Prospects of the Application of Garlic Extracts and Selenium and Silicon Compounds for Plant Protection against Herbivorous Pests: A Review
Abstract
:1. Introduction
2. Selenium Compounds
3. Silicon
4. Garlic Extracts
5. Comparative Evaluation of the Efficiency of Raphanus sativus var. Lobo Protection against Cruciferous Gall Midge (Contariana nastirtii) Using Foliar Application of Selenium, Silicon and Garlic Extract
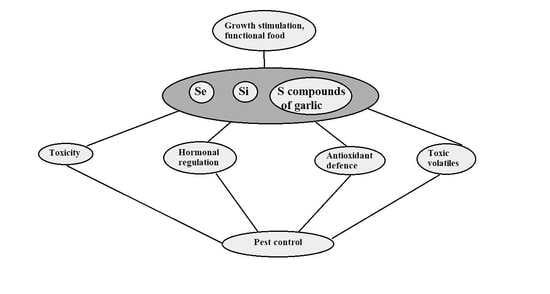
6. Prospects and Constraints of Plant Protection against Herbivory Pests via Se, Si and Garlic Extract Application
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abass, A.B.; Ndunguru, G.; Mamiro, P.; Alenkhe, B.; Mlingi, N.; Bekunda, M. Post-harvest food losses in a maize-based farming system of semi-arid savannah area of Tanzania. J. Stored Prod. Res. 2014, 57, 49–57. [Google Scholar] [CrossRef] [Green Version]
- Tapondjou, L.; Adler, C.; Bouda, H.; Fontem, D. Efficacy of powder and essential oil from Chenopodium ambrosioides leaves as post-harvest grain protectants against six-stored product beetles. J. Stored Prod. Res. 2002, 38, 395–402. [Google Scholar] [CrossRef]
- Kumar, D.; Kalita, P. Reducing Postharvest Losses during Storage of Grain Crops to Strengthen Food Security in Developing Countries. Foods 2017, 6, 8. [Google Scholar] [CrossRef] [Green Version]
- Singh, I.K.; Singh, A. Plant-Pest Interactions: From Molecular Mechanism to Chemical Ecology; Springer: Berlin/Heidelberg, Germany, 2021. [Google Scholar] [CrossRef]
- Hamann, E.; Blevins, C.; Franks, S.J.; Jameel, M.; Anderson, J.T. Climate change alters plant–herbivore interactions. New Phytol. 2021, 229, 1894–1910. [Google Scholar] [CrossRef] [PubMed]
- Skendžić, S.; Zovko, M.; Živkovi’c, I.P.; Leši’c, V.; Lemi´c, D. The impact of climate change on agricultural insects pests. Insects 2021, 12, 440. [Google Scholar] [CrossRef]
- Stankovic, S.; Kostic, M.; Kostic, I.; Krnjajic, S. Practical Approaches to Pest Control: The Use of Natural Compounds. In Pests, Weeds and Diseases in Agricultural Crop and Animal Husbandry Production; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef]
- War, A.R.; Paulraj, M.G.; Ahmad, T.; Buhroo, A.A.; Hussain, B.; Ignacimuthu, S.; Sharma, H.C. Mechanism of plant defense against insect herbivores. Plant Signal Behav. 2012, 7, 1306–1320. [Google Scholar] [CrossRef] [Green Version]
- Mitchell, C.; Brennan, R.M.; Graham, J.; Karley, A.J. Plant defense against herbivorous pests: Exploiting resistance and tolerance Traits for sustainable crop production. Front. Plant Sci. 2016, 7, 1132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schiavon, M.; Nardi, S.; dalla Vecchia, F.; Ertani, A. Selenium biofortification in the 21st century: Status and challenges for healthy human nutrition. Plant Soil 2020, 453, 245–270. [Google Scholar] [CrossRef]
- Kieliszek, M.; Bano, I.; Zare, H. A Comprehensive Review on Selenium and Its Effects on Human Health and Distribution in Middle Eastern Countries. Biol. Trace Elem. Res. 2021. [Google Scholar] [CrossRef]
- Golubkina, N.A.; Kharchenko, V.A.; Caruso, G. Selenium: Prospects of functional food production with high antioxidant activity in Reference Series in Phyto-chemistry. In Plant Antioxidants and Health; Ekiert, H., Ramawat, K.G., Arora, J., Eds.; Elsevier: Amsterdam, Switzerland, 2021. [Google Scholar]
- Kieliszek, M. Selenium–Fascinating Microelement, Properties and Sources in Food. Molecules 2019, 24, 1298. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Liu, Y. Potential interventions for novel coronavirus in China: A systematic review. J. Med. Virol. 2020, 92, 479–490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alfthan, G.; Eurola, M.; Ekholm, P.; Venäläinen, E.R.; Root, T.; Korkalainen, K.; Hartikainen, H.; Salminene, P.; Hietaniemi, V.; Aspila, P.; et al. Effects of nationwide addition of selenium to fertilizers on foods, and animal and human health in Finland: From deficiency to optimal selenium status of the population. J. Trace Elem. Med. Biol. 2015, 31, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Kovalsky, Y.G.; Golubkina, N.A.; Papazyan, T.T.; Senkevich, O.A. The human selenium status of Khabarovsk land population 2018. Trace Elem. Med. 2019, 20, 45–53. (In Russian) [Google Scholar] [CrossRef]
- Gupta, M.; Gupta, S. An Overview of Selenium Uptake, Metabolism, and Toxicity in Plants. Front. Plant Sci. 2017, 7, 2074. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jensen, P.D.; Rivas, M.D.; Trumble, J.T. Developmental responses of a terrestrial insect detritivore, Megaselia scalaris (Loew) to four selenium species. Ecotoxicology 2005, 14, 313. [Google Scholar] [CrossRef] [Green Version]
- Trumble, J.T.; Kund, G.S.; White, K.K. Influence of form and quantity of selenium on the development and survival of an insect herbivore. Environ. Pollut. 1998, 101, 175–182. [Google Scholar] [CrossRef]
- Vickerman, D.B.; Trumble, J.T. Feeding preferences of Spodoptera exigua in response to form and concentration of selenium. Arch. Insect Biochem. Physiol. 1999, 42, 64–73. [Google Scholar] [CrossRef]
- Mogren, C.L.; Trumble, J.T. The ipacts of metals and metalloids on insect behabior. Entomol. Exp. Applicata. 2010, 135, 1–17. [Google Scholar] [CrossRef]
- Vickerman, D.B.; Shannon, M.C.; Bañuelos, G.S.; Grieve, C.M.; Trumble, J.T. Evaluation of Atriplex lines for selenium accumulation, salt tolerance and suitability for a key agricultural insect pest. Environ. Pollut. 2002, 120, 463–473. [Google Scholar] [CrossRef] [Green Version]
- Vickerman, D.B.; Trumble, J.T. Biotransfer of selenium: Effects on an insect predator, Podisus maculiventris. Ecotoxicology 2003, 12, 497–504. [Google Scholar] [CrossRef]
- Zhou, C.; Li, D.; Shi, X.; Zhang, J.; An, Q.; Wu, Y.; Kang, L.; Li, J.-Q.; Pan, C. Nanoselenium Enhanced Wheat Resistance to Aphids by Regulating Biosynthesis of DIMBOA and Volatile Components. Agric. Food Chem. 2021, 69, 14103–14114. [Google Scholar] [CrossRef]
- Golubkina, N.; Moldovan, A.; Kekina, H.; Kharchenko, V.; Sekara, A.; Vasileva, V.; Skrypnik, L.; Tallarita, A.; Caruso, G. Joint Biofortification of Plants with Iodine and Selenium: A New Field of Discoveries. Plants 2021, 10, 1352. [Google Scholar] [CrossRef] [PubMed]
- Pilon-Smits, E.A.H. On the ecology of selenium accumulation in pants. Plants 2019, 8, 197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freeman, J.L.; Quinn, C.F.; Lindblom, S.D.; Klamper, E.M.; Pilon-Smits, E.A.H. Selenium protects the hyperaccumulator Stanleya pinnata against black-tailed prairie dog herbivory in native seleniferous habitats. Am. J. Bot. 2009, 96, 1075–1085. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Mehdawi, A.F.; Pilon-Smits, E.A.H. Ecological aspects of plant selenium hyperaccumulation. Plant Biol. 2012, 14, 1–10. [Google Scholar] [CrossRef]
- Mechora, Š.; Ugrinović, K. Can Plant—Herbivore Interaction be affected by Selenium? Austin J. Environ. Toxicol. 2015, 1, 5. [Google Scholar]
- Moxon, A.L. Selenium: Its occurrence in the rocks and soils, absorption by plants, toxic action in animals, and possible essential role in animal nutrition. In Trace Elements: Proceedings of the Conference, Agricultural Experiment Station, Wooster, Ohio, 14–16 October 1957; Lamb, C.A., Bentley, O.G., Beattie, J.M., Eds.; Academic Press: New York, NY, USA, 1958; pp. 175–191. [Google Scholar]
- Hanson, B.; Lindblom, S.D.; Loeffler, M.L.; Pilon-Smits, E.A.H. Selenium protects plants from phloem-feeding aphids due to both deterrence and toxicity. New Phytol. 2004, 162, 655–662. [Google Scholar] [CrossRef] [PubMed]
- Hanson, B.; Garifullina, G.F.; Lindblom, S.D.; Wangeline, A.; Ackley, A.; Kramer, K.; Norton, A.P.; Lawrence, C.B.; Pilon-Smits, E.A.H. Selenium accumulation protects Brassica juncea from invertebrate herbivory and fungal infection. New Phytol. 2003, 159, 461–469. [Google Scholar] [CrossRef] [Green Version]
- Freeman, J.L.; Lindblom, S.D.; Quinn, C.F.; Fakra, S.; Marcus, M.A.; Pilon-Smits, E.A. Selenium accumulation protects plants from herbivory by Orthoptera via toxicity and deterrence. New Phytol. 2007, 175, 490–500. [Google Scholar] [CrossRef] [Green Version]
- Golubkina, N.; Sheshnitsan, S.; Kapitalchuk, M. Ecological Importance of Insects in Selenium Biogenic Cycling. Int. J. Ecol. 2014, 2014, 835636. [Google Scholar] [CrossRef] [Green Version]
- Mechora, S. Selenium as a Protective Agent Against Pests: A Review. Plants 2019, 8, 262. [Google Scholar] [CrossRef] [Green Version]
- Prins, C. Effect of Elevated Plant Selenium Levels on Reproduction and Root-Nematode Interaction. Master’s Thesis, Colorado State University, Fort Collins, CO, USA, 2011. [Google Scholar]
- Mechora, Š.; Placido Torres, D.; Bruns, R.E.; Škof, M.; Ugrinović, K. Effect of selenium treated broccoli on herbivory and oviposition preferences of Delia radicum and Phyllotreta spp. Sci. Hortic. 2017, 225, 445–453. [Google Scholar] [CrossRef]
- Golubkina, N.; Skryabin, K. Anomalous accumulation of selenium by genetically modified potato, stable to Colorado beetle. J. Food Comp. Anal. 2010, 23, 190–193. [Google Scholar] [CrossRef]
- Quinn, C.F.; Freeman, J.L.; Galeas, M.L.; Klamper, E.M.; Pilon-Smits, E.A.H. The role of selenium in protecting plants against prairie dog herbivory: Implications for the evolution of selenium hyperaccumulation. Oecologia 2008, 155, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Quinn, C.F.; Freeman, J.L.; Reynolds, R.J.B.; Cappa, J.J.; Fakra, S.C.; Marcus, M.A.; Lindblom, S.D.; Quinn, E.K.; Bennett, L.E.; Pilon-Smits, E.A.H. Selenium hyperaccumulation offers protection from cell disruptor herbivores. BMC Ecol. 2010, 10, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Javeed, H.M.R.; Qamar, R.; Zamir, S.I.; Atique-ur-Rehman; Nawaz, F.; Faheem, M.M.; Ali, M.; Rehman, A.; Farooq, A.; Zakir, A. Selenate uptake through roots improves the tolerance to cell distuptors and fungal infection in Canola seedlings after exogenous selenium application. Pak. J. Agric. Sci. 2019, 56, 329–337. [Google Scholar] [CrossRef]
- Hogan, G.R.; Razniak, H.G. Selenium-induced mortality and tissue distribution studies in Tenebrio molitor (Coleoptera: Tenebrionidae). Environ. Entomol. 1991, 20, 790–794. [Google Scholar] [CrossRef]
- Popham, H.J.R.; Shelbya, K.S.; Popham, T.W. Effect of dietary selenium supplementation on resistance to baculovirus infection. Biol. Control 2005, 32, 419–426. [Google Scholar] [CrossRef]
- Kastori, R.; Kadar, I. Effect of Selenium, Molybdenum and Zinc on Seedling Growth and Frequency of Grain Weevil (Sitophilus granarius) in Triticale grains. Pestic. I Fitomedicina 2009, 24, 133–138. [Google Scholar] [CrossRef]
- Quinn, C.F.; Prins, C.N.; Freeman, J.L.; Gross, A.M.; Hantzis, L.J.; Reynolds, R.J.B.; Yang, S.; Covey, P.A.; Bañuelos, G.S.; Pickering, I.J.; et al. Selenium accumulation in flowers and its effects on pollination. New Phytol. 2011, 192, 727–737. [Google Scholar] [CrossRef]
- Alburaki, M.; Smith, K.D.; Adamczyk, J.; Karim, S. Interplay between Selenium, selenoprotein genes, and oxidative stress in honey bee Apis mellifera L. J. Insect Physiol. 2019, 117, 103891. [Google Scholar] [CrossRef]
- Luyckx, M.; Hausman, J.-F.; Lutts, S.; Guerriero, G. Silicon and Plants: Current Knowledge and Technological Perspectives. Front. Plant Sci. 2017, 8, 411. [Google Scholar] [CrossRef] [Green Version]
- Ma, J.F. Role of silicon in enhancing the resistance of plants to biotic and abiotic stresses. Soil Sci. Plant Nutr. 2004, 50, 11–18. [Google Scholar] [CrossRef]
- Liang, Y.; Nikolic, M.; Belanger, R.; Gong, H.; Song, A. Silicon and insect pest resistance. In Silicon in Agriculture; Springer: Berlin, Germany, 2015; pp. 197–207. [Google Scholar] [CrossRef]
- Hou, M.L.; Han, Y.Q. Si-mediated rice plant resistance to the Asiatic rice borer: Effects of silicon amendment and rice varietal resistance. J. Econ. Entomol. 2010, 103, 1412–1419. [Google Scholar] [CrossRef]
- Dias, P.A.S.; Sampaio, M.V.; Rodrigues, M.P.; Korndorfer, A.P.; Oliveira, R.S.; Ferreira, S.E.; Korndörfer, G.H. Induction of resistance by silicon in wheat plants to alate and apterous morphs of Sitobion avenae (Hemiptera: Aphididae). Environ. Entomol. 2014, 43, 949–956. [Google Scholar] [CrossRef]
- Leroy, N.; de Tombeur, F.; Walgraffe, Y.; Cornélis, J.-T.; Verheggen, F.J. Silicon and Plant Natural Defenses against Insect Pests: Impact on Plant Volatile Organic Compounds and Cascade Effects on Multitrophic Interactions. Plants 2019, 8, 444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, A.; Kumar, A.; Hartley, S.; Singh, I. Silicon: Its ameliorative effect on plant defense against herbivory. J. Exp. Bot. 2020, 71, 6730–6743. [Google Scholar] [CrossRef] [PubMed]
- Islam, W.; Tayyab, M.; Khalil, F.; Hua, Z.; Huang, Z.; Chen, H.Y.H. Silicon-mediates plants defense against pathogens and insect pests. Pestic Biochem. Physiol. 2020, 168, 104641. [Google Scholar] [CrossRef]
- Waterman, J.M.; Cibils-Stewart, X.; Cazzonelli, C.I.; Hartley, S.E.; Johnson, S.N. Short-term exposure to silicon rapidly enhances plant resistance to herbivory. Ecology 2021, 102, e03438. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.N.; Hartley, S.E.; Ryalls, J.M.W.; Frew, A.; Hall, C.R. Targeted plant defense: Silicon conserves hormonal defense signaling impacting chewing but not fluid-feeding herbivores. Ecology 2021, 102, e03250. [Google Scholar] [CrossRef] [PubMed]
- Thabet, A.F.; Boraei, H.A.; Galal, O.A.; El-Samahy, M.F.M.; Mousa, K.M.; Zhang, Y.Z.; Tuda, M.; Helmy, E.A.; Wen, J.; Nozaki, T. Silica nanoparticles as pesticide against insects of different feeding types and their non-target attraction of predators. Sci. Rep. 2021, 11, 14484. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, A.; Tripathi, D.K.; Yadav, S.; Chauhan, D.K.; Zivcak, M.; Ghorbanpour, M.; El-Sheery, N.I.; Brestic, M. Application of silicon nanoparticles in agriculture. 3 Biotech 2019, 9, 90. [Google Scholar] [CrossRef] [Green Version]
- Bhat, J.A.; Rajora, N.; Raturi, G.; Sharma, S.; Dhiman, P.; Sanand, S.; Shivaraj, S.M.; Sonah, H.; Deshmukh, R. Silicon nanoparticles (SiNPs) in sustainable agriculture: Major emphasis on the practicality, efficacy and concerns. Nanoscale Adv. 2021, 3, 4019. [Google Scholar] [CrossRef]
- He, W.; Yang, M.; Li, Z.; Qui, J.; Liu, F.; Qu, X.; Qiu, Y.; Li, R. High levels of silicon provided as a nutrient in hydroponic culture enhances rice plant resistance to brown planthopper. Crop Prot. 2015, 6, 20–25. [Google Scholar] [CrossRef]
- Parrella, M.P.; Costamagna, T.P.; Kaspi, R. The addition of potassium silicate to the fertilizer mix to suppress Liriomyza leafminers attacking chrysanthemums. Acta Hortic. 2007, 747, 365–369. [Google Scholar] [CrossRef]
- Reynolds, O.L.; Keeping, M.G.; Meyer, J.H. Silicon-augmented resistance of plants to herbivorous insects: A review. Ann. Appl. Biol. 2009, 155, 171–186. [Google Scholar] [CrossRef]
- Alhousari, F.; Greger, M. Silicon and mechanism of plant resistance to insect pests. Plants 2018, 7, 33. [Google Scholar] [CrossRef] [Green Version]
- Peixtot, M.L.; Moraes, J.C.; Silva, A.A.; Assis, F.A. Effect of silicon on the oviposition preference of Bemisia tabaci Biotype B (GENN.) (Hemiptera: Aleyrodidae) on bean (Phaseolus vulgaris L.) plants. Cienc. Agrotec. 2011, 35, 478–481. [Google Scholar] [CrossRef]
- Correa, R.S.B.; Moraes, J.C.; Auad, A.M.; Carvalho, G.A. Silicon and acibenzolar-S-methyl as resistance inducers in cucumber, against the whitefly Bemisia tabaci (Gennadius) (Hemiptera: Aleyrodidae) biotype B. Neotrop. Entomol. 2005, 34, 429–433. [Google Scholar] [CrossRef]
- Faraone, N.; Hillier, N.K. Preliminary evaluation of a granite rock dust product for pest herbivore management in field conditions. Insects 2020, 11, 877. [Google Scholar] [CrossRef]
- Acevedo, F.E.; Peiffer, M.; Ray, S.; Tan, C.-W.; Felton, G.V. Silicon-mediated enhancement of herbivore resistance in agricultural crops. Front. Plant Sci. 2021, 2, 631824. [Google Scholar] [CrossRef]
- Assis, F.A.; Moraes, J.C.; Auad, A.M.; Coelho, M. The effects of foliar spray application of silicon on plant damage levels and components of larval biology of the pest butterfly Chlosyne lacinia saundersii (Nymphalidae). Int. J. Pest Manag. 2013, 59, 128–134. [Google Scholar] [CrossRef]
- Frew, A.; Powell, J.R.; Hiltpold, I.; Allsopp, P.G.; Sallam, N.; Johnson, S.N. Host plant colonisation by arbuscular mycorrhizal fungi stimulates immune function whereas high root silicon concentrations diminish growth in a soil-dwelling herbivore. Soil Biol. Biochem. 2017, 112, 117–126. [Google Scholar] [CrossRef]
- Assis, F.A.E.; Jair, C.M.; Luis, C.P.S.; Jonas, F.; Amanda, M.N.; Cristiana, S.A. Inducers of resistance in potato and its effects on defoliators and predatory insects. Rev. Colomb. Entomol. 2012, 38, 30–34. [Google Scholar]
- Wang, J.; Xue, R.; Ju, X.; Yan, H.; Gao, Z.; Elzaki, M.E.A.; Hu, L. Silicon-mediated multiple interactions: Simultaneous induction of rice defense and inhibition of larval performance and insecticide tolerance of Chilo suppressalis by sodium silicate. Ecol. Evol. 2020, 10, 4816–4827. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Han, Y.Q.; Li, P.; Wen, L.; Hou, M. Silicon amendment to rice plants impairs sucking behaviors and population growth in the phloem feeder Nilaparvata lugens (Hemiltera: Delphacidae). Sci. Rep. 2017, 7, 1101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costa, R.R.; Moraes, J.C.; DaCosta, R.R. Feeding behaviour of the greenbug Schizaphis graminum on wheat plants treated with imidacloprid and/or silicon. J. Appl. Entomol. 2011, 135, 115–120. [Google Scholar] [CrossRef]
- Gomes, F.B.; Jair, C.D.M.; Custódio, D.D.S.; Márcio, M.G. Resistance induction in wheat plants by silicon and aphids. Sci. Agric. 2005, 62, 547–551. [Google Scholar] [CrossRef]
- Bayan, L.; Koulivand, P.H.; Gorji, A. Garlic: A review of potential therapeutic effects. Avicenna J. Phytomed. 2014, 4, 1–14. [Google Scholar]
- Fesseha, H.; Goa, E. Therapeutic value of garlic (Allium sativum): A Review. Adv. Food Technol. Nutr. Sci. Open J. 2019, 5, 107–117. [Google Scholar] [CrossRef]
- Ali, M.; Ahmad, H.; Hayat, S.; Chani, M.I.; Aim, B.; Atif, M.J.; Wali, K.; Cgeng, A. Application of garlic allelochemisals improves frowth and induces defense repsonses in eggplant (Solanum melongena) against Verticillium dahliae. Ecotoxicol. Environ. Saf. 2021, 215, 112132. [Google Scholar] [CrossRef]
- Wanyika, H.N.; Gachanja, A.N.; Kenji, G.M.; Keriko, J.M.; Mwangi, A.N. A rapid method based on UV spectrophotometry for quantitative determination of allicin in aqueous garlic extracts. J. Agric. Sci. Technol. 2011, 12, 77–84. [Google Scholar]
- Blanchard, A.; Limache, F. Les Stimulateurs des Defenses Naturelles des Plantes (SDN). 2005. Available online: https://hal.archives-ouvertes.fr/hal-01857661/document (accessed on 15 October 2021).
- Hardiansyah, M.Y.; Al Ridho, A.F. Nurhidayat The Effect of Garlic (Allium sativum) Extract Pesticides in Repelling Rice Eating Bird Pests. Indonesian J. Agric. Res. 2020, 3, 145–152. [Google Scholar] [CrossRef]
- Sharaby, A.; El-Nojiban, A. Evaluation of some plant essential oils against the black cutworm Agrotis ipsilon. Glob. J. Adv. Res. 2015, 2, 701–711. [Google Scholar]
- Kim, S.I.; Chae, S.H.; Youn, H.S.; Yeon, S.H.; Ahn, Y.J. Contact and fumigant toxicity of plant essential oils and efficacy of spray formulations containing the oils against B- and Q-biotypes of Bemisia tabaci. Pest Manag. Sci. 2011, 67, 1093–1099. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.N.; Zhang, H.; Zhang, X.C.; Luan, X.B.; Zhou, C.; Liu, Q.Z.; Shi, W.P.; Liu, Z.L. Evaluation of acute toxicity of essential oil of garlic (Allium sativum) and its selected major constituent compounds against overwintering Cacopsylla chinensis (Hemiptera: Psyllidae). J. Econ. Entomol. 2013, 102, 1349–1354. [Google Scholar] [CrossRef]
- Douri, A.; Bougdad, L.F.; Assobhei, O.; Moumni, M. Chemical composition and biological activity of Allium sativum essential oils against Callosobruchus maculatus. J. Environ. Sci. 2013, 3, 30–36. [Google Scholar] [CrossRef]
- Hincapie, C.A.; Lopez, G.E.; Torres, R. Comparison and characterization of garlic (Allium sativum L.) bulbs extracts and their effect on mortality and repellency of Tetranychus urticae Koch (Acari: Tetranychidae). Chil. J. Agric. Res. 2008, 68, 317–327. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.; Singh, B.; Singh, A.P. Nematodes: A threat to sustainability of agriculture. Procedia Environ. Sci. 2015, 9, 215–216. [Google Scholar] [CrossRef] [Green Version]
- Chaubey, M. Fumigant and contact toxicity of Allium sativum (Alliaceae) essential oil against Sitophilus oryzae L. (Coleoptera: Dryophthoridae). Entomol. Appl. Sci. Lett. 2016, 3, 43–48. [Google Scholar]
- Vassiliou, V.A. Botanical Insecticides in Controlling Kelly’s Citrus Thrips (Thysanoptera: Thripidae) on Organic Grapefruits. J. Econ. Entomol. 2011, 104, 1979–1985. [Google Scholar] [CrossRef]
- Denloy, A.A. Bioactivity of Powder and Extracts from Garlic, Allium sativum L. (Alliaceae) and Spring Onion, Allium fistulosum L. (Alliaceae) against Callosobruchus maculatus F. (Coleoptera: Bruchidae) on Cowpea, Vigna unguiculata (L.) Walp (Leguminosae) Seeds. J. Enthomol. 2010, 2010, 958348. [Google Scholar] [CrossRef] [Green Version]
- Al-Shuraym, L.A.M.; Al-Keridis, L.A.; Al-Dakhil, A.A.; Al-Qahtani, W.S. The impact of onion-garlic mixture to control of Rhynchophorus ferrugineus in Saudi Arabia. J. Saudi Soc. Agric. Sci. 2020, 19, 521–527. [Google Scholar] [CrossRef]
- Baidoo, P.K.; Mochiah, M.B. Comparing the Effectiveness of Garlic (Allium sativum L.) and Hot Pepper (Capsicum frutescens L.) in the Management of the Major Pests of Cabbage Brassica oleracea (L.). Int. Sustain. Agric. Res. 2016, 5, 83–91. [Google Scholar] [CrossRef] [Green Version]
- González-Macedo, M.; Cabiro, L.N.; Rojas-Oropeza, M. Assessment of the ancestral use of garlic (Allium sativum) and nettle (Urtica dioica) as botanical insecticides in the protection of mesquite (Prosopis laevigata) seeds against bruchins. J. Plant Prot. Res. 2021, 61, 170–175. [Google Scholar] [CrossRef]
- Rinaldi, S.; Casorri, L.; Masciarelli, E.; Beni, C. Prospects of using garlic extracts for pest control in sustainable agriculture. Fres. Environ. Bull. 2019, 28, 535–540. [Google Scholar]
- Nwachukwu, I.D.; Asawalam, E.F. Evaluation of freshly prepared juice from garlic (Allium sativum L.) as a biopesticide against the maize weevil, Sitophilus zeamais (Motsch.) (Coleoptera: Curculionidae). J. Plant Prot. Res. 2014, 54, 132–138. [Google Scholar] [CrossRef] [Green Version]
- Prowse, G.M.; Galloway, T.S.; Foggo, A. Insecticidal activity of garlic juice in two dipteran pests. Agric. For. Entomol. 2006, 8, 1–6. [Google Scholar] [CrossRef]
- Park, I.K.; Choi, K.S.; Kim, D.H.; Choi, I.H.; Kim, L.S.; Bak, W.C.; Choi, J.W.; Shin, S.C. Fumigant activity of plant essential oils and components from horseradish (Armoracia rusticana), anise (Pimpinella anisum) and garlic (Allium sativum) oils against Lycoriella ingenua (Diptera: Sciaridae). Pest Manag. Sci. 2006, 62, 723–728. [Google Scholar] [CrossRef] [PubMed]
- Macial, C.M.; Shikano, I.; Smirle, M.; Bradbury, R.; Isman, M.B. Evaluation of the toxicity of 17 essential oils against Choristoneura rosaceana (Lepidoptera: Tortricidae) and Trichoplusia. ni (Lepidoptera: Noctuidae). Pest Manag. Sci. 2010, 66, 1116–1121. [Google Scholar] [CrossRef]
- Anwar, A.; Gould, E.; Tinson, R.; Groom, M.; Hamilton, C.J. Think Yellow and Keep Green—Role of Sulfanes from Garlic in Agriculture. Antioxidants 2017, 6, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, Y.; Lee, S.-H.; Na, J.H.; Chang, P.-S.; Han, J. Protection of Grain Products from Sitophilus oryzae (L.) Contamination by Anti-Insect Pest Repellent Sachet Containing Allyl Mercaptan Microcapsule. J. Food Sci. 2017, 82, 2634–2642. [Google Scholar] [CrossRef]
- Gagné, R.J. A Catalog of the Cecidomyiidae (Diptera) of the World; The Entomological Society of Washington: Washington, DC, USA, 2004. [Google Scholar]
- Harris, M.O.; Foster, S.P. “Gall Midges,” in Pheromones of Non-Lepidopteran Insects Associated with Agricultural Plants; Hardie, J., Ed.; CAB International: Oxford, UK, 1999; pp. 27–49. [Google Scholar]
- Stireman, J.O., III; Janson, E.M.; Carr, T.G.; Devlin, H.; Abbot, P. Evolutionary radiation of Asteromyia Carbonifera (Diptera: Cecidomyiidae) gall morphotypes on the goldenrod Solidago Altissima (Asteraceae). Biol. J. Linnean Soc. 2008, 95, 840–858. [Google Scholar] [CrossRef] [Green Version]
- Mishima, M.; Sato, S.; Tsuda, K.; Yukawa, J. Sexual isolation between two known intraspecific populations of Hartigiola (Diptera: Cecidomyiidae) that induce leaf galls on upper and lower surfaces of Fagus crenata (Fagales: Fagaceae), indicating possible diversification into sibling species. Arthropod Biol. 2013, 107, 789–798. [Google Scholar] [CrossRef] [Green Version]
- Yukawa, J.; Uechi, N.; Tokuda, M.; Sato, S. Radiation of gall midges (Diptera: Cecidomyiidae) in Japan. Basic Appl. Ecol. 2005, 6, 453–461. [Google Scholar] [CrossRef]
- Keunen, E.; Peshev, D.; Vangronsveld, J.; Van Den Ende, W.; Cuypers, A. Plant sugars are crucial players in the oxidative challenge during abiotic stress: Extending the traditional concept. Plant Cell Environ. 2013, 36, 1242–1255. [Google Scholar] [CrossRef]
- Hayat, S.H.; Ali, A.M.; Hayat, K.; Khan, M.A.; Cheng, Z. Aqueous Garlic Extract as a Plant Biostimulant Enhances Physiology, Improves Crop Quality and Metabolite Abundance, and Primes the Defense Responses of Receiver Plants. Appl. Sci. 2018, 8, 1505. [Google Scholar] [CrossRef] [Green Version]
- Massey, F.P.; Hartley, S.E. Physical defenses wear you down: Progressive and irreversible impacts of silica on insect herbivores. J. Anim. Ecol. 2009, 78, 281–291. [Google Scholar] [CrossRef]
- Frew, A.; Weston, L.A.; Reynolds, O.L.; Gurr, G.M. The role of silicon in plant biology: A paradigm shift in research approach. Ann. Bot. 2018, 121, 1265–1273. [Google Scholar] [CrossRef] [Green Version]
- Shivaraj, S.M.; Mandlik, R.; Bhat, J.A.; Raturi, G.; Elbaum, R.; Alexander, L.; Tripathi, D.K.; Deshmukh, R.; Sonah, H. Outstanding Questions on the Beneficial Role of Silicon in Crop Plants. Plant Cell Physiol. PCAB 2021, 145, 1–15. [Google Scholar] [CrossRef]
- Golubkina, N. Selenium Biorhythms and Hormonal Regulation Chapter 2 in Selenium: Sources, Functions and Health Effects; Aomori, C., Hokkaido, M., Eds.; Nova Science Pubishers Inc.: New York, NY, USA, 2012; pp. 33–74. ISBN 989-1-61942-061-8. [Google Scholar]
- Elzaawely, A.A.; Ahmed, M.E.; Maswada, H.F.; Al-Araby, A.A.; Xuan, T.D. Growth traits, physiological parameters and hormonal status of snap bean (Phaseolus vulgaris L.) sprayed with garlic cloves extract. Arch. Agron. Soil Sci. 2018, 62, 1068–1082. [Google Scholar] [CrossRef]
- Ding, H.; Ali, A.; Cheng, Z. An allelopathic role of garlic root exudares on regulation of carbohydrate metabolism in cucumber in a hydroponic co-culture systen. Plants 2020, 9, 45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Rokick Kowther, G.; El-Masry, R.R.; Ahmed, S.A.A.; Mohan, S.A.; Messiha, N.K. Allelopathic effects of Allium sativum cloves on growth and yield of Helianthus annuus plants associating Cyperus rotundus. Int. J. Environ. 2018, 7, 78–86. [Google Scholar]
- Kleiber, T.; Borowiak, K.; Kosiada, T.; Bre’s, W.; Ławniczak, B. Application of selenium and silicon to alleviate short-term drought stress in French marigold (Tagetes patula L.) as a model plant species. Open Chem. 2020, 18, 1468–1480. [Google Scholar] [CrossRef]
- Shalaby, T.A.; Abd-Alkarim, E.; El-Aidy, F.; El-Samahy, H.; Sharaf-Eldin, M.; Taha, N.; El-Ramady, H.; Bayoumi, Y.; Reis, A.R. Nano-selenium, silicon and H2O2 boost growth and productivity of cucumber under combined salinity and heat stress. Ecotoxicol. Environ. Saf. 2021, 212, 111962. [Google Scholar] [CrossRef] [PubMed]
- Zahedi, S.M.; Moharrami, F.; Sarikhani, S.; Padervand, M. Selenium and silica nanostructure-based recovery of strawberry plants subjected to drought stress. Sci. Rep. 2021, 10, 17672. [Google Scholar] [CrossRef]
- Guo, K.; Hu, A.; Wang, K.; Wang, L.; Fu, D.; Hao, Y.; Wang, Y.; Ali, A.; Adeel, M.; Rui, Y.; et al. Effects of spraying nano-materials on the absorption of metal(loid)s in cucumber. IET Nanobiotechnol. 2019, 13, 712–719. [Google Scholar] [CrossRef]
- Badawy, S.A.; Zayed, B.A.; Bassiouni, S.M.A.; Mahdi, A.H.A.; Majrashi, A.; Ali, E.F.; Seleiman, M.F. Influence of Nano Silicon and Nano Selenium on Root Characters, Growth, Ion Selectivity, Yield, and Yield Components of Rice (Oryza sativa L.) under Salinity Conditions. Plants 2021, 10, 1657. [Google Scholar] [CrossRef]
- Golubkina, N.; Moldovan, A.; Fedotov, M.; Kekina, H.; Kharchenko, V.; Folmanis, G.; Alpatov, A.; Caruso, G. Iodine and Selenium Biofortification of Chervil Plants Treated with Silicon Nanoparticles. Plants 2021, 10, 2528. [Google Scholar] [CrossRef]




| Insects, Birds | Plant | Stage of Development | LC50 (mg L−1) | References |
|---|---|---|---|---|
| Garlic oil | ||||
| Black cutworm (Agrotis ipsilon) | Rice, beet, cotton, blackberry | Eggs, Larvae, Pupae | 60 190 90 | [81] |
| Silverleaf whitefly (Benisia tabaci) | Tomato, cucumber, pumpkin, cotton, melon, Brassica | Imago | 150 | [82] |
| Cacopsylla chinensis | Plum | Imago | 142 | [83] |
| Cowpea weevil (Calloso-bruchus maculatus) | Vigna chinensis | Imago | 0.25 | [84] |
| Grasshopper (Heteracris littoralis) | Maize, rice, vegetables, cotton | First instar Larvae | 670 | [85] |
| Cabbage looper (Trichoplusia ni) | Cabbage | Larvae | 3300 | [86] |
| Rice weevil (Sitophilus oryzae) | Rice | Imago | 0.017 | [87] |
| Garlic extracts | ||||
| Kelly’s citrus thrips (Pezothrips kellyanus) | Citrus | Larvae | Low protection level | [88] |
| Cowpea weevil (Callosobruchus maculatus) | Chickpea seeds, Vigna unguiculata | Imago | 0.11 g L−1 | [89] |
| red palm weevil Rhynchophorus ferrugineus | Palms | Larvae | * 44 µg mL−1 | [90] |
| Diamondback moth (Plutella xylostella), Cabbage aphid (Brevicoryne brassicae), Cabbage webworm (Hellula undalis), Cabbage looper (Trichoplusia ni) | Cabbage (Brassica oleracea) | Larvae, imago | 200 g L−1 | [91] |
| Coleoptera, Bruchinae | Prosopis laevigata seeds soaking in 5% during 3 days at 20 °C | Larvae, imago | [92] | |
| Spontaneous herbivory attacks in field conditions | Cucurbita pepo (1% water extract) | - | ** | [93] |
| Garlic juice | ||||
| Maize weevil (Sitophilus zeamais) | Storage of maize grain | Imago | 90% lethal mortality | [94] |
| Cabbage root fly (Delium radicum) | Eggs Larvae Imago | 0.8 % (7 days) 6.8 % (4 days) 0.4 % (2 days) | [95] | |
| Housefly (Musca domestica) | Eggs Larvae Imago | 1.6 % (7 days) 4.5 % (4 days) 2.2% (2 days) | [95] | |
| Diallyl polysulfides | ||||
| Cacopsylla chinensis | Plum | Imago | 11.04 DADS 0.640 DATS | [83] |
| Sciarid fly (Lycoriella ingénue) | Mushrooms, herbs | Larvae | 0.25 DAS 0.087 DADS 0.25 DATS | [96] |
| Maize weevil (Sitophilua zeamais) | Maize | Imago | 5.54 DATS | [97] |
| Red flour beetle (Tribolium castaneum) | Cereals, flour | Imago | 1.02 DATS | |
| Parameter | Control | Siliplant | Se | Se + Siliplant | Garlic |
|---|---|---|---|---|---|
| Presence of live larvae | ++ | − | + | − | − |
| Dry matter, % | 12.29a | 11.67a | 11.25a | 12.48a | 11.56a |
| AOA *, mg GAE g−1 d.w. | 46.1a | 44.1a | 48.6a | 46.1a | 50.5a |
| TP **, mg GAE g−1 d.w. | 14.0b | 15.0ab | 16.0ab | 16.1ab | 17.1a |
| Se, µg Kg−1 d.w. | 146d | 213c | 1080b | 1295a | 150d |
| Monosaccharides, % | 17.0a | 16.9a | 12.6b | 13.9b | 16.5a |
| Disaccharides, % | 0.2d | 1.0c | 2.2b | 2.5b | 3.3a |
| Total sugar, % | 16.8bc | 17.9ab | 14.8c | 16.4bc | 19.8a |
| Water-soluble compounds TDS, mg g−1 | 57.1b | 72.8a | 70.4a | 56.3b | 58.0b |
| Nitrates, mg Kg−1 d.w. | 2968b | 3112ab | 3306a | 2326c | 2750bc |
| Ash, % | 10.0a | 10.9a | 11.3a | 9.1a | 9.1a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Golubkina, N.; Zayachkovsky, V.; Sheshnitsan, S.; Skrypnik, L.; Antoshkina, M.; Smirnova, A.; Fedotov, M.; Caruso, G. Prospects of the Application of Garlic Extracts and Selenium and Silicon Compounds for Plant Protection against Herbivorous Pests: A Review. Agriculture 2022, 12, 64. https://doi.org/10.3390/agriculture12010064
Golubkina N, Zayachkovsky V, Sheshnitsan S, Skrypnik L, Antoshkina M, Smirnova A, Fedotov M, Caruso G. Prospects of the Application of Garlic Extracts and Selenium and Silicon Compounds for Plant Protection against Herbivorous Pests: A Review. Agriculture. 2022; 12(1):64. https://doi.org/10.3390/agriculture12010064
Chicago/Turabian StyleGolubkina, Nadezhda, Vladimir Zayachkovsky, Sergei Sheshnitsan, Liubov Skrypnik, Marina Antoshkina, Anna Smirnova, Mikhail Fedotov, and Gianluca Caruso. 2022. "Prospects of the Application of Garlic Extracts and Selenium and Silicon Compounds for Plant Protection against Herbivorous Pests: A Review" Agriculture 12, no. 1: 64. https://doi.org/10.3390/agriculture12010064
APA StyleGolubkina, N., Zayachkovsky, V., Sheshnitsan, S., Skrypnik, L., Antoshkina, M., Smirnova, A., Fedotov, M., & Caruso, G. (2022). Prospects of the Application of Garlic Extracts and Selenium and Silicon Compounds for Plant Protection against Herbivorous Pests: A Review. Agriculture, 12(1), 64. https://doi.org/10.3390/agriculture12010064