | mg/L | 0.013 | 0.007 |
| Fructose | mg/L | 0.010 | 0.004 |
| Xylose | mg/L | - | 0.213 |
| BMP Bottle | Operating Condition | Substrate |
|---|---|---|
| 1, 2, 3 | WoF | CM |
| 4, 5, 6 | WoF | MR |
| 7, 8, 9 | WoF | CM + MR |
| 10, 11, 12 | WF | CM |
| 13, 14, 15 | WF | MR |
| 16, 17, 18 | WF | CM + MR |
| Reactor | Period (Days) | OLR (g/L/Day) | ACoD Ratio |
|---|---|---|---|
| CSTR | 12 | 1 | 100% CM |
| 18 | 2 | 100% CM | |
| 6 | 3 | 100% CM | |
| 6 | 4 | 100% CM | |
| 10 | 4 | 50% CM/50% MR | |
| 11 | 5 | 50% CM/50% MR | |
| 11 | 6 | 50% CM/50% MR | |
| 11 | 7 | 50% CM/50% MR | |
| 11 | 7 | 30% CM/70% MR | |
| 11 | 7 | 70% CM/30% MR | |
| AF | 12 | 1 | 100% CM |
| 18 | 2 | 100% CM | |
| 6 | 3 | 100% CM | |
| 6 | 4 | 100% CM | |
| 10 | 4 | 50% CM/50% MR | |
| 11 | 5 | 50% CM/50% MR | |
| 11 | 6 | 50% CM/50% MR |
| Model | Mathematical Definition | Source | Equation |
|---|---|---|---|
| First Order | [40,41] | (1) | |
| Modified Gompertz Model | [40] | (2) | |
| Logistic Model | [41] | (3) |
| Parameters | ANN1 | ANN2 |
|---|---|---|
| No. of layers | 2 | 2 |
| No. of neurons first hidden layer | 20 | 20 |
| No. of neurons second hidden layer | 20 | 20 |
| Activation function first hidden layer | Tan-sigmoid | Log-sigmoid |
| Activation function second hidden layer | Log-sigmoid | Tan-sigmoid |
| CM | MR | CM + MR (50:50) | ||||
|---|---|---|---|---|---|---|
| WoF | WF | WoF | WF | WoF | WF | |
| IA/PA ratio | 0.147 ± 0.002 | 0.228 ± 0.005 | 0.155 ± 0.006 | 0.139 ± 0.010 | 0.250 ± 0.008 | 0.192 ± 0.011 |
| pH | 6.75 ± 0.05 | 6.93 ± 0.15 | 7.76 ± 0.11 | 8.21 ± 0.08 | 7.10 ± 0.05 | 7.26 ± 0.09 |
| TAN (mg/L) | 168 ± 20 | 255 ± 60 | 235 ± 40 | 198 ± 55 | 170 ± 30 | 295 ± 65 |
| SMP (mL CH4/VS) | 25.90 ± 8.20 | 35.28 ± 5.80 | 33.65 ± 3.30 | 45.05 ± 7.40 | 32.92 ± 6.20 | 31.24 ± 5.30 |
| VS removal (%) | 38.5 ± 5.50 | 49.6 ± 2.80 | 42.3 ± 3.60 | 67.7 ± 2.40 | 51.4 ± 1.80 | 64.5 ± 0.91 |
| Volume of biogas (mL) | 860 ± 80 | 1520 ± 120 | 1310 ± 180 | 1650 ± 60 | 720 ± 100 | 1310 ± 200 |
| Substrates | Unit | OLR 4 (Mono) | OLR 4 (Co) | OLR 7 (Co) | Optimum OLR | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| CSTR | AF | CSTR | AF | CSTR | CSTR | AF | ||||
| 100:0 | 100:0 | 50:50 | 50:50 | 50:50 | 30:70 | 70:30 | OLR 5 (Co) (50:50) | OLR 2 (Mono) (100:0) | ||
| Lactic acid | mg/L | 5.684 | 147.826 | 122.885 | 66.7 | - | - | - | 187.653 | 144.328 |
| Acetic acid | mg/L | - | 352.459 | 122.885 | - | 328.982 | 144.672 | 150.954 | 31.471 | - |
| Butyric acid | mg/L | - | - | - | - | 55.554 | - | - | - | - |
| Propionic acid | mg/L | - | - | - | - | 371.419 | 326.082 | 199.539 | 88.789 | - |
| Model | Parameter | Units | Sample | |||||
|---|---|---|---|---|---|---|---|---|
| CM WoF | CM WF | MR WoF | MR WF | CM + MR WoF | CM + MR WF | |||
| Modified Gompertz | R-square | 0.9853 | 0.9857 | 0.1398 | 0.9896 | 0.9379 | 0.679 | |
| RMSE | 47.658 | 74.363 | 415.908 | 110.942 | 76.554 | 152.183 | ||
| Rm | L/day | 21.763 | 98.327 | 700.001 | 217.266 | 57.70 | 102.599 | |
| Mean R-square | 0.83937 | |||||||
| Mean RMSE | 146.268 | |||||||
| Logistic | R-square | 0.9792 | 0.7580 | 0.9895 | 0.4859 | 0.8608 | 0.742 | |
| RMSE | 32.849 | 187.128 | 43.597 | 227.667 | 120.517 | 122.868 | ||
| Rm | L/day | 33.121 | 45.556 | 50.030 | 37.195 | 175.455 | 33.175 | |
| Mean R-square | 0.80257 | |||||||
| Mean RMSE | 122.438 | |||||||
| First Order | R-square | 0.9932 | 0.8239 | 0.9512 | 0.9943 | 0.9413 | 0.9382 | |
| RMSE | 12.451 | 16.596 | 140.132 | 24.216 | 74.343 | 75.483 | ||
| Rate Constant (k) | d−1 | 0.0507 | 0.0958 | 0.002 | 0.219 | 0.210 | 0.208 | |
| Mean R-square | 0.94035 | |||||||
| Mean RMSE | 57.204 | |||||||
| Sample | OLR (g/L/Day) | SMP (LCH4/gVSadded) | Energy (KWh/kgVSadded) | Electrical Energy Generation (kWh) [LS] | Electrical Energy Generation (kWh) [OFS] | ||||
|---|---|---|---|---|---|---|---|---|---|
| CSTR | AF | CSTR | AF | CSTR | AF | CSTR | AF | ||
| CM | 1 | 0.14 | 0.09 | 1.39 | 0.90 | 0.0024 | 0.0018 | 9.45 | 3.00 |
| 2 | 0.58 | 0.35 | 5.78 | 3.49 | 0.0199 | 0.0140 | 78.61 | 23.27 | |
| 3 | 0.53 | 0.48 | 5.28 | 4.78 | 0.0273 | 0.0287 | 105.6 | 47.80 | |
| 4 | 0.51 | 0.51 | 5.08 | 5.08 | 0.0351 | 0.0203 | 140.21 | 67.73 | |
| CM + MR | 4 (50:50) | 1.19 | 0.53 | 11.85 | 5.28 | 0.0818 | 0.0422 | 145.73 | 70.40 |
| 5 (50:50) | 1.24 | 0.51 | 12.35 | 5.08 | 0.1065 | 0.0508 | 425.83 | 84.67 | |
| 6 (50:50) | 1.01 | 0.51 | 10.06 | 5.08 | 0.1040 | 0.0610 | 416.08 | 101.6 | |
| 7 (50:50) | 0.39 | 3.88 | - | 0.0468 | - | 187.32 | - | ||
| 7 (30:70) | 0.47 | 4.68 | - | 0.0565 | - | 224.64 | - | ||
| 7 (70:30) | 0.53 | 5.28 | - | 0.0637 | - | 253.44 | - | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jaman, K.; Idrus, S.; Wahab, A.M.A.; Harun, R.; Daud, N.N.N.; Ahsan, A.; Shams, S.; Uddin, M.A. Influence of Molasses Residue on Treatment of Cow Manure in an Anaerobic Filter with Perforated Weed Membrane and a Conventional Reactor: Variations of Organic Loading and a Machine Learning Application. Membranes 2023, 13, 159. https://doi.org/10.3390/membranes13020159
Jaman K, Idrus S, Wahab AMA, Harun R, Daud NNN, Ahsan A, Shams S, Uddin MA. Influence of Molasses Residue on Treatment of Cow Manure in an Anaerobic Filter with Perforated Weed Membrane and a Conventional Reactor: Variations of Organic Loading and a Machine Learning Application. Membranes. 2023; 13(2):159. https://doi.org/10.3390/membranes13020159
Chicago/Turabian StyleJaman, Khairina, Syazwani Idrus, Abdul Malek Abdul Wahab, Razif Harun, Nik Norsyahariati Nik Daud, Amimul Ahsan, Shahriar Shams, and Md. Alhaz Uddin. 2023. "Influence of Molasses Residue on Treatment of Cow Manure in an Anaerobic Filter with Perforated Weed Membrane and a Conventional Reactor: Variations of Organic Loading and a Machine Learning Application" Membranes 13, no. 2: 159. https://doi.org/10.3390/membranes13020159
APA StyleJaman, K., Idrus, S., Wahab, A. M. A., Harun, R., Daud, N. N. N., Ahsan, A., Shams, S., & Uddin, M. A. (2023). Influence of Molasses Residue on Treatment of Cow Manure in an Anaerobic Filter with Perforated Weed Membrane and a Conventional Reactor: Variations of Organic Loading and a Machine Learning Application. Membranes, 13(2), 159. https://doi.org/10.3390/membranes13020159