Surface Properties of a Biocompatible Thermoplastic Polyurethane and Its Anti-Adhesive Effect against E. coli and S. aureus
Abstract
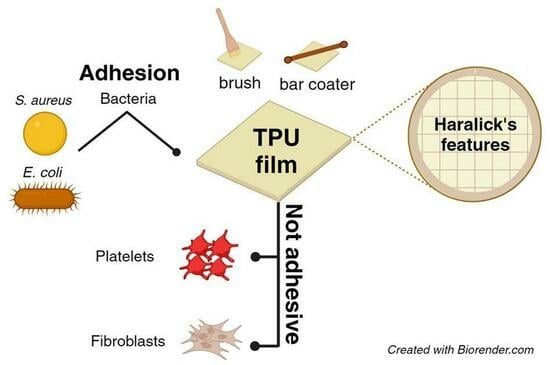
:1. Introduction
2. Materials and Methods
2.1. Material Preparation and Characterization
2.2. Scanning Electron Microscopy (SEM)
2.3. Atomic Force Microscopy (AFM)
2.4. Bacterial Cell Adhesion and Biofilm Formation
2.4.1. Bacterial Strains and Culture Conditions
2.4.2. MTT Assay
2.4.3. Biofilm Formation
2.5. Texture Analysis of SEM Images
2.6. Platelets’ Adhesion
2.7. Enzyme-Linked Immunosorbent Assay (ELISA)
2.8. Fibroblasts’ Viability
2.8.1. Indirect Experiment
2.8.2. Direct Experiment
2.9. SEM of Cells and Platelets
2.10. Statistical Analysis
3. Results
3.1. Evaluation of Morphological and Topographical Properties of TPUs
3.2. Evaluation of Planktonic Bacterial Adhesion on TPUs
3.3. TEXTURE Analysis of the SEM Images for the Prediction of the Bacteria Number in Planktonic Cultures
3.4. Effect of TPU Surfaces on Bacterial Biofilms
3.5. Evaluation of TPU Surface Effect on Platelets, Fibrinogen, and Cells Adhesion
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xu, L.C.; Siedlecki, C.A. Submicron topography design for controlling staphylococcal bacterial adhesion and biofilm formation. J. Biomed. Mater. Res. A 2022, 110, 1238–1250. [Google Scholar] [CrossRef]
- Muszanska, A.K.; Rochford, E.T.J.; Gruszka, A.; Bastian, A.A.; Busscher, H.J.; Norde, W.; Van Der Mei, H.C.; Herrmann, A. Antiadhesive polymer brush coating functionalized with antimicrobial and RGD peptides to reduce biofilm formation and enhance tissue integration. Biomacromolecules 2014, 15, 2019–2026. [Google Scholar] [CrossRef] [PubMed]
- Skovdal, S.M.; Jørgensen, N.P.; Petersen, E.; Jensen-Fangel, S.; Ogaki, R.; Zeng, G.; Johansen, M.I.; Wang, M.; Rohde, H.; Meyer, R.L. Ultra-dense polymer brush coating reduces Staphylococcus epidermidis biofilms on medical implants and improves antibiotic treatment outcome. Acta Biomater. 2018, 76, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Ramasamy, M.; Lee, J. Recent nanotechnology approaches for prevention and treatment of biofilm-associated infections on medical devices. Biomed. Res. Int. 2016, 2016, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Stewart, P.S.; Bjarnsholt, T. Risk factors for chronic biofilm-related infection associated with implanted medical devices. Clin. Microbiol. Infect. 2020, 26, 1034–1038. [Google Scholar] [CrossRef]
- Uddin, T.M.; Chakraborty, A.J.; Khusro, A.; Zidan, B.R.M.; Mitra, S.; Emran, T.B.; Dhama, K.; Ripon, M.K.H.; Gajdács, M.; Sahibzada, M.U.K.; et al. Antibiotic resistance in microbes: History, mechanisms, therapeutic strategies and future prospects. J. Infect. Public Health 2021, 14, 1750–1766. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, J. Tackling drug resistant infections globally: Final report and recommendations. Rev. Antimicrob. Resist. 2016, 1–84. Available online: https://amr-review.org/sites/default/files/160518_Final%20paper_with%20cover.pdf (accessed on 11 January 2024).
- Wang, C.G.; Surat’man, N.E.B.; Mah, J.J.Q.; Qu, C.; Li, Z. Surface antimicrobial functionalization with polymers: Fabrication, mechanisms and applications. J. Mater. Chem. B 2022, 10, 9349–9368. [Google Scholar] [CrossRef]
- Nhu Hieu, V.; Thanh Van, T.T.; Hang, C.T.T.; Mischenko, N.P.; Sergey, A.F.; Truong, H. Polyhydroxynaphthoquinone Pigment from Vietnam Sea Urchins as a Potential Bioactive Ingredient in Cosmeceuticals. Nat. Prod. Commun. 2020, 15, 1–8. [Google Scholar] [CrossRef]
- Zayed, N.; Munjaković, H.; Aktan, M.K.; Simoens, K.; Bernaerts, K.; Boon, N.; Braem, A.; Pamuk, F.; Saghi, M.; Van Holm, W.; et al. Electrolyzed Saline Targets Biofilm Periodontal Pathogens In Vitro. J. Dent. Res. 2024, 1–10. [Google Scholar] [CrossRef]
- Káčerová, S.; Muchová, M.; Doudová, H.; Münster, L.; Hanulíková, B.; Valášková, K.; Kašpárková, V.; Kuřitka, I.; Humpolíček, P.; Víchová, Z.; et al. Chitosan/dialdehyde cellulose hydrogels with covalently anchored polypyrrole: Novel conductive, antibacterial, antioxidant, immunomodulatory, and anti-inflammatory materials. Carbohydr. Polym. 2024, 327, 121640. [Google Scholar] [CrossRef]
- Restivo, E.; Pugliese, D.; Gallichi-Nottiani, D.; Sammartino, J.C.; Bloise, N.; Peluso, E.; Percivalle, E.; Janner, D.; Milanese, D.; Visai, L. Effect of Low Copper Doping on the Optical, Cytocompatible, Antibacterial, and SARS-CoV-2 Trapping Properties of Calcium Phosphate Glasses. ACS Omega 2023, 8, 42264–42274. [Google Scholar] [CrossRef]
- Cai, S.; Wu, C.; Yang, W.; Liang, W.; Yu, H.; Liu, L. Recent advance in surface modification for regulating cell adhesion and behaviors. Nanotechnol. Rev. 2020, 9, 971–989. [Google Scholar] [CrossRef]
- Tudureanu, R.; Handrea-Dragan, I.M.; Boca, S.; Botiz, I. Insight and Recent Advances into the Role of Topography on the Cell Differentiation and Proliferation on Biopolymeric Surfaces. Int. J. Mol. Sci. 2022, 23, 7731. [Google Scholar] [CrossRef]
- Clarke, D.E.; Mccullen, S.D.; Chow, A.; Stevens, M.M. 5.02 Functional Biomaterials. In Comprehensive Biotechnology, 2nd ed.; Pergamon: Oxford, UK, 2011; pp. 3–10. [Google Scholar]
- Kulangara, K.; Leong, K.W. Substrate topography shapes cell function. Soft Matter 2009, 5, 4072–4076. [Google Scholar] [CrossRef]
- Yu, H.; Xu, Y.; Zhang, M.; Zhang, L.; Wu, W.; Huang, K. Magnetically-separable cobalt catalyst embedded in metal nitrate-promoted hierarchically porous N-doped carbon nanospheres for hydrodeoxygenation of lignin-derived species. Fuel 2023, 331, 125917. [Google Scholar] [CrossRef]
- Yu, H.; Xu, Y.; Havener, K.; Zhang, L.; Wu, W.; Liao, X.; Huang, K. Efficient catalysis using honeycomb-like N-doped porous carbon supported Pt nanoparticles for the hydrogenation of cinnamaldehyde in water. Mol. Catal. 2022, 525, 112343. [Google Scholar] [CrossRef]
- Vishnoi, M.; Kumar, P.; Murtaza, Q. Surface texturing techniques to enhance tribological performance: A review. Surf. Interfaces 2021, 27, 101463. [Google Scholar] [CrossRef]
- Haralick, R.; Shanmugam, K.; Dinstein, I. Textural Features for Image Classification. IEEE Trans. Syst. Man. Cybern. 1973, 3, 610–621. [Google Scholar] [CrossRef]
- Brynolfsson, P.; Nilsson, D.; Torheim, T.; Asklund, T.; Karlsson, C.T.; Trygg, J.; Nyholm, T.; Garpebring, A. Haralick texture features from apparent diffusion coefficient (ADC) MRI images depend on imaging and pre-processing parameters. Sci. Rep. 2017, 7, 4041. [Google Scholar] [CrossRef]
- Vrbik, I.; Van Nest, S.J.; Meksiarun, P.; Loeppky, J.; Brolo, A.; Lum, J.J.; Jirasek, A. Haralick texture feature analysis for quantifying radiation response heterogeneity in murine models observed using Raman spectroscopic mapping. PLoS ONE 2019, 14, e0212225. [Google Scholar] [CrossRef]
- Das, A.; Mahanwar, P. A brief discussion on advances in polyurethane applications. Adv. Ind. Eng. Polym. Res. 2020, 3, 93–101. [Google Scholar] [CrossRef]
- Maestri, C.; Plancher, L.; Duthoit, A.; Hébert, R.L.; Di Martino, P. Fungal Biodegradation of Polyurethanes. J. Fungi 2023, 9, 760. [Google Scholar] [CrossRef]
- Borcan, F.; Vlase, T.; Vlase, G.; Popescu, R.; Soica, C.M. The Influence of an Isocyanate Structure on a Polyurethane Delivery System for 2′-Deoxycytidine-5′-monophosphate. J. Funct. Biomater. 2023, 14, 526. [Google Scholar] [CrossRef]
- Wang, J.; Dai, D.; Xie, H.; Li, D.; Xiong, G.; Zhang, C. Biological Effects, Applications and Design Strategies of Medical Polyurethanes Modified by Nanomaterials. Int. J. Nanomed. 2022, 17, 6791–6819. [Google Scholar] [CrossRef]
- Cortella, L.R.X.; Cestari, I.A.; Guenther, D.; Lasagni, A.F.; Cestari, I.N. Endothelial cell responses to castor oil-based polyurethane substrates functionalized by direct laser ablation. Biomed. Mater. 2017, 12, 065010. [Google Scholar] [CrossRef] [PubMed]
- Navas-Gómez, K.; Valero, M.F. Why polyurethanes have been used in the manufacture and design of cardiovascular devices: A systematic review. Materials 2020, 13, 3250. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, K.R.; Zhang, Y.; Magri, A.M.P.; Renno, A.C.M.; Van Den Beucken, J.J.J.P. Biomaterial Property Effects on Platelets and Macrophages: An in Vitro Study. ACS Biomater. Sci. Eng. 2017, 3, 3318–3327. [Google Scholar] [CrossRef]
- Pavithra, D.; Doble, M. Biofilm formation, bacterial adhesion and host response on polymeric implants—Issues and prevention. Biomed. Mater. 2008, 3, 034003. [Google Scholar] [CrossRef] [PubMed]
- Εkonomou, S.; Soe, S.; Stratakos, A.C. An explorative study on the antimicrobial effects and mechanical properties of 3D printed PLA and TPU surfaces loaded with Ag and Cu against nosocomial and foodborne pathogens. J. Mech. Behav. Biomed. Mater. 2023, 137, 105536. [Google Scholar] [CrossRef]
- Feng, Q.; Fan, B.; He, Y.-C.; Ma, C. Antibacterial, antioxidant and fruit packaging ability of biochar-based silver nanoparticles-polyvinyl alcohol-chitosan composite film. Int. J. Biol. Macromol. 2023, 256, 128297. [Google Scholar] [CrossRef]
- Fu, Y.; Dudley, E.G. Antimicrobial-coated films as food packaging: A review. Compr. Rev. Food Sci. Food Saf. 2021, 20, 3404–3437. [Google Scholar] [CrossRef]
- Villani, M.; Consonni, R.; Canetti, M.; Bertoglio, F.; Iervese, S.; Bruni, G.; Visai, L.; Iannace, S.; Bertini, F. Polyurethane-based composites: Effects of antibacterial fillers on the physical-mechanical behavior of thermoplastic polyurethanes. Polymers 2020, 12, 362. [Google Scholar] [CrossRef] [PubMed]
- Villani, M.; Bertoglio, F.; Restivo, E.; Bruni, G.; Iervese, S.; Arciola, C.R.; Carulli, F.; Iannace, S.; Bertini, F.; Visai, L. Polyurethane-based coatings with promising antibacterial properties. Materials 2020, 13, 4296. [Google Scholar] [CrossRef] [PubMed]
- Sader, J.E.; Chon, J.W.M.; Mulvaney, P. Calibration of rectangular atomic force microscope cantilevers. Rev. Sci. Instrum. 1999, 70, 3967–3969. [Google Scholar] [CrossRef]
- Trespidi, G.; Scoffone, V.C.; Barbieri, G.; Marchesini, F.; Abualsha’ar, A.; Coenye, T.; Ungaro, F.; Makarov, V.; Migliavacca, R.; De Rossi, E.; et al. Antistaphylococcal activity of the FtsZ inhibitor C109. Pathogens 2021, 10, 886. [Google Scholar] [CrossRef]
- Pallavicini, P.; Arciola, C.R.; Bertoglio, F.; Curtosi, S.; Dacarro, G.; D’Agostino, A.; Ferrari, F.; Merli, D.; Milanese, C.; Rossi, S.; et al. Silver nanoparticles synthesized and coated with pectin: An ideal compromise for anti-bacterial and anti-biofilm action combined with wound-healing properties. J. Colloid Interface Sci. 2017, 498, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Bloise, N.; Fassina, L.; Focarete, M.L.; Lotti, N.; Visai, L. Haralick’s texture analysis to predict cellular proliferation on randomly oriented electrospun nanomaterials. Nanoscale Adv. 2022, 4, 1330–1335. [Google Scholar] [CrossRef]
- Guerra-Flórez, D.Y.; Valencia-Osorio, L.M.; Zapata-González, A.F.; Álvarez-Láinez, M.L.; Cadavid-Torres, E.; Meneses-Ramírez, E.A.; Torres-Osorio, V.; Botero-Valencia, J.S.; Pareja-López, A. In vitro toxicity of fine and coarse particulate matter on the skin, ocular and lung microphysiological cell-culture systems. Toxicology 2023, 500, 153685. [Google Scholar] [CrossRef] [PubMed]
- Gatto, M.L.; Furlani, M.; Giuliani, A.; Cabibbo, M.; Bloise, N.; Fassina, L.; Petruczuk, M.; Visai, L.; Mengucci, P. Combined Effects of HA Concentration and Unit Cell Geometry on the Biomechanical Behavior of PCL/HA Scaffold for Tissue Engineering Applications Produced by LPBF. Materials 2023, 16, 4950. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, L.; Levänen, E. Superhydrophobic surfaces for the reduction of bacterial adhesion. RSC Adv. 2013, 3, 12003–12020. [Google Scholar] [CrossRef]
- Sharma, S.; Jaimes-Lizcano, Y.A.; McLay, R.B.; Cirino, P.C.; Conrad, J.C. Subnanometric Roughness Affects the Deposition and Mobile Adhesion of Escherichia coli on Silanized Glass Surfaces. Langmuir 2016, 32, 5422–5433. [Google Scholar] [CrossRef]
- Wu, X.; Jia, H.; Fu, W.; Li, M.; Pan, Y. Enhanced Tensile Properties, Biostability, and Biocompatibility of Siloxane–Cross-Linked Polyurethane Containing Ordered Hard Segments for Durable Implant Application. Molecules 2023, 28, 2464. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Ju, Y.M.; Kim, D.M. Platelet adhesion onto segmented polyurethane film surfaces modified by addition and crosslinking of PEO-containing block copolymers. Biomaterials 2000, 21, 683–691. [Google Scholar] [CrossRef]
- Shin, E.K.; Park, H.; Noh, J.Y.; Lim, K.M.; Chung, J.H. Platelet shape changes and cytoskeleton dynamics as novel therapeutic targets for anti-thrombotic drugs. Biomol. Ther. 2017, 25, 223–230. [Google Scholar] [CrossRef]
- Cho, J.; Kim, H.; Song, J.; Cheong, J.W.; Shin, J.W.; Yang, W.I.; Kim, H.O. Platelet storage induces accelerated desialylation of platelets and increases hepatic thrombopoietin production. J. Transl. Med. 2018, 16, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Apte, G.; Börke, J.; Rothe, H.; Liefeith, K.; Nguyen, T.H. Modulation of Platelet-Surface Activation: Current State and Future Perspectives. ACS Appl. Bio. Mater. 2020, 3, 5574–5589. [Google Scholar] [CrossRef] [PubMed]
- Al Nakib, R.; Toncheva, A.; Fontaine, V.; Vanheuverzwijn, J.; Raquez, J.M.; Meyer, F. Design of Thermoplastic Polyurethanes with Conferred Antibacterial, Mechanical, and Cytotoxic Properties for Catheter Application. ACS Appl. Bio. Mater. 2022, 5, 5532–5544. [Google Scholar] [CrossRef]
- Ensoylu, M.; Deliormanli, A.M.; Atmaca, H. Preparation, Characterization, and Drug Delivery of Hexagonal Boron Nitride-Borate Bioactive Glass Biomimetic Scaffolds for Bone Tissue Engineering. Biomimetics 2023, 8, 10. [Google Scholar] [CrossRef]
- Gautrot, J.E.; Trappmann, B.; Oceguera-Yanez, F.; Connelly, J.; He, X.; Watt, F.M.; Huck, W.T.S. Exploiting the superior protein resistance of polymer brushes to control single cell adhesion and polarisation at the micron scale. Biomaterials 2010, 31, 5030–5041. [Google Scholar] [CrossRef]
- Robotti, F.; Bottan, S.; Fraschetti, F.; Mallone, A.; Pellegrini, G.; Lindenblatt, N.; Starck, C.; Falk, V.; Poulikakos, D.; Ferrari, A. A micron-scale surface topography design reducing cell adhesion to implanted materials. Sci. Rep. 2018, 8, 10887. [Google Scholar] [CrossRef]
- Gorji Kandi, S.; Panahi, B.; Zoghi, N. Impact of surface texture from fine to coarse on perceptual and instrumental gloss. Prog. Org. Coat. 2022, 171, 107028. [Google Scholar] [CrossRef]
- Rastogi, V.K.; Samyn, P. Bio-based coatings for paper applications. Coatings 2015, 5, 887–930. [Google Scholar] [CrossRef]
- Crawford, R.J.; Webb, H.K.; Truong, V.K.; Hasan, J.; Ivanova, E.P. Surface topographical factors influencing bacterial attachment. Adv. Colloid Interface Sci. 2012, 179–182, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Zhang, B.; Liu, Y.; Suo, X.; Li, H. Influence of surface topography on bacterial adhesion: A review (Review). Biointerphases 2018, 13, 60801. [Google Scholar] [CrossRef] [PubMed]
- Silhavy, T.J.; Kahne, D.; Walker, S. The bacterial cell envelope. Cold Spring Harb. Perspect. Biol. 2010, 2, a000414. [Google Scholar] [CrossRef]
- Zheng, S.; Bawazir, M.; Dhall, A.; Kim, H.E.; He, L.; Heo, J.; Hwang, G. Implication of Surface Properties, Bacterial Motility, and Hydrodynamic Conditions on Bacterial Surface Sensing and Their Initial Adhesion. Front. Bioeng. Biotechnol. 2021, 9, 643722. [Google Scholar] [CrossRef]
- Xu, L.; Dara, Y.; Magar, S.; Badughaish, A.; Xiao, F. Morphological and rheological investigation of emulsified asphalt/polymer composite based on gray-level co-occurrence matrix. Int. J. Transp. Sci. Technol. 2023, 1–18. [Google Scholar] [CrossRef]
- Liang, Y.; Kou, W.; Lai, H.; Wang, J.; Wang, Q.; Xu, W.; Wang, H.; Lu, N. Improved estimation of aboveground biomass in rubber plantations by fusing spectral and textural information from UAV-based RGB imagery. Ecol. Indic. 2022, 142, 109286. [Google Scholar] [CrossRef]
- Mansour, I.R.; Thomson, R.M. Haralick texture feature analysis for characterization of specific energy and absorbed dose distributions across cellular to patient length scales. Phys. Med. Biol. 2023, 68, 075006. [Google Scholar] [CrossRef]
- Pathak, R.; Bierman, S.F.; D’arnaud, P. Inhibition of bacterial attachment and biofilm formation by a novel intravenous catheter material using an in vitro percutaneous catheter insertion model. Med. Devices Evid. Res. 2018, 11, 427–432. [Google Scholar] [CrossRef]
- Schelin, J.; Wallin-Carlquist, N.; Cohn, M.T.; Lindqvist, R.; Barker, G.C.; Rådström, P. The formation of Staphylococcus aureus enterotoxin in food environments and advances in risk assessment. Virulence 2011, 2, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Uneputty, A.; Dávila-Lezama, A.; Garibo, D.; Oknianska, A.; Bogdanchikova, N.; Hernández-Sánchez, J.F.; Susarrey-Arce, A. Strategies applied to modify structured and smooth surfaces: A step closer to reduce bacterial adhesion and biofilm formation. Colloids Interface Sci. Commun. 2022, 46, 100560. [Google Scholar] [CrossRef]
- Weber, M.; Steinle, H.; Golombek, S.; Hann, L.; Schlensak, C.; Wendel, H.P.; Avci-Adali, M. Blood-Contacting Biomaterials: In Vitro Evaluation of the Hemocompatibility. Front. Bioeng. Biotechnol. 2018, 6, 99. [Google Scholar] [CrossRef] [PubMed]
- Khorasani, M.T.; MoemenBellah, S.; Mirzadeh, H.; Sadatnia, B. Effect of surface charge and hydrophobicity of polyurethanes and silicone rubbers on L929 cells response. Colloids Surf. B Biointerfaces 2006, 51, 112–119. [Google Scholar] [CrossRef]








| TPU Surface | Adhesion | ||||||
|---|---|---|---|---|---|---|---|
| Planktonic Behavior Predicted by Haralick Analysis | Biofilm | PLTs | hFg | NIH-3T3 | |||
| E. coli | S. aureus | E. coli | S. aureus | ||||
| Film | ~1% | ~50% | 80% | 50% | <0.5% | ~15% | <2.5% |
| Brush | ~1% | ~70% | 70% | 70% | <0.5% | ~15% | <2.5% |
| Bar Coater | ~1% | ~25% | 60% | 20% | <0.5% | ~20% | <2.5% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Restivo, E.; Peluso, E.; Bloise, N.; Bello, G.L.; Bruni, G.; Giannaccari, M.; Raiteri, R.; Fassina, L.; Visai, L. Surface Properties of a Biocompatible Thermoplastic Polyurethane and Its Anti-Adhesive Effect against E. coli and S. aureus. J. Funct. Biomater. 2024, 15, 24. https://doi.org/10.3390/jfb15010024
Restivo E, Peluso E, Bloise N, Bello GL, Bruni G, Giannaccari M, Raiteri R, Fassina L, Visai L. Surface Properties of a Biocompatible Thermoplastic Polyurethane and Its Anti-Adhesive Effect against E. coli and S. aureus. Journal of Functional Biomaterials. 2024; 15(1):24. https://doi.org/10.3390/jfb15010024
Chicago/Turabian StyleRestivo, Elisa, Emanuela Peluso, Nora Bloise, Giovanni Lo Bello, Giovanna Bruni, Marialaura Giannaccari, Roberto Raiteri, Lorenzo Fassina, and Livia Visai. 2024. "Surface Properties of a Biocompatible Thermoplastic Polyurethane and Its Anti-Adhesive Effect against E. coli and S. aureus" Journal of Functional Biomaterials 15, no. 1: 24. https://doi.org/10.3390/jfb15010024
APA StyleRestivo, E., Peluso, E., Bloise, N., Bello, G. L., Bruni, G., Giannaccari, M., Raiteri, R., Fassina, L., & Visai, L. (2024). Surface Properties of a Biocompatible Thermoplastic Polyurethane and Its Anti-Adhesive Effect against E. coli and S. aureus. Journal of Functional Biomaterials, 15(1), 24. https://doi.org/10.3390/jfb15010024