Electrochemical Behaviour of Ti/Al2O3/Ni Nanocomposite Material in Artificial Physiological Solution: Prospects for Biomedical Application
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
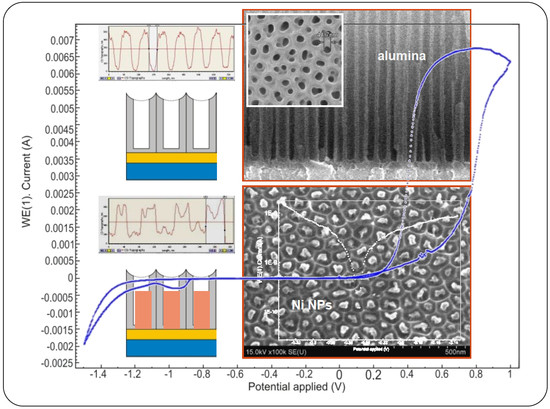
3.1. Microstructure (Topological) Characteristics of Ni–PAAT Composite
3.2. Potentiodynamic Polarization Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Han, S.; Su, L.; Zhai, M.; Ma, L.; Liu, S.; Teng, Y. A moleculary imprinted composite based on graphene oxide for target drug delivery to tumor cells. J. Mater. Sci. 2019, 54, 3331–3341. [Google Scholar] [CrossRef]
- Gerrard, E.; Poinern, J.; Gerrard, E.; Nurshahidah, A.; Derek, F. Progress in nano-engineered anodic aluminum oxide membrane development. Materials 2011, 4, 487–526. [Google Scholar]
- Proenca, M.P.; Sousa, C.T.; Ventura, J.; Vazquez, M.; Araujo, J.P. Distinguishing nanowire and nanotube formation by the deposition current transients. Nanoscale Res. Lett. 2012, 7, 280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dukenbayev, K.; Korolkov, I.V.; Tishkevich, D.I.; Kozlovskiy, A.L.; Trukhanov, S.V.; Gorin, Y.G.; Shumskaya, E.E.; Kaniukov, E.Y.; Vinnik, D.A.; Zdorovets, M.V.; et al. Fe3O4 nanoparticles for complex targeted delivery and boron neutron capture therapy. Nanomaterials 2019, 9, 494. [Google Scholar] [CrossRef] [Green Version]
- Vorobyova, A.I.; Outkina, E.A.; Komar, O.M. Study of metal pillar nanostructure formation with thin porous alumina template. Thin Solid Films 2013, 548, 109–117. [Google Scholar] [CrossRef]
- Vorobyova, A.I.; Outkina, E.A.; Khodin, A.A. Nickel/alumina nanocomposites by AC electrochemical processing. Appl. Phys. A Mater. Sci. Process 2016, 122, 1. [Google Scholar] [CrossRef]
- Bucak, S.; Yavuzturk, B.; Demir, A. Magnetic nanoparticles: Synthesis, surface modifications and application in drug delivery. Recent Adv. Nov. Drug Carr. Syst. 2012, 7, 165–200. [Google Scholar]
- Kaniukov, E.; Shumskaya, A.; Yakimchuk, B.; Kozlovskiy, A.; Korolkov, I.; Ibragimova, M.; Zdorovets, M.; Kadyrzhanov, K.; Rusakov, V.; Fadeev, M.; et al. FeNi nanotubes: Perspective tool for targeted delivery. Appl. Nanosci. 2019, 9, 835–844. [Google Scholar] [CrossRef]
- Kozlovskiy, A.; Zdorovets, M.; Kadyrzhanov, K.; Korolkov, I.; Rusakov, V.; Nikolaevich, L.; Fesenko, O.; Budnyk, O.; Yakimchuk, D.; Shumskaya, A.; et al. FeCo nanotubes: Possible tool for targeted delivery of drugs and proteins. Appl. Nanosci. 2019, 9, 1091. [Google Scholar] [CrossRef]
- Paduano, F.; Marrelli, M.; Alom, N.; Amer, M.; White, L.J.; Shakesheff, K.V.; Tatullo, M. Decellularized bone extracellular matrix and human dental pulp stem cells as a construct for bone regeneration. J. Biomater. Sci. Polym. Ed. 2007, 28, 730. [Google Scholar] [CrossRef] [Green Version]
- Kerativitayanan, P.; Tatullo, M.; Khariton, M.; Joshi, P.; Perniconi, B.; Gaharwar, A.K. Nanoengineered osteoinductive and elastomeric scaffolds for bone tissue engineering. ACS Biomater. Sci. Eng. 2017, 3, 590–600. [Google Scholar] [CrossRef]
- Tishkevich, D.I.; Grabchikov, S.S.; Lastovskii, S.B.; Trukhanov, S.V.; Vasin, D.S.; Zubar, T.I.; Kozlovskiy, A.L.; Zdorovets, M.V.; Sivakov, V.A.; Muradyan, T.R.; et al. Function composites materials for shielding applications: Correlation between phase separation and attenuation properties. J. All. Comp. 2019, 771, 238–245. [Google Scholar] [CrossRef]
- Tishkevich, D.I.; Grabchikov, S.S.; Lastovskii, S.B.; Trukhanov, S.V.; Zubar, T.I.; Vasin, D.S.; Trukhanov, A.V.; Kozlovskiy, A.L.; Zdorovets, M.V. Effect of the synthesis conditions and microstructure for highly effective electron shields production based on Bi coatings. ACS Appl. Energy Mater. 2018, 1, 1695–1702. [Google Scholar] [CrossRef]
- Barry, M.; Pearce, H.; Cross, L.; Tatullo, M.; Gaharwar, A.K. Advances in nanotechnology for the treatment of osteoporosis. Curr. Osteoporos. Rep. 2016, 14, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Tatullo, M.; Marelli, M.; Scacco, S.; Lorusso, M.; D’Oria, S.; Sabatini, R.; Auteri, P.; Cagiano, R.; Inchingolo, F. Relationship between oxidative stress and “burning mouth syndrome” in female patients: A scientific hypothesis. Eur. Rev. Med. Pharmacol. Sci. 2012, 16, 1218. [Google Scholar] [PubMed]
- Chiolerio, A.; Ferrante, I.; Ricci, A.; Marasso, S.L.; Tiberto, P.; Canavese, G.; Ricciardi, C.; Allia, P. Toward mechano-spintronics: Nanostructured magnetic multilayers for the realization of microcantilever sensors featuring wireless actuation for liquid environments. J. Intell. Mater. Syst. Struct. 2012, 24, 2189–2196. [Google Scholar] [CrossRef]
- Sulka, D.G.; Zaraska, L.; Stępniowski, W. Anodic porous alumina as a template for nanofabrication. In Encyclopedia of Nanoscience and Nanotechnology; Nalwa, H.S., Ed.; American Scientific Publishers: Valencia, CA, USA, 2011; Volume 11, p. 261. [Google Scholar]
- Mishra, R.; Basu, B.; Balasubramaniam, R. Effect of grain size on the tribological behavior of nanocrystalline nickel. Mater. Sci. Eng. A 2004, 373, 370–373. [Google Scholar] [CrossRef]
- Mushtaq, M.W.; Kanwai, F.; Batool, A.; Jamil, T.; Zia-ul-Haq, M.; Ijaz, B.; Huang, Q.; Ullah, Z. Polymer-coated CoFe2O4 nanoassemblies as biocompatible magnetic nanocarriers for anticancer drug delivery. J. Mater. Sci. 2017, 52, 9282. [Google Scholar] [CrossRef]
- Vorobjova, A.I.; Shimanovich, D.L.; Sycheva, O.A.; Ezovitova, T.I.; Tishkevich, D.I.; Trykhanov, A.V. Studying the thermodynamic properties of composite magnetic material based on anodic alumina. Rus. Microelectron. 2019, 48, 107–118. [Google Scholar] [CrossRef]
- Bundyukova, V.D.; Yakimchuk, D.V.; Kozlovskiy, A.; Shlimas, D.I.; Tishkevich, D.I.; Kaniukov, E.Y. Synthesis of gold nanostructures using wet chemical deposition in SiO2/Si template. Lith. J. Phys. 2019, 59, 139–145. [Google Scholar] [CrossRef] [Green Version]
- Yakimchuk, D.V.; Khubezhov, S.A.; Bundyukova, V.D.; Kozlovskiy, A.L.; Zdorovets, M.V.; Shlimas, D.I.; Tishkevich, D.I.; Kaniukov, E.Y. Copper nanostructures into pores of SiO2/Si template: Galvanic displacement, chemical and structural characterization. Mater. Res. Express. 2019, 6, 105058. [Google Scholar] [CrossRef]
- Yang, L.; Bashir, K. Electrical/electrochemical impedance for rapid detection of foodborne pathogenic bacteria. Biotechnol. Adv. 2008, 26, 135–150. [Google Scholar] [CrossRef] [PubMed]
- Varshney, M.; Li, Y. Interdigitated array microelectrodes based impedance biosensors for detection of bacterial cells. Biosens. Bioelectron. 2009, 24, 2951–2960. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ng, H.T.; Cassell, A.; Fan, W.; Chen, H.; Ye, Q.; Koehne, J.; Han, J.; Meyyappan, M. Carbon nanotube nanoelectrode array for ultrasensitive DNA detection. Nano Lett. 2003, 3, 597–602. [Google Scholar] [CrossRef]
- Neri, G.; Bonavita, A.; Galvagno, S.; Donato, A.; Caddemi, A. Electrical modeling of Fe2O3 humidity sensors doped with Li+, Zn2+ and Au3+ ions. Sens. Actuators B 2005, 111, 71. [Google Scholar] [CrossRef]
- Moreno-Hagelsiebaa, L.; Nizetb, Y.; Tanga, X.; Raskin, J.P.; Flandre, D.; Francis, L.A. CMOS compatible anodic Al2O3 based sensors for bacteria detection. Proc. Chem. 2009, 1, 1283–1286. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Zhu, X.; Sudagar, J.; Diao, W.; Yu, S. Increased corrosion resistance of closed-cell aluminum foams by electroless Ni-P coatings. Mater. Trans. 2011, 52, 2282–2284. [Google Scholar] [CrossRef] [Green Version]
- Meng, G.; Li, Y.; Shao, Y.; Zhang, T.; Wang, Y.; Wang, F.; Cheng, X.; Dong, C.; Li, X. Effect of microstructures on corrosion behavior of nickel coatings: (II) competitive effect of grain size and twins density on corrosion behavior. J. Mater. Sci. Technol. 2016, 32, 465–469. [Google Scholar] [CrossRef]
- Sudagar, J.; Bi, G.; Jiang, Z.; Li, G.; Jiang, Q.; Lian, J. Electrochemical polarization behaviour of electroless Ni-P deposits with different chromium-free pre-treatment on magnesium alloy. Int. J. Electrochem. Sci. 2011, 6, 2767–2788. [Google Scholar]
- Tishkevich, D.I.; Vorobjova, A.I.; Vinnik, D.A. Formation and corrosion behavior of nickel/alumina nanocomposites. Sol. State. Phen. 2020, 299, 100–106. [Google Scholar] [CrossRef]
- Cheng, X.; Ma, H.; Chen, S.; Chen, X.; Yao, Z. Corrosion of nickel in acid solutions with hydrogen sulphide. Corros. Sci. 2000, 42, 299–311. [Google Scholar] [CrossRef]
- De Gromoboy, T.S.; Shreir, L.L. The formation of nickel oxides during the passivation of nickel in relation to the potential/pH diagram. Electrochim. Acta 1966, 11, 895–904. [Google Scholar] [CrossRef]
- Gu, C.; Lian, J.; He, J.; Jiang, J.; Jiang, Q. High corrosion-resistance nanocrystalline Ni coating on AZ91D magnesium alloy. Surf. Coat.Technol. 2006, 200, 5413–5418. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, J.; Gao, Y.; Xue, Q.; Hu, L.; Xu, T. Grain size effect in corrosion behavior of electrodeposited nanocrystalline Ni coatings in alkaline solution. Scr. Mater. 2006, 55, 657–660. [Google Scholar] [CrossRef]
- Benea, L.; Bonora, P.L.; Borello, A.; Martelli, F.; Wenger, F.; Pontiaux, P.; Galland, J. Composite electrodeposition to obtain nanostructured coatings. J. Electrochem. Soc. 2001, 148, C461–C465. [Google Scholar] [CrossRef]
- Danişman, M.; Cansever, N. Effect of Cr content on mechanical and electrical properties of Ni-Cr thin films. J. Alloys Compd. 2010, 493, 649–653. [Google Scholar] [CrossRef]
- Tishkevich, D.I.; Vorobjova, A.I.; Trukhanov, A.V. Thermal stability of nano-crystalline nickel electrodeposited into porous alumina. Sol. State. Phen. 2020, 299, 281–286. [Google Scholar] [CrossRef]
- Goodwin, S.; Peterson, C.; Hoh, C.; Bitther, K. Targeting and retention of magnetic targeted carriers (MTCs) enhancing intra-arterial chemotherapy. J. Magn. Magn. Mater. 1999, 194, 132–139. [Google Scholar] [CrossRef]
- Hirota, Y.; Akiyama, Y.; Izumi, Y.; Nishijma, S. Fundamental study for development magnetic drug delivery system. Phys. C Supercond. 2009, 469, 1853–1856. [Google Scholar] [CrossRef]
- Zubar, T.I.; Fedosyuk, V.M.; Trukhanov, A.V.; Kovaleva, N.N.; Astapovich, K.A.; Vinnik, D.A.; Trukhanova, E.L.; Kozlovskiy, A.L.; Zdorovets, M.V.; Solobai, A.A.; et al. Control of growth mechanism of electrodeposited nanocrystalline NiFe films. J. Electrochem. Soc. 2019, 166, D173–D180. [Google Scholar] [CrossRef]
- Kozlovskiy, A.L.; Kenzhina, I.E.; Zdorovets, M.V.; Saiymova, M.; Tishkevich, D.I.; Trukhanov, S.V.; Trukhanov, A.V. Synthesis, phase composition and structural and conductive properties of ferroelectric microparticles based on ATiOx (A = Ba, Ca, Sr). Ceram. Int. 2019, 45, 17236–17242. [Google Scholar] [CrossRef]
- Kozlovskiy, A.L.; Korolkov, I.V.; Kalkabay, G.; Ibragimova, M.A.; Ibrayeva, A.D.; Zdorovets, M.V.; Mikulich, V.S.; Yakimchuk, D.V.; Shumskaya, A.E.; Kaniukov, E.Y. Comprehensive study of Ni nanotubes for bioapplications: From synthesis to payloads attaching. J. Nanomater. 2017, 2017, 3060972. [Google Scholar] [CrossRef] [Green Version]
- O’Grady, K. Progress in applications of magnetic nanoparticles in biomedicine. J. Phys. D Appl. Phys. 2009, 42, 220301. [Google Scholar] [CrossRef] [Green Version]
- Tishkevich, D.I.; Korolkov, I.V.; Kozlovskiy, A.L.; Anisovich, M.; Vinnik, D.A.; Ermekova, A.E.; Vorobjova, A.I.; Shumskaya, E.E.; Zubar, T.I.; Trukhanov, S.V.; et al. Immobilization of boron-rich compound on Fe3O4 nanoparticles: Stabillity and cytotoxity. J. Alloys Compd. 2019, 797, 573–581. [Google Scholar] [CrossRef]
- Tishkevich, D.I.; Vorobjova, A.I.; Vinnik, D.A. Template assisted Ni nanowires fabrication. Mater. Sci. Forum 2019, 946, 235–241. [Google Scholar] [CrossRef]
- Nilsch, K.; Muller, F.; Li, A.P.; Gosele, U. Uniform nickel deposition into ordered alumina pores by pulsed electrodeposition. Adv. Mater. 2000, 12, 582. [Google Scholar] [CrossRef]
- Shaban, M.; Hamdy, H.; Shahin, F.; Park, J.; Ryu, S.W. Uniform and reproducible barrier layer removal of porous anodic alumina membrane. J. Nanosci. Nanotechnol. 2010, 10, 3380–3384. [Google Scholar] [CrossRef]
- Zhao, X.; Seo, S.K.; Lee, U.J.; Lee, K.H. Controlled electrochemical dissolution of anodic aluminum oxide for preparation of open-through pore structure. J. Electrochem. Soc. 2007, 154, C553. [Google Scholar] [CrossRef] [Green Version]
- Vorob’eva, A.I.; Utkina, E.A.; Komar, O.M. Homogeneous deposition of nickel in pores of the ordered thin aluminum oxide. Russ. Microelectron. 2013, 42, 79–88. [Google Scholar] [CrossRef]
- Dvornikov, A.V.; Mi, Y.C.; Chan, C.K. Transient analysis of force–frequency relationships in rat hearts perfused by Krebs-Henseleit and Tyrode solutions with different [Ca2+]o. Cardiovasc. Eng. Technol. 2012, 3, 203–210. [Google Scholar] [CrossRef]
- Nagalakshmi, R.; Rajendran, S.; Sathiyabama, O.; Pandiarajan, M.; Christy, J.L. Corrosion behaviour of biomaterials in synthetic biological solutions. Eur. Chem. Bull. 2013, 2, 171–179. [Google Scholar]
- Pan, H.; Liu, B.; Yi, J.; Poh, C.; Lim, S.; Ding, J.; Feng, Y.; Huan, C.H.A.; Lin, J. Growth of single-crystalline Ni and Co nanowires via electrochemical deposition and their magnetic properties. J. Phys. Chem. B 2005, 109, 3094–3098. [Google Scholar] [CrossRef] [PubMed]
- McCafferty, E. Introduction to Corrosion Science; Springer: New York, NY, USA, 2010; p. 501. [Google Scholar]
- Stansbury, E.E.; Buchanan, R.A. Fundamentals of the Electrochemical Corrosion; ASM International: Geauga, OH, USA, 2000; p. 487. [Google Scholar]
- Scully, J.R. Polarization resistance method for determination of instantaneous corrosion rates. Corossion 2000, 56, 199–218. [Google Scholar] [CrossRef]
- Beleevskii, V.S.; Konev, K.A.; Novosadov, V.V.; Vasil’ev, V.Y. Estimating corrosion current and tafel constants from the curvature of voltammetric curves near the free-corrosion potential. Prot. Met. 2004, 40, 566–569. [Google Scholar] [CrossRef]
- Stern, M.; Geary, A.L. Electrochemical polarization. J. Electrochem. Soc. 1957, 104, 56. [Google Scholar] [CrossRef]
- Mansfeld, F. Advances in Corrosion Science and Technology of Corrosion Protection; Metallurgiya: Moscow, Russia, 1980; p. 173. [Google Scholar]
- Rybalka, K.V.; Beketaeva, L.A.; Davydov, A.D. Estimation of corrosion current by the analysis of polarization curves: Electrochemical kinetics mode. Rus. J. Electrochem. 2014, 50, 108–113. [Google Scholar] [CrossRef]
- Onyeachu, B.I.; Peng, X.; Oguzie, E.E.; Ogukwe, C.E.; Digbo, I. Characterizing the electrochemical corrosion behavior of a Ni–28wt.%Al composite coating in 3.5% NaCl Solution. Port. Electrochim. Acta 2015, 33, 69. [Google Scholar] [CrossRef]
- Qin, L.Y.; Lian, J.; Jiang, Q. Effect of grain size on corrosion behavior of electrodeposited bulk nanocrystalline Ni. Trans. Nonferrous Met. Soc. China 2010, 20, 82–89. [Google Scholar] [CrossRef]
- Wang, X.; Wang, B.; Zhang, L.; Yang, C.; Yang, Y. Effect of different welding processes on electrochemical and corrosion behavior of pure nickel in 1 M NaCl solution. Metals 2017, 7, 532. [Google Scholar] [CrossRef] [Green Version]
- Ayoub, H.; Griveau, S.; Lair, L.; Brunswick, P.; Cassir, M.; Bedioui, F. Electrochemical characterization of nickel electrodes in phosphate and carbonate electrolytes in view of assessing a medical diagnostic device for the detection of early diabetes. Electroanalysis 2010, 22, 2483–2490. [Google Scholar] [CrossRef]
- Srinivasan, V.; Weidner, J.V. An electrochemical route for making porous nickel oxide electro-chemical capacitors. Electrochim. Soc. 1997, 144, L210. [Google Scholar] [CrossRef]
- Shimanovich, D.L.; Vorobjova, A.I.; Tishkevich, D.I.; Trukhanov, A.V.; Zdorovets, M.V.; Kozlovskiy, A.L. Preparation and morphology-dependent wettability of porous alumina membranes. Beilst. J. Nanotechnol. 2018, 9, 1423–1436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milosev, I.; Kosec, T. Study of Cu-18Ni-20Zn nickel silver and other Cu-based alloys in artificial sweat and physiological solution. Electrochim. Acta 2007, 52, 6799–6810. [Google Scholar] [CrossRef]
- Real, S.G.; Barbosa, M.R.; Vilche, J.R.; Arvia, A.J. Influence of chloride concentration on the active dissolution. J. Electrochem. Soc. 1990, 137, 1696. [Google Scholar] [CrossRef]
- Keddam, M.; Takenouti, H.; Yu, N. New data on the kinetics of passive nickel from low frequency impedance measurements. Corros. Sci. 1987, 27, 107–110. [Google Scholar] [CrossRef]
- Chopra, N.; Wu, J.; Agrawal, P. Synthesis of nanoscale heterostructures comprised of metal nanowires, carbon nanotubes, and metal nanoparticles: Investigation of their structure and electrochemical properties. J. Nanomater. 2015, 16, 2. [Google Scholar] [CrossRef]
- Parshutin, V.V.; Bogdashkina, N.L.; Chernova, G.P. Influence of the environment on corrosion and electrochemical behavior of nickel. Prot. Met. 2007, 1, 64. [Google Scholar]
- Ren, B.; Fan, M.Q.; Liu, Q.; Wang, J.; Song, D.; Bai, X. NiO nanofibers modified by citric acid and the performances as supercapacitor electrode. Electrochim. Acta 2013, 92, 197–204. [Google Scholar] [CrossRef]
- Yuan, B.; Xu, C.; Deng, D.; Xing, Y.; Liu, L.; Pang, H.; Zhang, D. Graphene oxide/nickel oxide modified glassy carbon electrode for supercapacitor and nonenzymatic glucose sensor. Electrochim. Acta 2013, 88, 708–712. [Google Scholar] [CrossRef]
- Tishkevich, D.I.; Vorobjova, A.I.; Shimanovich, D.L.; Vinnik, D.A.; Zubar, T.I.; Kozlovskiy, A.L.; Zdorovets, M.V.; Yakimchuk, D.V.; Trukhanov, S.V.; Trukhanov, A.V. Formation and corrosion properties of Ni-based composite material in the anodic alumina porous matrix. J. All. Comp. 2019, 804, 139–146. [Google Scholar] [CrossRef]
- Zubar, T.; Trukhanov, A.; Vinnik, D.; Astapovich, K.; Tishkevich, D.; Kaniukov, E.; Kozlovskiy, A.; Zdorovets, M.; Trukhanov, S. Features of the growth processes and magnetic domain structure of nife nano-objects. J. Phys. Chem. C 2019, 123, 26957–26964. [Google Scholar] [CrossRef]










| Characteristic | Value |
|---|---|
| Diameter of pillars d | (50–75) ± 10 [nm] |
| Length between pillars D | 105 ± 10 [nm] |
| Pillars height h, [nm] | (400–1000) ± 15 |
| Aspect ratio (h/d) | 5–20 |
| Pillars density N, [per cm2] | 5 × 109–1.5 × 1010 |
| Crystal Orientation | 2 Theta, [deg.] * | Intensity I, [%] | Size of Crystallites D, [nm] |
|---|---|---|---|
| Ni (111) | 43.82(44.51) | 100 | 18 |
| Ni (200) | 51.07(51.85) | 8.9 | 9 |
| Ni (220) | 74.90(76.37) | 3.2 | 20 |
| Sample No and Mode of Test | Ecorr, V (Ag/AgCl) | Icorr, [µA] | Jcorr, [µA /cm2] | ba, [mV/decade] | bc, [mV/decade] | Rp, [Ω/cm2] |
|---|---|---|---|---|---|---|
| No1 Ti/PAAT/Ni (1st mode) | −0.213 | 0.065 | 0.433 | ‒ | ‒ | ‒ |
| No1 Ti/PAAT/Ni (2nd mode) | −0.213 | 0.091 | 0.610 | 193 | 155 | 1.25 × 106 |
| No2 Ti/PAAT/Ni (1st mode) | −0.265 | 1.22 | 0.339 | ‒ | ‒ | ‒ |
| No2 Ti/PAAT/Ni (2nd mode) | −0.265 | 1.65 | 0.458 | 172 | 162 | 0.61 × 106 |
| Ni [61] 1 bulk electrodeposited | −0.303 | ‒ | 2.226 | 185 | 273 | ‒ |
| Ni [62] 2 bulk nano electrodeposited | −0.494 | ‒ | 5.210 | ‒ | ‒ | ‒ |
| Ni [63] 3 bulk metallurgical | −0.428 | – | 7.059 | ‒ | ‒ | ‒ |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vorobjova, A.; Tishkevich, D.; Shimanovich, D.; Zdorovets, M.; Kozlovskiy, A.; Zubar, T.; Vinnik, D.; Dong, M.; Trukhanov, S.; Trukhanov, A.; et al. Electrochemical Behaviour of Ti/Al2O3/Ni Nanocomposite Material in Artificial Physiological Solution: Prospects for Biomedical Application. Nanomaterials 2020, 10, 173. https://doi.org/10.3390/nano10010173
Vorobjova A, Tishkevich D, Shimanovich D, Zdorovets M, Kozlovskiy A, Zubar T, Vinnik D, Dong M, Trukhanov S, Trukhanov A, et al. Electrochemical Behaviour of Ti/Al2O3/Ni Nanocomposite Material in Artificial Physiological Solution: Prospects for Biomedical Application. Nanomaterials. 2020; 10(1):173. https://doi.org/10.3390/nano10010173
Chicago/Turabian StyleVorobjova, Alla, Daria Tishkevich, Dmitriy Shimanovich, Maxim Zdorovets, Artem Kozlovskiy, Tatiana Zubar, Denis Vinnik, Mengge Dong, Sergey Trukhanov, Alex Trukhanov, and et al. 2020. "Electrochemical Behaviour of Ti/Al2O3/Ni Nanocomposite Material in Artificial Physiological Solution: Prospects for Biomedical Application" Nanomaterials 10, no. 1: 173. https://doi.org/10.3390/nano10010173
APA StyleVorobjova, A., Tishkevich, D., Shimanovich, D., Zdorovets, M., Kozlovskiy, A., Zubar, T., Vinnik, D., Dong, M., Trukhanov, S., Trukhanov, A., & Fedosyuk, V. (2020). Electrochemical Behaviour of Ti/Al2O3/Ni Nanocomposite Material in Artificial Physiological Solution: Prospects for Biomedical Application. Nanomaterials, 10(1), 173. https://doi.org/10.3390/nano10010173