Foray into Concepts of Design and Evaluation of Microemulsions as a Modern Approach for Topical Applications in Acne Pathology
Abstract
:1. Introduction
2. Skin Structure and Implications in Topical Delivery of Active Molecules
2.1. The Epidermis
2.2. The Dermis
2.3. The Hypodermis
2.4. Topical Therapy as a Route of Administration
3. Current Topical Formulations in Acne Therapy
3.1. Short Characterization and Prevalence among Population
3.2. Current Therapeutic Models and Challenges
4. Microemulsions in a New Vision for an Optimized Acne Therapy
4.1. General Concepts
4.2. Physicochemical Concepts
- In a system composed of oil and water, the surfactant will promote a low interfacial tension at the interface between oil and water, resulting thus a monomolecular film [186].
- The system will be characterized by a free energy that will assure the microemulsifying process of droplets with a size of 1–100 nm which cannot be observed at a macroscale level, but only using fine experimental techniques like transmission electron microscopy (TEM) [185,188]. In this way, will be discovered particles with a specific assembly. As an example, in a study of Reis et al. were formulated O/W microemulsion systems that can load babassu oil 12.2% as an active oil component with anti-inflammatory effects. The analysed formulation was based on a mixture of two surfactants, Span 80 and Kolliphor EL, in combination with propylene glycol as a cosurfactant with a total amount S/CoS mix of 48.8%, in a water medium of 39%. Using TEM imaging, the internal structure was visualized, confirming the particle architecture, dimensional data and the type of MEs. Thus, were observed babassu oil phase droplets covered with the monolayer stratum of tensioactive mixture and embedded in the water medium [189]. Furthermore, O/W microemulsions for dermal and transdermal delivery with a 2% flavone extract of rhizoma arisaematis with analgesic properties were formulated using a vehicle composed of a S/CoS mixture with Cremophor EL 9–27% and Transcutol 8–27% with stabilizing properties for ethyl oleate particles 4–8% in a medium of 60% water. TEM analysis revealed the presence of spherical particles of oil phase with a dimension under 100 nm, stabilized in the aqueous medium [190]. In the same direction, confocal laser microscopy can be a useful tool with application in material structure analysis, being largely accessed for microemulsion characterization [191].
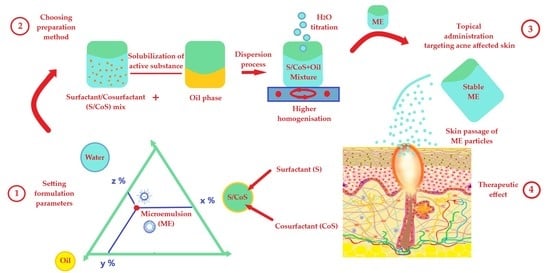
4.3. Formulation of Microemulsions
4.4. Methods for Microemulsion Preparation
4.5. The Mechanism of Action for Microemulsions at Skin Level
4.6. Application of Microemulsions for Anti-Acne Drug Delivery
| No. | API ME Type Method | Composition | Observations | Ref. |
|---|---|---|---|---|
| 1. | Tretinoin O/W Water titration method | S1: Tween 80 30% S2: Lecithin 1% CoS: Ethanol 10% Oil: isopropyl myristate (IPM) 5% Water: PBS1 pH 5.5 | Mean particle size: 110 nm. The release (µg/cm2/h): maximum for ME and MEgel compared with other formulations: ME > MEgel > gel > comm. gel > solution 33.92 > 31.54 > 28.67 > 24.28 > 22.33 The retention (g/cm2): greater for MEs, in the following order: MEgel > ME > Solution > comm. gel > gel 96.28 > 82.13 > 32.40 > 29.32 > 21.54. | [231] |
| 2. | Tretinoin 0.05% O/W Water titration method | S1: Tween 80 S2: Labrasol CoS: PG Amount S1/S2CoS: 55–90% IPM + Transcutol P as oil phase: 5–30% Water: 5–15% Km 3:1 and 4:1 ME model: S/CoS 65%/Oil 30%/Water 5% | Mean particle size: 14–60 nm. The pH was situated in the near physiological range: 6.1–6.87. PDI1 values: 0.352–0.411, defining the homogeneity of the systems. Refractive index variation: 1.4397–1.4505, as a mark of isotropy. The release in 8 h from ME model was 49%. | [285] |
| 3. | Tretinoin O/W Oil titration method | For screening: S: Tween 20, 40, 80, glyceryl stearate, stearyl alcohol, Span 20 or 80 CoS: PG, ethanol, isopropanol, PEG 4000 or PEG 6000 S/CoS amount: 45% Oil: olive oil, castor oil or IPM 10–17% Water: 38–45% | O/W MEs are suitable systems for tretinoin delivery. Span 20 and 80: not preferred for O/W systems. Glyceryl stearate and stearyl alcohol modified the viscosity of the systems. PEG 4000 and 6000 may increase the viscosity due to their high molecular weight. Ethanol and isopropanol may be reconsidered due to their fluidity promotion in ME systems. PG was preferred, along with Tween 80 and olive oil, promoting a maximum release of tretinoin of 82% in 24 h. | [264] |
| 4. | Isotretinoin 0.5% O/W Water titration method | S: Caprylocaproyl macrogol-8-glyceride 31.5% CoS: Polyglyceryl oleate 10.5% Oil: IPM 4.0% Water: q.s. and optimal Km 3:1 | Mean particle size (ME model): 45 ± 0.5 nm PDI1 value (ME model) was 0.145 ± 0.027, defining the homogeneity of the system. Refractive index (ME model): 1.329, as a mark of isotropy. | [288] |
| 5. | Isotretinoin 0.05% O/W or W/O Water titration method | S: Labrasol 24–54% CoS: Plurol oleique 8–18% Oil: IPM 8–18% Water: 10–60%, and optimal Km 3:1 ME model had the following formula: S 24%/CoS 8%/Oil 8%/Water 60% Carbopol 971P NF 2% was added to create a MEgel | Mean particle size: 22.4 ± 0.2 nm (ME model)–85.9 ± 3.7 nm. pH was situated in the near physiological range: 6.30 ± 0.01–6.67 ± 0.06 (ME model). Conductivity values: 2.4 ± 0.1–68.6 ± 0.4 µS/cm, correlated with the amount of water and the type of ME. O/W type: attributed to the ME model. Viscosity for ME model increased from a fluid type (38 ± 0.07 cP) to a high viscosity (6.5 × 103 ± 2 × 103 cP), correlated with the addition of the gelling agent. Skin drug deposition was improved. | [289] |
| 6. | Isotretinoin 0.05% O/W Water titration method | S: Labrasol 26.25–46.80% CoS: Kolliphor HS 7–12.5% or Kolliphor EL 7–12.5% or Plurol oleique 10–14.63% Oil: IPM 3.8–6.5% Water 34.94–61.14% and Km 3:1 and 4:1 | Kolliphor EL and HS: promote a diminishing in particle dimension. API embedding did not affect ME’s properties. Conductivity values: 7.50 ± 0.06–77.65 ± 0.21 µS/cm, correlated with the amount of water and the type of ME. Viscosity: 22.15 ± 0.06–88.00 ± 0.19 cP. The Newtonian behaviour of MEs was correlated with MEs fluidity. | [291] |
| 7. | Isotretinoin 0.05% W/O Water titration method | ME model formula: Kolliphor 22.5%/Ethanol 7.5%/Oil 8%/Water 61.95%/API 0.05%, Carbopol ETD 2020 0.75% and TEA 1 q.s. were added for ME gel | The ME model: used to prepare a ME based spray with isotretinoin and a ME based gel which. Particle size for MEspray: 68.79 nm The release (µg/cm2/h): maximum for MEspray: MEspray > MEgel > commercial gel 27.67 ± 0.12 > 21.81 ± 0.103 > 19.29 ± 0.34. | [292] |
| 8. | Adapalene 0.1% O/W Water titration method | 3 MEs types: 1. Plantacare 2000 15.9%/PG 30%/C90 15%/Water 39.1% 2. Plantacare 810 17.8%/PG 28%/C90 14%/Water 40.2% 3. Emanon 28%/Transcutol P 28%/C90 14%/Water 30% | Oil solubility of alkyl polyglucoside can be increased using CoS; Adapalene: good solubility in Plantacare tensioactives and Emanon EV and also in the oil. Adapalene embedding did not affect ME properties. pH values 7.38 ± 0.01–8.49 ± 0.01. Conductivity values: 61.6 ± 0.6 µS/cm (ME with Emanon) and 1022.0 ± 14.0 µS/cm (ME with Plantacare), correlated with the O/W type. Viscosity: 19.6 ± 0.27–27.90 ± 0.32 cP. The Newtonian behaviour of MEs was correlated with MEs fluidity. Skin application: favorable. | [300] |
| 9. | Erythromycin 0.5% W/O Water titration method | S: Tween 20 CoS: PEG 400 Oil: avocado oil and IPM Water: 5–15% and Km 1:1 | pH values: 5.02–5.35. Conductivity values: 11.98 ± 1.21–29.39 ± 1.92 µS/cm, correlated with the O/W type. Viscosity: 33.32 ± 1.58–58.50 ± 1.74 mPa·s. The Newtonian behaviour of MEs was correlated with MEs fluidity. PEG 400: implied in hydration and ME spreadability. The release: <40%/6 h (in vitro), influenced by the low water amount of 10%. PEG 400, an elevated water content and the adjusting of oil fraction should be reconsidered. | [305] |
| 10. | Nadifloxacin O/W Water titration method | S: Tween 80 CoS: Transcutol P, with S/CoS 30–60% Oil: C90 10–20% and optimal Km 1:1 Were formulated 2 MEgels, using 1. xanthan gum or 2.Carbopol ETD 2020 | Mean particle size: 65 ± 1.35–121.64 ± 1.35 nm. PDI1 values: 0.890 ± 0.124–1.132 ± 0.006, defining the homogeneity of the systems. S and CoS c% influence the particle dimension and drug entrapment. A high oil content will increase the particle diameter. Highest drug loading: for 10% oil and 60%S/CoS mix. S/CoS mix exerted penetration activity, altering the lipidic structure, assuring the passage of nadifloxacin. | [307] |
| 11. | Azelaic acid O/W Water titration method | S: Tween 80 and Labrasol 60–70% CoS: C90 Oil: oleic acid and Transcutol P (10:1) 5–10% Water: 20–30% Transcutol P was added in the oil phase due to its solubilization power And Km: 6:1 and 4:1 | Mean particle size: 48.05 ± 0.75–151 ± 1.1 nm. Viscosity: 72.5 ± 1.45–83 ± 1.7 cP. The Newtonian behaviour of MEs was correlated with MEs fluidity. Release: up to 42% in 24 h. | [309] |
| 12. | Metronidazole 0.75% W/O Water titration method | Optimal ME: S: Lecithin 35.75% CoS: Butanol 17.86% Oil: IPM 26.86% Water: 18.86% and Km 2:1 | Mean particle size: 11.6 nm. Conductivity: 1.5µS/cm, correlated with the W/O type. Viscosity: 457.3 mPa·s The Newtonian behaviour of MEs was correlated with MEs fluidity. Stability over 6 months. | [311] |
| 13. | Metronidazole 1% O/W Water titration method | Optimal emulgel: Acconon 16.67% PG 8.33%, Capmul 10%, Preservatives 0.22%, Xanthan gum 1%, TEA 1 q.s, Water q.s. and Km 2:1 | pH values: 6.0–6.9. Viscosity (optimal emulgel): 4568 ± 0.32 mPa·s (at 10 rpm) through 1087 ± 0.43 mPa·s (at 100 rpm). Rheological behaviour: expressed as shear thinning. The release (optimal emulgel): 93.16%. | [312] |
| 14. | Dapsone O/W Water titration method | S: Kolliphor EL CoS: PEG 400, and S/CoS 20–43% Oil: Capryol and N-methyl-2-pyrolidone (1:1) 3–10% Water: 50–70% and Km 1:1, 2:1, 1:2 ME model had the following formula: S/CoS 32%/Oil 6%/Water 62% and Km 1:1 | Mean particle size: 27.53 (ME model)–64.40 nm. pH: 5.6–6 (ME model); Conductivity: 13–15.8 µS/cm, correlated with the O/W type. Drug entrapment for ME model: 97.99 ± 0.040%. The release: 70 ± 0.09%. | [313] |
| 15. | Dapsone 5.15% O/W Water titration method | For optimal ME: S: Tween 80 18% CoS: Transcutol P 20% and ethanol 18% Oil: IPM 4% Water: 34.85% Two ME models derived from the first optimal: by adding menthol 2.5–5% Carbomer 940 0.5% was added. | Mean particle size (ME without menthol): 48.3 nm; Menthol has increased the particle size due to its hydrophobicity, being localized at the interface. pH values: 5.46–5.50. Conductivity: 25.40–28.25 µS/cm, correlated with the O/W type. Permeation values for MEgel: 74.38 ± 0.70–106.25 ± 4.84 µg/cm2/10 h. Stability over 6 months. | [314] |
5. Final Resolutions Considering Microemulsion Design
- It can be appreciated that microemulsion properties are closely influenced by the formulation parameters, where each of the selected phases will sustain the final action of the API in the targeted zone.
- Microemulsion systems will improve the localization of APIs in skin layers and can be optimized using Quality by Design principles.
- The use of combined systems like microemulsion-based gels or emulgels and the use of natural derived excipients will improve the tolerability and the biocompatibility at the application zone.
- Modulation of the total concentration of tensioactive mixture, without modifications in the quality profile of the systems, using natural surfactants.
- Integration of suitable substances that can enrich the quality profile of ME, based on their special properties, and here gelling agents, vegetable oils or biopolymers can be remembered.
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Slominski, A.T.; Zmijewski, M.A.; Skobowiat, C.; Zbytek, B.; Slominski, R.M.; Steketee, J.D. Sensing the environment: Regulation of local and global homeostasis by the skin’s neuroendocrine system. Adv. Anat. Embryol. Cell Biol. 2012, 212, 1–98. [Google Scholar] [CrossRef]
- Stalder, J.F.; Tennstedt, D.; Deleuran, M.; Fabbrocini, G.; de Lucas, R.; Haftek, M.; Taieb, C.; Coustou, D.; Mandeau, A.; Fabre, B.; et al. Fragility of epidermis and its consequence in dermatology. J. Eur. Acad. Dermatol. Venereol. 2014, 28, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Duarte, I.; Silveira, J.E.P.S.; Hafner, M.F.S.; Toyota, R.; Pedroso, D.M.M. Sensitive skin: Review of an ascending concept. An. Bras. Dermatol. 2017, 92, 521–525. [Google Scholar] [CrossRef] [PubMed]
- Oranges, T.; Dini, V.; Romanelli, M. Skin Physiology of the Neonate and Infant: Clinical Implications. Adv. Wound Care 2015, 4, 587–595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blume-Peytavi, U.; Kottner, J.; Sterry, W.; Hodin, M.W.; Griffiths, T.W.; Watson, R.E.B.; Hay, R.J.; Griffiths, C.E.M. Age-Associated Skin Conditions and Diseases: Current Perspectives and Future Options. Gerontologist 2016, 56, 230–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, H.W.; Collins, S.; Resneck, J.S.; Bolognia, J.L.; Hodge, J.A.; Rohrer, T.A.; Van Beek, M.J.; Margolis, D.J.; Sober, A.J.; Weinstock, M.A.; et al. The burden of skin disease in the United States. J. Am. Acad. Dermatol. 2017, 76, 958–972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karimkhani, C.; Dellavalle, R.P.; Coffeng, L.E.; Flohr, C.; Hay, R.J.; Langan, S.M.; Nsoesie, E.O.; Ferrari, A.J.; Erskine, H.E.; Silverberg, J.I.; et al. Global Skin Disease Morbidity and Mortality: An Update from the Global Burden of Disease Study 2013. JAMA Dermatol. 2017, 153, 406–412. [Google Scholar] [CrossRef]
- Seth, D.; Cheldize, K.; Brown, D.; Freeman, E.F. Global Burden of Skin Disease: Inequities and Innovations. Curr. Dermatol. Rep. 2017, 6, 204–210. [Google Scholar] [CrossRef]
- Hay, R.J.; Johns, N.E.; Williams, H.C.; Bolliger, I.W.; Dellavalle, R.P.; Margolis, D.J.; Marks, R.; Naldi, L.; Weinstock, M.A.; Wulf, S.K.; et al. The global burden of skin disease in 2010: An analysis of the prevalence and impact of skin conditions. J. Investig. Dermatol. 2014, 134, 1527–1534. [Google Scholar] [CrossRef] [Green Version]
- DeStefano, G.M.; Christiano, A.M. The Genetics of Human Skin Disease. Cold Spring Harb. Perspect. Med. 2014, 4, 1–25. [Google Scholar] [CrossRef] [Green Version]
- Truchuelo, M.T.; Vitale, M.A.; Bettoli, V.; Estebaranz, J.L. Acne Relapses and Maintenance Therapy: An Update on Definition and Prevention. Sci. J. Clin. Res. Dermatol. 2017, 2, 18–27. [Google Scholar]
- Shen, X.; Zhang, J.; Yan, C.; Zhou, H. An Automatic Diagnosis Method of Facial Acne Vulgaris Based on Convolutional Neural Network. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef]
- Barbieri, J.S.; Spaccarelli, N.; Margolis, D.J.; James, W.D. Approaches to limit systemic antibiotic use in acne: Systemic alternatives, emerging topical therapies, dietary modification, and laser and light-based treatments. J. Am. Acad. Dermatol. 2019, 80, 538–549. [Google Scholar] [CrossRef] [PubMed]
- Nastiti, C.M.; Ponto, T.; Abd, E.; Grice, J.E.; Benson, H.A.E.; Roberts, M.S. Topical Nano and Microemulsions for Skin Delivery. Pharmaceutics 2017, 9, 37. [Google Scholar] [CrossRef] [PubMed]
- Sapra, B.; Thatai, P.; Bhandari, S.; Sood, J.; Jindal, M.; Tiwary, A.K. A critical appraisal of microemulsions for drug delivery: Part II. Ther. Deliv. 2014, 5, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Heuschkel, S.; Goebel, A.; Neubert, R.H. Microemulsions-modern colloidal carrier for dermal and transdermal drug delivery. J. Pharm. Sci. 2008, 97, 603–631. [Google Scholar] [CrossRef]
- Nam, N.H.; Luong, N.H. Nanoparticles: Synthesis and applications. In Materials for Biomedical Engineering; Grumzescu, V., Grumzescu, M.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 211–240. [Google Scholar] [CrossRef]
- Do, L.D.; Withayyapayanon, A.; Harwell, J.H.; Sabatini, D.A. Environmentally Friendly Vegetable Oil Microemulsions Using Extended Surfactants and Linkers. J. Surfact. Deterg. 2009, 12, 91–99. [Google Scholar] [CrossRef]
- Schwarz, J.C.; Klang, V.; Hoppel, M.; Mahrhauser, D.; Valenta, C. Natural microemulsions: Formulation design and skin interaction. Eur. J. Pharm. Biopharm. 2012, 81, 557–562. [Google Scholar] [CrossRef]
- Alkrad, J.A.; Mrestani, Y.; Neubert, R.H. Development and characterization of microemulsions containing hyaluronic acid. Eur. J. Pharm. Sci. 2016, 86, 84–90. [Google Scholar] [CrossRef]
- Constantinides, P.P.; Welzel, G.; Ellens, H.; Smith, P.L.; Sturgis, S.; Yiv, S.H.; Owen, A.B. Water-in-oil microemulsions containing medium-chain fatty acids/salts: Formulation and intestinal absorption enhancement evaluation. Pharm. Res. 1996, 13, 210–215. [Google Scholar] [CrossRef]
- Malcolmson, C.; Satra, C.; Kantaria, S.; Sidhu, A.; Lawrence, M.J. Effect of oil on the level of solubilization of testosterone propionate into nonionic oil-in-water microemulsions. J. Pharm. Sci. 1998, 87, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Radomska-Soukharev, A.; Wojciechowska, J. Microemulsions as potential ocular drug delivery systems: Phase diagrams and physical properties depending on ingredients. Acta. Pol. Pharm. 2005, 62, 465–471. [Google Scholar] [PubMed]
- Radomska, A.; Dobrucki, R. The use of some ingredients for microemulsion preparation containing retinol and its esters. Int. J. Pharm. 2000, 196, 131–134. [Google Scholar] [CrossRef]
- Laihia, J.; Järvinen, R.; Wylęgała, E.; Kaarniranta, K. Disease aetiology-based design of multifunctional microemulsion eye drops for moderate or severe dry eye: A randomized, quadruple-masked and active-controlled clinical trial. Acta Ophthalmol. 2020, 98, 244–254. [Google Scholar] [CrossRef] [Green Version]
- Carvalho, F.C.; Barbi, M.S.; Sarmento, V.H.; Chiavacci, L.A.; Netto, F.M.; Gremião, M.P. Surfactant systems for nasal zidovudine delivery: Structural, rheological and mucoadhesive properties. J. Pharm. Pharmacol. 2010, 62, 430–439. [Google Scholar] [CrossRef]
- Patil, N.H.; Devarajan, P.V. Enhanced insulin absorption from sublingual microemulsions: Effect of permeation enhancers. Drug Deliv. Transl. Res. 2014, 4, 429–438. [Google Scholar] [CrossRef] [PubMed]
- Okur, M.E.; Ayla, Ş.; Yozgatlı, V.; Aksu, N.B.; Yoltaş, A.; Orak, D.; Sipahi, H.; Üstündağ Okur, N. Evaluation of burn wound healing activity of novel fusidic acid loaded microemulsion based gel in male Wistar albino rats. Saudi Pharm. J. 2020, 28, 338–348. [Google Scholar] [CrossRef]
- Bachhav, Y.G.; Patravale, V.B. Microemulsion based vaginal gel of fluconazole: Formulation, in vitro and in vivo evaluation. Int. J. Pharm. 2009, 365, 175–179. [Google Scholar] [CrossRef]
- Date, A.A.; Patravale, V.B. Microemulsions: Applications in transdermal and dermal delivery. Crit. Rev. Ther. Drug Carrier Syst. 2007, 24, 547–596. [Google Scholar] [CrossRef]
- Azeem, A.; Rizwan, M.; Ahmad, F.J.; Khan, Z.I.; Khar, R.K.; Aqil, M.; Talegaonkar, S. Emerging role of microemulsions in cosmetics. Recent Pat. Drug Deliv. Formul. 2008, 2, 275–289. [Google Scholar] [CrossRef] [PubMed]
- Krstić, M.; Medarević, Đ.; Đuriš, J.; Ibrić, S. Self-nanoemulsifying drug delivery systems (SNEDDS) and self-microemulsifying drug delivery systems (SMEDDS) as lipid nanocarriers for improving dissolution rate and bioavailability of poorly soluble drugs. In Lipid Nanocarriers for Drug Targeting; Grumzescu, A.M., Ed.; William Andrew (Elsevier): Oxford, UK, 2018; pp. 473–508. [Google Scholar] [CrossRef]
- Benigni, M.; Pescina, S.; Grimaudo, M.A.; Padula, C.; Santi, P.; Nicoli, S. Development of microemulsions of suitable viscosity for cyclosporine skin delivery. Int. J. Pharm. 2018, 545, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Sahle, F.F.; Wohlrab, J.; Neubert, R.H. Controlled penetration of ceramides into and across the stratum corneum using various types of microemulsions and formulation associated toxicity studies. Eur. J. Pharm. Biopharm. 2014, 86, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Pescina, S.; Garrastazu, G.; Del Favero, E.; Rondelli, V.; Cantù, L.; Padula, C.; Santi, P.; Nicoli, S. Microemulsions based on TPGS and isostearic acid for imiquimod formulation and skin delivery. Eur. J. Pharm. Sci. 2018, 125, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Guo, C.; Yu, A.; Gao, Y.; Cao, F.; Zhai, G. Microemulsion-based hydrogel formulation of penciclovir for topical delivery. Int. J. Pharm. 2009, 378, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Kaur, A.; Sharma, G.; Gupta, V.; Ratho, R.K.; Katare, O.P. Enhanced acyclovir delivery using w/o type microemulsion: Preclinical assessment of antiviral activity using murine model of zosteriform cutaneous HSV-1 infection. Artif. Cells Nanomed. Biotechnol. 2018, 46, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Goindi, S.; Narula, M.; Kalra, A. Microemulsion-Based Topical Hydrogels of Tenoxicam for Treatment of Arthritis. AAPS PharmSciTech. 2016, 17, 597–606. [Google Scholar] [CrossRef]
- Vyas, A.; Sonker, A.K.; Gidwani, B. Carrier-Based Drug Delivery System for Treatment of Acne. Sci. World J. 2014, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Bullinger, H.-J. Technology Guide: Principles-Applications-Trends; Springer: Berlin/Heidelberg, Germany, 2009; pp. 450–453. [Google Scholar] [CrossRef]
- Talakoub, L.; Neuhaus, I.M.; Yu, S.S. Cosmeceuticals. In Requisites in Dermatology, 1st ed.; Cosmetic Dermatology; Alam, M., Gladstone, H.B., Tung, R.C., Eds.; Saunders Elsevier (Elsevier): Philadelphia, PA, USA, 2009; pp. 7–35. ISBN 978-0-7020-3143-4. [Google Scholar]
- Wickett, R.R.; Visscher, M.O. Structure and function of the epidermal barrier. Am. J. Infect. Control 2006, 34, 98–110. [Google Scholar] [CrossRef]
- Abdallah, F.; Mijouin, L.; Pichon, C. Skin Immune Landscape: Inside and Outside the Organism. Mediators Inflam. 2017, 2017, 1–17. [Google Scholar] [CrossRef]
- Wong, R.; Geyer, S.; Weninger, W.; Guimberteau, J.-C.; Wong, J.K. The dynamic anatomy and patterning of skin. Exp. Dermatol. 2016, 25, 92–98. [Google Scholar] [CrossRef] [Green Version]
- Farage, M.A.; Miller, K.W.; Elsner, P.; Maibach, H.I. Structural Characteristics of the Ageing Skin: A Review. Cutan. Ocul. Toxicol. 2007, 26, 343–357. [Google Scholar] [CrossRef] [PubMed]
- Roger, M.; Fullard, N.; Costello, L.; Bradbury, S.; Markiewicz, E.; O’Reilly, S.; Darling, N.; Ritchie, P.; Määttä, A.; Karakesisoglou, I.; et al. Bioengineering the microanatomy of human skin. J. Anat. 2019, 234, 438–455. [Google Scholar] [CrossRef] [PubMed]
- Pillai, S.; Cornell, M.; Oresajo, C. Epidermal barrier. In Cosmetic Dermatology: Products and Procedures, 1st ed.; Draelos, Z.D., Ed.; Wiley-Blackwell: Oxford, UK, 2010; pp. 3–11. [Google Scholar] [CrossRef]
- Flynn, L.E.; Woodhouse, K.A. Burn Dressing Biomaterials and Tissue Engineering. Physiology of the Skin. In Biomedical Materials; Narayan, R., Ed.; Springer: New York, NY, USA, 2009; pp. 371–404. [Google Scholar] [CrossRef]
- Bouwstra, J.A.; Gooris, G.S. The Lipid Organization in Human Stratum Corneum and Model Systems. Open Dermatol. J. 2010, 4, 11–13. [Google Scholar] [CrossRef]
- Boncheva, M. The physical chemistry of the stratum corneum lipids. Int. J. Cosmet. Sci. 2014, 36, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Fowler, J. Understanding the Role of Natural Moisturizing Factor in Skin Hydration. Pract. Dermatol. 2012, 9, 36–40. [Google Scholar]
- Ishida-Yamamoto, A.; Igawa, S. The biology and regulation of corneodesmosomes. Cell Tissue Res. 2015, 360, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Haftek, M. Epidermal barrier disorders and corneodesmosome defects. Cell Tissue Res. 2015, 360, 480–490. [Google Scholar] [CrossRef] [Green Version]
- Harding, C.R.; Watkinson, A.; Rawlings, A.V.; Scott, I.R. Dry skin, moisturization and corneodesmolysis. Int. J. Cosmet. Sci. 2000, 22, 21–52. [Google Scholar] [CrossRef]
- Pashkovski, E.; Trumbull, C.T.; Lips, A.; Petko, M. Mechanisms of natural moisturizing factors for skin hydration. J. Am. Acad. Dermatol. 2009, 60, AB37. [Google Scholar] [CrossRef]
- Lynde, C.W.; Andriessen, A.; Barankin, B.; De Gannes, G.; Gulliver, W.; Haber, R.; Mccuaig, C.; Rajan, P.; Skotnicki, S.P.; Thomas, R.; et al. Moisturizers and Ceramide-containing Moisturizers May Offer Concomitant Therapy with Benefits. J. Clin. Aesthet. Dermatol. 2014, 7, 18–26. [Google Scholar]
- Kobielak, K.; Kandyba, E.; Leung, Y. Skin and Skin Appendage Regeneration. In Translational Regenerative Medicine; Atala, A., Allickson, J.G., Eds.; Academic Press (Elsevier): London, UK, 2015; pp. 269–286. ISBN 978-0-12-410396-2. [Google Scholar]
- Wang, Y.; Viennet, C.; Robin, S.; Berthon, J.-Y.; He, L.; Humbert, P. Precise role of dermal fibroblasts on melanocyte pigmentation. J. Dermatol. Sci. 2017, 88, 159–166. [Google Scholar] [CrossRef] [Green Version]
- Rippa, A.L.; Kalabusheva, E.P.; Vorotelyak, E.A. Regeneration of Dermis: Scarring and Cells Involved. Cells 2019, 8, 607. [Google Scholar] [CrossRef] [Green Version]
- Litwiniuk, M.; Krejner-Bienias, A.; Speyrer, M.S.; Gauto, A.R.; Grzela, T. Hyaluronic Acid in Inflammation and Tissue Regeneration. Wounds 2016, 28, 78–88. [Google Scholar]
- Yagi, M.; Yonei, Y. Glycative stress and anti-aging: 7. Glycative stress and skin aging. Glycative Stress Res. 2018, 5, 50–54. [Google Scholar] [CrossRef]
- Laverdet, B.; Danigo, A.; Girard, D.; Laurent, M.; Demiot, C.; Desmoulière, A. Skin innervation: Important roles during normal and pathological cutaneous repair. Histol. Histopathol. 2015, 30, 875–892. [Google Scholar] [CrossRef] [PubMed]
- Lai-Cheong, J.E.; McGrath, J.A. Structure and function of skin, hair and nails. Medicine 2017, 45, 347–351. [Google Scholar] [CrossRef]
- Perera, E.; Sinclair, R. Hyperhidrosis and bromhidrosis: A guide to assessment and management. Aust. Fam. Physician 2013, 42, 266–269. [Google Scholar] [PubMed]
- Kieser, D.; Carr, D.; Jermy, M.; Mabbott, A.; Kieser, J. Injury Biomechanics. In Forensic Epidemiology: Principles and Practice; Freeman, M.D., Zeegers, M.P., Eds.; Academic Press (Elsevier): London, UK, 2016; pp. 201–230. [Google Scholar] [CrossRef]
- Tran, T.-N.T. Cutaneous Drug Delivery: An Update. J. Investig. Dermatol. Symp. Proc. 2013, 16, 567–569. [Google Scholar] [CrossRef] [Green Version]
- Meaike, J.D.; Agrawal, N.; Chang, D.; Lee, E.I.; Nigro, M.G. Noninvasive Facial Rejuvenation. Part 3: Physician-Directed-Lasers, Chemical Peels, and Other Noninvasive Modalities. Semin. Plast. Surg. 2016, 30, 143–150. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.J.; Hyun, M.Y.; Park, K.Y.; Kim, B.J. A tip for performing intralesional triamcinolone acetonide injections in acne patients. J. Am. Acad. Dermatol. 2014, 71, 127–128. [Google Scholar] [CrossRef]
- Riviere, J.E.; Monteiro-Riviere, N.A. Dermal Exposure and Absorption of Chemicals and Nanomaterials. In Comprehensive Toxicology, 2nd ed.; McQueen, C.A., Ed.; Elsevier: Oxford, UK, 2010; Volume 1, pp. 111–121. [Google Scholar] [CrossRef]
- Jorge, L.L.; Feres, C.C.; Teles, V.E. Topical preparations for pain relief: Efficacy and patient adherence. J. Pain Res. 2011, 4, 11–24. [Google Scholar] [CrossRef]
- Sharadha, M.; Gowda, D.V.; Vishal Gupta, N.; Akhila, A.R. An overview on topical drug delivery system—Updated review. Int. J. Res. Pharm. Sci. 2020, 11, 368–385. [Google Scholar] [CrossRef]
- Wohlrab, J. Topical preparations and their use in dermatology. J. Dtsch. Dermatol. Ges. 2016, 14, 1061–1069. [Google Scholar] [CrossRef] [PubMed]
- Narasimha Murthy, S.; Shivakumar, H.N. Topical and Transdermal Drug Delivery. In Handbook of Non-Invasive Drug Delivery Systems, 1st ed.; Kulkarni, V.S., Ed.; William Andrew (Elsevier): Oxford, UK, 2010; pp. 1–36. [Google Scholar] [CrossRef]
- Singh Malik, D.; Mital, N.; Kaur, G. Topical Drug Delivery Systems: A Patent Review. Expert Opin. Ther. Pat. 2016, 26, 213–228. [Google Scholar] [CrossRef]
- Korting, H.A.; Schäfer-Korting, M. Carriers in the Topical Treatment of Skin Diseases. In Drug Delivery (Handbook of Experimental Pharmacology 197); Schäfer-Korting, M., Ed.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 435–460. [Google Scholar] [CrossRef]
- Weiss, S.C. Conventional topical delivery systems. Dermatol. Ther. 2011, 24, 471–476. [Google Scholar] [CrossRef]
- Ng, K.W.; Lau, W.M. Skin Deep: The Basics of Human Skin Structure and Drug Penetration. In Percutaneous Penetration Enhancers Chemical Methods in Penetration Enhancement: Drug Manipulation Strategies and Vehicle Effects; Dragicevic, N., Maibach, H.I., Eds.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 3–11. [Google Scholar] [CrossRef]
- Dayan, N. Delivery System Design in Topically Applied Formulations: An Overview. In Delivery System Handbook for Personal Care and Cosmetic Products; Rosen, M.R., Ed.; William Andrew Publishing: Norwich, VA, USA, 2005; pp. 101–118. [Google Scholar] [CrossRef]
- Uche, L.E.; Gooris, G.S.; Beddoes, C.M.; Bouwstra, J.A. New insight into phase behavior and permeability of skin lipid models based on sphingosine and phytosphingosine ceramides. Biochim. Biophys. Acta Biomembr. 2019, 1861, 1317–1328. [Google Scholar] [CrossRef]
- Güngör, S.; Erdal, M.S.; Güngördük, S. Colloidal carriers in the topical treatment of dermatological diseases. In Nano Based Drug Delivery; Naik, J., Ed.; IAPC Publishing: Zagreb, Croatia, 2015; pp. 391–409. [Google Scholar] [CrossRef]
- Ruiz, M.A.; Arias, J.L.; Gallardo, V. Skin creams made with olive oil. Factors determining the dermic absorbtion. In Olives and Olive Oil in Health and Disease Prevention, 1st ed.; Preedy, V.R., Watson, R.R., Eds.; Academic Press (Elsevier): London, UK, 2010; pp. 1133–1141. [Google Scholar] [CrossRef]
- Roberts, M.S.; Mohammed, Y.; Pastore, M.N.; Namjoshi, S.; Yousef, S.; Alinaghi, A.; Haridass, I.N.; Abd, E.; Leite-Silva, V.R.; Benson, H.; et al. Topical and cutaneous delivery using nanosystems. J. Control Release 2017, 247, 86–105. [Google Scholar] [CrossRef] [Green Version]
- Kasting, G.B.; Miller, M.A.; LaCount, T.D.; Jaworska, J. A Composite Model for the Transport of Hydrophilic and Lipophilic Compounds Across the Skin: Steady-State Behavior. J. Pharm. Sci. 2019, 108, 337–349. [Google Scholar] [CrossRef] [Green Version]
- Verma, A.; Jain, A.; Hurkat, P.; Jain, S.K. Transfollicular drug delivery: Current perspectives. Res. Rep. Transderm. Drug Deliv. 2016, 5, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Meidan, V.M.; Bonner, M.C.; Michniak, B.B. Transfollicular drug delivery—Is it a reality? Int. J. Pharm. 2005, 306, 1–14. [Google Scholar] [CrossRef]
- Yamamoto, A.; Takenouchi, K.; Ito, M. Impaired water barrier function in acne vulgaris. Arch. Dermatol. Res. 1995, 287, 214–218. [Google Scholar] [CrossRef] [PubMed]
- Thiboutot, D.; Del Rosso, J.Q. Acne Vulgaris and the Epidermal Barrier: Is Acne Vulgaris Associated with Inherent Epidermal Abnormalities that Cause Impairment of Barrier Functions? Do Any Topical Acne Therapies Alter the Structural and/or Functional Integrity of the Epidermal Barrier? J. Clin. Aesthet. Dermatol. 2013, 6, 18–24. [Google Scholar] [PubMed]
- Latter, G.; Grice, J.E.; Mohammed, Y.; Roberts, M.S.; Benson, H.A.E. Targeted Topical Delivery of Retinoids in the Management of Acne Vulgaris: Current Formulations and Novel Delivery Systems. Pharmaceutics 2019, 11, 490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, C.H.; Fang, Y.P.; Al-Suwayeh, S.A.; Yang, S.Y.; Fang, J.Y. Percutaneous absorption and antibacterial activities of lipid nanocarriers loaded with dual drugs for acne treatment. Biol. Pharm. Bull. 2013, 36, 276–286. [Google Scholar] [CrossRef] [Green Version]
- Makhmalzade, B.S.; Chavoshy, F. Polymeric micelles as cutaneous drug delivery system in normal skin and dermatological disorders. J. Adv. Pharm. Technol. Res. 2018, 9, 2–8. [Google Scholar] [CrossRef]
- Kotova, T.G.; Tsybusov, S.N.; Kochenov, V.I.; Tcyganov, M.I. Application of Cryogenic Methods in Skin Diseases of Different Etiology. In Dermatologic Surgery and Procedures; Vereecken, P., Ed.; IntechOpen: Rijeka, Croatia, 2017; pp. 35–75. [Google Scholar] [CrossRef] [Green Version]
- Aydemir, E.H. Acne vulgaris. Turk. Arch. Pediatr. Turk Pediatri Arşivi 2014, 49, 13–16. [Google Scholar] [CrossRef]
- Zaenglein, A.L.; Pathy, A.L.; Schlosser, B.J.; Alikhan, A.; Baldwin, H.E.; Berson, D.S.; Bowe, W.P.; Graber, E.M.; Harper, J.C.; Kang, S.; et al. Guidelines of care for the management of acne vulgaris. J. Am. Acad. Dermatol. 2016, 74, 945–973. [Google Scholar] [CrossRef] [Green Version]
- Zeichner, J.A.; Baldwin, H.E.; Cook-Bolden, F.E.; Eichenfield, L.F.; Fallon-Friedlander, S.; Rodriguez, D.A. Emerging Issues in Adult Female Acne. J. Clin. Aesthet. Dermatol. 2017, 10, 37–46. [Google Scholar]
- Collier, C.N.; Harper, J.C.; Cafardi, J.A.; Cantrell, W.C.; Wang, W.; Foster, K.W.; Elewski, B.E. The prevalence of acne in adults 20 years and older. J. Am. Acad. Dermatol. 2008, 58, 56–59. [Google Scholar] [CrossRef]
- Jappe, U. Pathological Mechanisms of Acne with Special Emphasis on Propionibacterium acnes and Related Therapy. Acta Derm. Venerol. 2003, 83, 241–248. [Google Scholar] [CrossRef] [Green Version]
- Dréno, B. What is new in the pathophysiology of acne, an overview. J. Eur. Acad. Dermatol. Venerol. 2017, 31, 8–12. [Google Scholar] [CrossRef]
- Ferreira, B.R.; Cardoso, J.C.; Reis, J.P.; Figueiredo, A. Acne. In Advances in Integrative Dermatology, 1st ed.; França, K., Lotti, T., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2019; pp. 33–56. [Google Scholar] [CrossRef]
- Tan, A.U.; Schlosser, B.J.; Paller, A.S. A review of diagnosis and treatment of acne in adult female patients. Int. J. Womens Dermatol. 2017, 4, 56–71. [Google Scholar] [CrossRef] [PubMed]
- Park, C.; Kim, G.; Patel, I.; Chang, J.; Tan, X. Improving adherence to acne treatment: The emerging role of application software. Clin. Cosmet. Investig. Dermatol. 2014, 7, 65–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Menditto, E.; Orlando, V.; De Rosa, G.; Minghetti, P.; Musazzi, U.M.; Cahir, C.; Kurczewska-Michalak, M.; Kardas, P.; Costa, E.; Sousa Lobo, J.M.; et al. Patient Centric Pharmaceutical Drug Product Design-The Impact on Medication Adherence. Pharmaceutics 2020, 12, 44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moradi Tuchayi, S.; Alexander, T.M.; Nadkarni, A.; Feldman, S.R. Interventions to increase adherence to acne treatment. Patient Prefer. Adherence 2016, 10, 2091–2096. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kircik, L.H. Importance of vehicles in acne therapy. J. Drugs Dermatol. 2011, 10, 17–23. [Google Scholar]
- Thielitz, A.; Gollnick, H. Recent therapeutic developments for acne. Expert Rev. Dermatol. 2013, 8, 37–50. [Google Scholar] [CrossRef]
- Simonart, T.; Dramaix, M. Treatment of acne with topical antibiotics: Lessons from clinical studies. Br. J. Dermatol. 2005, 153, 395–403. [Google Scholar] [CrossRef] [Green Version]
- Walsh, T.R.; Efthimiou, J.; Dréno, B. Systematic review of antibiotic resistance in acne: An increasing topical and oral threat. Lancet. Infect. Dis. 2016, 16, 23–33. [Google Scholar] [CrossRef] [Green Version]
- Morgan, A.J.; Lewis, G.; Van den Hoven, W.E.; Akkerboom, P.J. The effect of zinc in the form of erythromycin-zinc complex (Zineryt® lotion) and zinc acetate on metallothionein expression and distribution in hamster skin. Br. J. Dermatol. 1993, 129, 563–570. [Google Scholar] [CrossRef]
- Bettoli, V.; Tosti, G.; Virgili, A. Erythromycin 2% + isotretinoin 0.05% gel vs erythromycin 3% + zinc lotion: Comparative split-face evaluation of efficacy and tolerability in acne patients. Gior. Ital. Dermatol. Venereol. 2002, 137, 363–368. [Google Scholar]
- Gupta, M.; Mahajan, V.K.; Mehta, K.S.; Chauhan, P.S. Zinc therapy in dermatology: A review. Dermatol. Res. Pract. 2014, 2014, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, N.; Gans, E.H. Clindamycin 1.2% Tretinoin 0.025% Gel versus Clindamycin Gel Treatment in Acne Patients: A Focus on Fitzpatrick Skin Types. J. Clin. Aesthet. Dermatol. 2011, 4, 31–40. [Google Scholar] [PubMed]
- Shahmoradi, Z.; Iraji, F.; Siadat, A.H.; Ghorbaini, A. Comparison of topical 5% nicotinamid gel versus 2% clindamycin gel in the treatment of the mild-moderate acne vulgaris: A double-blinded randomized clinical trial. J. Res. Med. Sci. 2013, 18, 115–117. [Google Scholar] [PubMed]
- Dos, S.K.; Barbhuiya, J.N.; Jana, S.; Dey, S.K. Comparative evaluation of clindamycin phosphate 1% and clindamycin phosphate 1% with nicotinamide gel 4% in the treatment of acne vulgaris. Indian J. Dermatol. Venerol. Leprol. 2003, 69, 8–9. [Google Scholar]
- Gold, M.H. Clindamycin Phosphate 1.2% and Benzoyl Peroxide 2.5% Gel for the Treatment of Moderate-to-severe Acne: An Update. J. Clin. Aesthet. Dermatol. 2012, 5, 30–35. [Google Scholar]
- Khodaeiani, E.; Fouladi, R.F.; Yousefi, N.; Amirnia, M.; Babaeinejad, S.; Shokri, J. Efficacy of 2% Metronidazole Gel in Moderate Acne Vulgaris. Indian J. Dermatol. 2012, 57, 279–281. [Google Scholar] [CrossRef]
- Beutner, K.; Calvarese, M.S.; Graeber, M. A multi-center, investigator-blind clinical trial to assess the safety and efficacy of metronidazole gel 1% as compared to metronidazole gel vehicle and metronidazole cream 1% in the treatment of rosacea. J. Am. Acad. Dermatol. 2005, 52. [Google Scholar] [CrossRef]
- Schaller, M.; Dirschka, T.; Kemény, L.; Briantais, P.; Jacovella, J. Superior Efficacy with Ivermectin 1% Cream Compared to Metronidazole 0.75% Cream Contributes to a Better Quality of Life in Patients with Severe Papulopustular Rosacea: A Subanalysis of the Randomized, Investigator-Blinded ATTRACT Study. Dermatol. Ther. 2016, 6, 427–436. [Google Scholar] [CrossRef] [Green Version]
- Del Rosso, J.Q. Oral Doxycycline in the Management of Acne Vulgaris: Current Perspectives on Clinical Use and Recent Findings with a New Double-scored Small Tablet Formulation. J. Clin. Aesthet. Dermatol. 2015, 8, 19–26. [Google Scholar]
- Raoof, T.J.; Hooper, D.; Moore, A.; Zaiac, M.; Sullivan, T.; Kircik, L.; Lain, E.; Jankicevic, J.; Stuart, I. Efficacy and safety of a novel topical minocycline foam for the treatment of moderate to severe acne vulgaris: A phase 3 study. J. Am. Acad. Dermatol. 2020, 82, 832–837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garrett, J.P.-D.; Margolis, D.J. Impact of Long-Term Antibiotic Use for Acne on Bacterial Ecology and Health Outcomes: A Review of Observational Studies. Curr. Dermatol. Rep. 2012, 1, 23–28. [Google Scholar] [CrossRef] [Green Version]
- Leyden, J.J.; Del Rosso, J.Q. Oral antibiotic therapy for acne vulgaris: Pharmacokinetic and pharmacodynamic perspectives. J. Clin. Aesthet. Dermatol. 2011, 4, 40–47. [Google Scholar] [PubMed]
- Adişen, E.; Kaymak, Y.; Gurer, M.A.; Durukan, E. Topical tetracycline in the treatment of acne vulgaris. J. Drug. Dermatol. 2008, 7, 953–955. [Google Scholar]
- McCarty, M.; Del Rosso, J.Q. Chronic administration of oral trimethoprim-sulfamethoxazole for acne vulgaris. J. Clin. Aesthet. Dermatol. 2011, 4, 58–66. [Google Scholar]
- Del Rosso, J.Q. Azelaic Acid Topical Formulations: Differentiation of 15% Gel and 15% Foam. J. Clin. Aesthet. Dermatol. 2017, 10, 37–40. [Google Scholar]
- Kede, M.P.V.; Guedes, L.S. Salicylic Acid Peel. In Clinical Approaches and Procedures in Cosmetic Dermatology: Chemical and Physical Procedures; Issa, M., Tamura, B., Eds.; Springer: Cham, Switzerland, 2017; Volume 2, pp. 1–6. [Google Scholar] [CrossRef]
- Lu, J.; Cong, T.; Wen, X.; Li, X.; Du, D.; He, G.; Jiang, X. Salicylic acid treats acne vulgaris by suppressing AMPK/SREBP1 pathway in sebocytes. Exp. Dermatol. 2019, 28, 786–794. [Google Scholar] [CrossRef]
- Chularojanamontri, L.; Tuchinda, P.; Kulthanan, K.; Pongparit, K. Moisturizers for Acne: What are their Constituents? J. Clin. Aesthet. Dermatol. 2014, 7, 36–44. [Google Scholar]
- Leyden, J.; Stein-Gold, L.; Weiss, J. Why Topical Retinoids Are Mainstay of Therapy for Acne. Dermatol. Ther. 2017, 7, 293–304. [Google Scholar] [CrossRef]
- Marazzi, P.; Boorman, G.C.; Donald, A.E.; Davies, H.D. Clinical evaluation of Double Strength Isotrexin versus Benzamycin in the topical treatment of mild to moderate acne vulgaris. J. Dermatolog. Treat. 2002, 13, 111–117. [Google Scholar] [CrossRef]
- Kolli, S.S.; Pecone, D.; Pona, A.; Cline, A.; Feldman, S.R. Topical Retinoids in Acne Vulgaris: A Systematic Review. Am. J. Clin. Dermatol. 2019, 20, 345–365. [Google Scholar] [CrossRef] [PubMed]
- Gregoriou, S.; Kritsotaki, E.; Katoulis, A.; Rigopoulos, D. Use of tazarotene foam for the treatment of acne vulgaris. Clin. Cosmet. Investig. Dermatol. 2014, 7, 165–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blume-Peytavi, U.; Fowler, J.; Kemény, L.; Draelos, Z.; Cook-Bolden, F.; Dirschka, T.; Eichenfield, L.; Graeber, M.; Ahmad, F.; Alió Saenz, A.; et al. Long-term safety and efficacy of trifarotene 50 μg/g cream, a first-in-class RAR-γ selective topical retinoid, in patients with moderate facial and truncal acne. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 166–173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hebert, A.; Thiboutot, D.; Stein Gold, L.; Cartwright, M.; Gerloni, M.; Fragasso, E.; Mazzetti, A. Efficacy and Safety of Topical Clascoterone Cream, 1%, for Treatment in Patients with Facial Acne: Two Phase 3 Randomized Clinical Trials. JAMA Dermatol. 2020, 156, 621–630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, G.K.; Del Rosso, J.Q. Oral Spironolactone in Post-teenage Female Patients with Acne Vulgaris: Practical Considerations for the Clinician Based on Current Data and Clinical Experience. J. Clin. Aesthet. Dermatol. 2012, 5, 37–50. [Google Scholar] [PubMed]
- Elsaie, M.L. Hormonal treatment of acne vulgaris: An update. Clin. Cosmet. Investig. Dermatol. 2016, 9, 241–248. [Google Scholar] [CrossRef] [Green Version]
- Thiboutot, D.M.; Kircik, L.; McMichael, A.; Cook-Bolden, F.E.; Tyring, S.K.; Berk, D.R.; Chang-Lin, J.E.; Lin, V.; Kaoukhov, A. Efficacy, Safety, and Dermal Tolerability of Dapsone Gel, 7.5% in Patients with Moderate Acne Vulgaris: A Pooled Analysis of Two Phase 3 Trials. J. Clin. Aesthet. Dermatol. 2016, 9, 18–27. [Google Scholar]
- Didona, D.; Paolino, G.; Donati, P.T.; Muscardin, L.M. Resolution of nodulocystic acne with oral dapsone. Dermatol. Ther. 2017, 30. [Google Scholar] [CrossRef] [Green Version]
- Rathi, S.K. Acne vulgaris treatment: The current scenario. Indian J. Dermatol. 2011, 56, 7–13. [Google Scholar] [CrossRef]
- Sevimli Dikicier, B. Topical treatment of acne vulgaris: Efficiency, side effects, and adherence rate. J. Int. Med. Res. 2019, 47, 2987–2992. [Google Scholar] [CrossRef]
- Nano.gov. National Nanotechnology Initiative. Available online: https://www.nano.gov/nanotech-101/what/definition (accessed on 7 February 2020).
- Cancino, J.; Marangoni, V.S.; Zucolotto, V. Nanotechnology in medicine: Concepts and concerns. Quím. Nova 2014, 37, 521–526. [Google Scholar] [CrossRef] [Green Version]
- Kotla, N.; Chandrasekar, B.; Rooney, P.; Sivaraman, G.; Larrañaga, A.; Kanala, V.K.; Pandi, A.; Rochev, Y. Biomimetic Lipid-Based Nanosystems for Enhanced Dermal Delivery of Drugs and Bioactive Agents. ACS Biomater. Sci. Eng. 2017, 3, 1262–1272. [Google Scholar] [CrossRef]
- Shah, S.M.; Ashtikar, M.; Jain, A.S.; Makhija, D.T.; Nikam, Y.; Gude, R.P.; Steiniger, F.; Jagtap, A.A.; Nagarsenker, M.S.; Fahr, A. LeciPlex, invasomes, and liposomes: A skin penetration study. Int. J. Pharm. 2015, 490, 391–403. [Google Scholar] [CrossRef] [PubMed]
- Jurairattanaporn, N.; Chalermchai, T.; Ophaswongse, S.; Udompataikul, M. Comparative Trial of Silver Nanoparticle Gel and 1% Clindamycin Gel when Use in Combination with 2.5% Benzoyl Peroxide in Patients with Moderate Acne Vulgaris. J. Med. Assoc. Thai. 2017, 100, 78–85. [Google Scholar] [PubMed]
- Sercombe, L.; Veerati, T.; Moheimani, F.; Wu, S.Y.; Sood, A.K.; Hua, S. Advances and Challenges of Liposome Assisted Drug Delivery. Front. Pharmacol. 2015, 6, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Al Sabaa, H.; Mady, F.M.; Hussein, A.K.; Abdel-Wahab, H.M.; Ragaie, M.H. Dapsone in topical niosomes for treatment of acne vulgaris. Afr. J. Pharm. Pharmacol. 2018, 12, 221–230. [Google Scholar] [CrossRef]
- Salama, A.; Badran, M.; Elmowafy, M.; Soliman, G.M. Spironolactone-Loaded LeciPlexes as Potential Topical Delivery Systems for Female Acne: In Vitro Appraisal and Ex Vivo Skin Permeability Studies. Pharmaceutics 2020, 12, 25. [Google Scholar] [CrossRef] [Green Version]
- Mistry, A.; Ravikumar, P. Development and Evaluation of Azelaic Acid Based Ethosomes for Topical Delivery for the Treatment of Acne. Indian J. Pharm. Educ. Res. 2016, 50, S232–S243. [Google Scholar] [CrossRef] [Green Version]
- El-Nabarawi, M.A.; Shamma, R.N.; Farouk, F.; Nasralla, S.M. Dapsone-Loaded Invasomes as a Potential Treatment of Acne: Preparation, Characterization, and In Vivo Skin Deposition Assay. AAPS PharmSciTech 2018, 19, 2174–2184. [Google Scholar] [CrossRef]
- Khan, S.; Jain, P.; Jain, S.; Jain, R.; Bhargava, S.; Jain, A. Topical Delivery of Erythromycin Through Cubosomes for Acne. Pharm. Nanotechnol. 2018, 6, 38–47. [Google Scholar] [CrossRef]
- Rahman, S.A.; Abdelmalak, N.S.; Badawi, A.; Elbayoumy, T.; Sabry, N.; El Ramly, A. Tretinoin-loaded liposomal formulations: From lab to comparative clinical study in acne patients. Drug Deliv. 2016, 23, 1184–1193. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Banga, A.K. Intradermal and follicular delivery of adapalene liposomes. Drug. Dev. Ind. Pharm. 2016, 42, 871–879. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhao, J.L.; Zhou, H.L. Study on the Preparation Process of Salicylic Acid Liposome Gel. Adv. Mat. Res. 2012, 554–556, 828–831. [Google Scholar] [CrossRef]
- Chorachoo, J.; Amnuaikit, T.; Voravuthikunchai, S.P. Liposomal Encapsulated Rhodomyrtone: A Novel Antiacne Drug. Evid. Based Complement. Alternat. Med. 2013, 2013, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thakur, N.; Jain, P.; Jain, V. Formulation Development and Evaluation of Transferosomal Gel. J. Drug. Deliv. Ther. 2018, 8, 168–177. [Google Scholar] [CrossRef] [Green Version]
- Yadav, A.V.; Murthy, M.S.; Shete, A.S.; Sakhare, S. Stability Aspects of Liposomes. Indian J. Pharm. Educ. Res. 2011, 45, 402–413. [Google Scholar]
- Chaurasia, L.; Singh, S.; Arora, K.; Saxena, C. Transferosome: A Suitable Delivery System for Percutaneous Administration. Curr. Res. Pharm. Sci. 2019, 9, 1–11. [Google Scholar] [CrossRef]
- McClements, D.J. Nanoemulsions versus microemulsions: Terminology, differences and similarities. Soft Matter 2012, 8, 1719–1729. [Google Scholar] [CrossRef]
- de Oca-Ávalos, J.M.M.; Candal, R.J.; Herrera, M.L. Nanoemulsions: Stability and physical properties. Curr. Opin. Food Sci. 2017, 16, 1–6. [Google Scholar] [CrossRef]
- Ngan, C.L.; Basri, M.; Tripathy, M.; Abedi, K.R.; Abdulmalek, E. Physicochemical Characterization and Thermodynamic Studies of Nanoemulsion-Based Transdermal Delivery System for Fullerene. Sci. World J. 2014, 2014, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Pavoni, L.; Perinelli, D.R.; Bonacucina, G.; Cespi, M.; Palmieri, G.F. An Overview of Micro- and Nanoemulsions as Vehicles for Essential Oils: Formulation, Preparation and Stability. Nanomaterials 2020, 10, 135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chircov, C.; Grumzescu, A.M. Nanoemulsion preparation, characterization, and application in the field of biomedicine. In Nanoarchitectonics in Biomedicine; Grumzescu, A.M., Ed.; William Andrew (Elsevier): Amsterdam, The Netherlands, 2019; pp. 169–188. [Google Scholar] [CrossRef]
- Hoar, T.P.; Schulman, J.H. Transparent water-oil dispersions, the oleophatic hydromycelle. Nature 1943, 152, 102–103. [Google Scholar] [CrossRef]
- Ruckenstein, E. Thermodynamics of Microemulsions II. In Handbook of Microemulsion Science and Technology; Kumar, P., Mittal, K.L., Eds.; Marcel Dekker: New York, NY, USA, 1999; pp. 45–58. [Google Scholar]
- Schulman, J.H.; Stoeckenius, W.; Prince, L.M. Mechanisms of Formation and Structure of Microemulsions by Electron Microscopy. J. Phys. Chem. 1959, 63, 1677–1680. [Google Scholar] [CrossRef]
- Najjar, R. Microemulsions—A Brief Introduction. In Microemulsions—An Introduction to Properties and Applications; Najjar, R., Ed.; IntechOpen: Rijeka, Croatia, 2012; pp. 3–30. [Google Scholar] [CrossRef] [Green Version]
- Dantas, T.N.C.; Santanna, V.C.; Souza, T.T.C.; Lucas, C.R.S.; Dantas Neto, A.A.; Aum, P.T.P. Microemulsios and Nanoemulsions Applied to Well Stimulation and Enhanced Oil Recovery. Brazil. J. Petrol. Gas 2018, 12, 251–265. [Google Scholar] [CrossRef]
- Khan, B.A.; Akhtar, N.; Khan, H.M.S.; Waseem, K.; Mahmood, T.; Rasul, A.; Iqbal, M.; Khan, H. Basics of pharmaceutical emulsions: A review. Afr. J. Pharm. Pharmacol. 2011, 5, 2715–2725. [Google Scholar] [CrossRef]
- Estanqueiro, M.; Conceição, J.; Amaral, M.H.; Santos, D.; Silva, J.B.; Lobo, J.M.S. Characterization and stability studies of emulsion systems containing pumice. Brazil. J. Pharm. Sci. 2014, 50, 361–369. [Google Scholar] [CrossRef] [Green Version]
- Talegaonkar, S.; Azeem, A.; Ahmad, F.J.; Khar, R.K.; Pathan, S.A.; Khan, Z.I. Microemulsions: A novel approach to enhanced drug delivery. Recent Pat. Drug. Deliv. Formul. 2008, 2, 238–257. [Google Scholar] [CrossRef] [PubMed]
- Karunaratne, D.N.; Pamunuwa, G.; Ranatunga, U. Introductory Chapter: Microemulsions. In Properties and Uses of Microemulsions; Karunaratne, D.N., Pamunuwa, G., Ranatunga, U., Eds.; IntechOpen: Rijeka, Croatia, 2017; pp. 3–15. [Google Scholar] [CrossRef] [Green Version]
- Muzaffar, F.; Singh, U.K.; Chauchan, L. Review on microemulsion as futuristic drug delivery. J. Pharm. Pharm. Sci. 2013, 5, 39–53. [Google Scholar]
- Tlusty, T.; Safran, S.A. Microemulsion networks: The onset of bicontinuity. J. Phys. Condens. Matter 2000, 12, 253–262. [Google Scholar] [CrossRef] [Green Version]
- Nordiyana, M.S.W.; Khalil, M.; Jan, B.M.; Ali, B.S.; Tong, C.W. Formation and Phase Behavior of Winsor Type III Jatropha curcas-Based Microemulsion Systems. J. Surfactants Deterg. 2016, 19, 701–712. [Google Scholar] [CrossRef]
- Salager, J.-L. Emulsion Phase Inversion Phenomena. In Emulsions and Emulsion Stability, 2nd ed.; Sjoblöm, J., Ed.; CRC Press (Taylor & Francis Group): Boca Raton, FL, USA, 2005; Volume 132, pp. 185–223. [Google Scholar] [CrossRef]
- Santos, P.; Watkinson, A.C.; Hadgraft, J.; Lane, M.E. Application of microemulsions in dermal and transdermal drug delivery. Skin Pharmacol. Physiol. 2008, 21, 246–259. [Google Scholar] [CrossRef] [PubMed]
- Callender, S.P.; Mathews, J.A.; Kobernyk, K.; Wettig, S.D. Microemulsion utility in pharmaceuticals: Implications for multi-drug delivery. Int. J. Pharm. 2017, 526, 425–442. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.K.; Garg, T.; Goyal, A.K.; Rath, G. Role of microemulsions in advanced drug delivery. Artif. Cells Nanomed. Biotechnol. 2016, 44, 1177–1185. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, P.K.; Murthy, R.S.R. Microemulsions: A potential drug delivery system. Curr. Drug Deliv. 2006, 3, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Alaoui, Y.E.; Fahry, A.; Rahali, Y.; Cherkaoui, N.; Bensouda, Y.; Laatiris, A. Formulation, optimization and characterization of ibuprofen loaded microemulsion systems using D-optimal mixture design. Int. J. Appl. Pharm. 2019, 11, 304–312. [Google Scholar] [CrossRef]
- Lopes, L.B. Overcoming the cutaneous barrier with microemulsions. Pharmaceutics 2014, 6, 52–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amarji, B.; Garg, N.K.; Singh, B.; Katare, O.P. Microemulsions mediated effective delivery of methotrexate hydrogel: More than a tour de force in psoriasis therapeutics. J. Drug Target. 2016, 24, 147–160. [Google Scholar] [CrossRef] [PubMed]
- Vadlamudi, H.C.; Yalavarthi, P.R.; Basaveswara Rao, M.V.; Sundaresan, C.R. Insights of Microemulsions – A Thermodynamic Comprehension. Jordan. J. Pharm. Sci. 2017, 10, 23–40. [Google Scholar]
- Lindman, B.; Shinoda, K.; Olsson, U.; Anderson, D.; Karlström, G.; Wennerström, H. On the demonstration of bicontinuous structures in microemulsions. Colloids Surf. 1989, 38, 205–224. [Google Scholar] [CrossRef]
- Jha, S.K.; Dey, S.; Karki, R. Microemulsions-potential carriers for improved drug delivery. Asian J. Biomed. Pharm. Sci. 2011, 1, 5–9. [Google Scholar]
- Mehta, S.K.; Kaur, G. Microemulsions: Thermodynamic and Dynamic Properties. In Thermodynamics; Mizutani, T., Ed.; IntechOpen: Rijeka, Croatia, 2011; pp. 381–407. [Google Scholar] [CrossRef] [Green Version]
- Kegel, W.K.; Overbeek, J.T.G.; Lekkerkerker, H.N.W. Thermodynamics of Microemulsions I. In Handbook of Microemulsion Science and Technology; Kumar, P., Mittal, K.L., Eds.; Marcel Dekker: New York, NY, USA, 1999; pp. 13–44. [Google Scholar] [CrossRef]
- De Gennes, P.G.; Taupin, C. Microemulsions and the flexibility of oil/water interfaces. J. Phys. Chem. 1982, 86, 2294–2304. [Google Scholar] [CrossRef]
- Del-real, A.; Garcóa-Garduño, M.V.; Castaño, V.M. Transmission Electron Microscopy Observations of Micelle Structure in a Vinyl Acetate-based Microemulsion. Int. J. Polym. Mater. Polym. Biomat. 1998, 42, 319–326. [Google Scholar] [CrossRef]
- Reis, M.Y.F.A.; dos Santos, S.M.; Silva, D.R.; Silva, M.V.; Correia, M.T.S.; Ferraz Navarro, D.M.A.; Santos, G.K.N.; Hallwass, F.; Bianchi, O.; Silva, A.G.; et al. Anti-Inflammatory Activity of Babassu Oil and Development of a Microemulsion System for Topical Delivery. Evid. Based Complentary Altern. Med. 2017, 2017, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, L.-N.; Zhang, Y.-T.; Wang, Q.; Xu, L.; Feng, N.-P. Preparation and evaluation of microemulsion-based transdermal delivery of total flavone of rhizoma arisaematis. Int. J. Nanomed. 2014, 9, 3453–3464. [Google Scholar] [CrossRef] [Green Version]
- Teng, X.; Li, F.; Lu, C. Visualization of materials using the confocal laser scanning microscopy technique. Chem. Soc. Rev. 2020, 49, 2408–2425. [Google Scholar] [CrossRef]
- Robbins, M.L.; Bock, J.; Shuang, J. Model for microemulsions: III. Interfacial tension and droplet size correlation with phase behavior of mixed surfactants. J. Colloids Interface Sci. 1988, 126, 114–133. [Google Scholar] [CrossRef]
- Bergström, L.M. Thermodynamics and Bending Energetics of Microemulsions. In Properties and Uses of Microemulsions; Karunaratne, D.N., Pamunuwa, G., Ranatunga, U., Eds.; IntechOpen: Rijeka, Croatia, 2017; pp. 117–138. [Google Scholar] [CrossRef] [Green Version]
- Binks, B.P.; Meunier, J.; Langevin, D. Characteristic sizes, film rigidity and interfacial tensions in microemulsion systems. In Trends in Colloid and Interface Science III. Progress in Colloid & Polymer Science; Bothorel, P., Dufourc, E.J., Eds.; Steinkopff: Darmstadt, Germany, 1989; Volume 79, pp. 208–213. [Google Scholar] [CrossRef]
- Kos, Ž.; Ravnik, M. Relevance of saddle-splay elasticity in complex nematic geometries. Soft Matter 2016, 12, 1313–1323. [Google Scholar] [CrossRef] [Green Version]
- Geethu, P.M.; Yadav, I.; Mani, E.; Aswal, V.K.; Satapathy, D.K. Saddle-splay modulus of reverse microemulsions: Experimental determination using small-angle neutron scattering and dielectric relaxation spectroscopy. Phys. Rev. E 2018, 98, 052604. [Google Scholar] [CrossRef]
- Langevin, D. Micelles and Microemulsions. Annu. Rev. Phys. Chem. 1992, 43, 341–369. [Google Scholar] [CrossRef]
- Chamieh, J.; Davanier, F.; Jannin, V.; Demarne, F.; Cottet, H. Size characterization of commercial micelles and microemulsions by Taylor dispersion analysis. Int. J. Pharm. 2015, 492, 46–54. [Google Scholar] [CrossRef]
- Chamieh, J.; Merdassi, H.; Rossi, J.-C.; Jannin, V.; Demarne, F.; Cottet, H. Size characterization of lipid-based self-microemulsifying pharmaceutical excipients durig lipolysis using Taylor dispersion analysis with fluorescence detection. Int. J. Pharm. 2018, 537, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Zemb, T.N.; Klossek, M.; Lopian, T.; Marcus, J.; Schöettl, S.; Horinek, D.; Prevost, S.F.; Touraud, D.; Diat, O.; Marčelja, S.; et al. How to explain microemulsions formed by solvent mixtures without conventional surfactants. Proc. Natl. Acad. Sci. USA 2016, 113, 4260–4265. [Google Scholar] [CrossRef] [Green Version]
- Klossek, M.L.; Touraud, D.; Zemb, T.; Kunz, W. Structure and Solubility in Surfactant-Free Microemulsions. Chemphyschem 2012, 13, 4116–4119. [Google Scholar] [CrossRef]
- Lucia, A.; Argudo, P.G.; Guzmán, E.; Rubio, R.G.; Ortega, F. Formation of surfactant free microemulsions in the ternary system water/eugenol/ethanol. Colloids Surf. A Physicochem. Eng. Asp. 2017, 521, 133–140. [Google Scholar] [CrossRef]
- François, G.; Katz, J.L. Nanoparticles and nanocapsules created using the Ouzo effect: Spontaneous emulisification as an alternative to ultrasonic and high-shear devices. Chemphyschem 2005, 6, 209–216. [Google Scholar] [CrossRef]
- Clausse, M.; Peyrelasse, J.; Heil, J.; Boned, C.; Lagourette, B. Bicontinuous structure zones in microemulsions. Nature 1981, 293, 636–638. [Google Scholar] [CrossRef]
- Kogan, A.; Shalev, D.E.; Raviv, U.; Aserin, A.; Garti, N. Formation and Characterization of Ordered Bicontinuous Microemulsions. J. Phys. Chem. B 2009, 113, 10669–10678. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Dominguez, M.; Aubery, C.; Solans, C. New Trends on the Synthesis of Inorganic Nanoparticles Using Microemulsions as Confined Reaction Media. In Smart Nanoparticles Technology; Hashim, A.A., Ed.; IntechOpen: Rijeka, Croatia, 2012; pp. 195–219. [Google Scholar] [CrossRef] [Green Version]
- Prévost, S.; Gradzielski, M.; Zemb, T. Self-assembly, phase behaviour and structural behaviour as observed by scattering for classical and non-classical microemulsions. Adv. Colloid Interface Sci. 2017, 247, 374–396. [Google Scholar] [CrossRef] [PubMed]
- Polarz, S.; Kunkel, M.; Donner, A.; Schlötter, M. Added-Value Surfactants. Chemistry 2018, 24, 18842–18856. [Google Scholar] [CrossRef] [Green Version]
- Schramm, L.L.; Stasiuk, E.N.; Marangonic, D.G. Surfactants and their applications. Annu. Rep. Prog. Chem. Sect. C Phys. Chem. 2003, 99, 3–48. [Google Scholar] [CrossRef]
- Dutt, S.; Siril, P.F.; Remita, S. Swollen liquid crystals (SLCs): A versatile template for the synthesis of nano structured materials. RSC Adv. 2017, 7, 5733–5750. [Google Scholar] [CrossRef] [Green Version]
- Sekhon, B.S. Surfactants: Pharmaceutical and Medicinal Aspects. JPTRM 2013, 1, 43–88. [Google Scholar] [CrossRef] [Green Version]
- Attwood, D.; Florence, A.T. Physical Pharmacy; Pharmaceutical Press: London, UK, 2008; pp. 43–47. ISBN 978-0-85369-725-1. [Google Scholar]
- Pandey, A.; Mittal, A.; Chauhan, N.; Alam, S. Role of Surfactants as Penetration Enhancer in Transdermal Drug Delivery System. J. Mol. Pharm. Org. Process Res. 2014, 2, 1–10. [Google Scholar] [CrossRef]
- Mohamed, A.I.A.; Sultan, A.S.; Hussein, I.A.; Al-Muntasheri, G.A. Influence of Surfactant Structure on the Stability of Water-in-Oil Emulsions under High-Temperature High-Salinity Conditions. J. Chem. 2017, 2017, 1–11. [Google Scholar] [CrossRef]
- Ruckenstein, E. Microemulsions, Macroemulsions, and the Bancroft Rule. Langmuir 1996, 12, 6351–6353. [Google Scholar] [CrossRef]
- Ramadan, E.; Borg, T.; Abdelghani, G.M.; Saleh, N.M. Formulation and evaluation of acyclovir microemulsions. Bull. Pharm. Sci. 2013, 36, 31–47. [Google Scholar]
- Pappinen, S.; Urtti, A. Microemulsions. In Percutaneous Penetration Enhancers Chemical Methods in Penetration Enhancement: Nanocarriers; Dragicevic, N., Maibach, H.I., Eds.; Springer: Berlin/Heidelberg, Germany, 2016; pp. 253–262. [Google Scholar] [CrossRef]
- Moroi, Y. Micelles: Theoretical and Applied Aspects; Plenum Press: New York, NY, USA, 1992; pp. 12–13. ISBN 978-1-4899-0702-8. [Google Scholar]
- Prieto, C.; Calvo, L. Performance of the Biocompatible Surfactant Tween 80, for the Formation of Microemulsions Suitable for New Pharmaceutical Processing. J. Appl. Chem. 2013, 2013, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Bowman, B.J.; Ofner, C.M.; Schott, H. Colloidal dispersions. In Remigton: The Science and Practice of Pharmacy, 21th ed.; Troy, D.B., Beringer, P., Eds.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2005; pp. 293–319. ISBN 0-683-306472. [Google Scholar]
- Chai, J.L.; Liu, N.; Bai, T.T.; Zhang, H.M.; Liu, N.N.; Wang, D. Compositions and Physicochemical Properties of Tween Type Surfactants-Based Microemulsions. J. Disper. Sci. Technol. 2014, 35, 441–447. [Google Scholar] [CrossRef]
- Neghi, J.S. Nanolipid Materials for Drug Delivery Systems: A Comprehensive Review. In Characterization and Biology of Nanomaterials for Drug Delivery: Nanoscience and Nanotechnology in Drug Delivery; Mohapatra, S.S., Ranjan, S., Dasgupta, N., Mishra, R.K., Thomas, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 137–163. [Google Scholar] [CrossRef]
- Ngawhirunpat, T.; Worachun, N.; Opanasopit, P.; Rojanarata, T.; Panomsuk, S. Cremophor RH40-PEG 400 microemulsions as transdermal drug delivery carrier for ketoprofen. Pharm. Dev. Technol. 2013, 18, 798–803. [Google Scholar] [CrossRef]
- Gelderblom, H.; Verweij, J.; Nooter, K.; Sparreboom, A. Cremophor EL: The drawbacks and advantages of vehicle selection for drug formulation. Eur. J. Cancer 2001, 37, 1590–1598. [Google Scholar] [CrossRef]
- Lo, J.-T.; Lee, T.-M.; Chen, B.-H. Nonionic Microemulsions as Solubilizers of Hydrophobic Drugs: Solubilization of Paclitaxel. Materials 2016, 9, 761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bevinakatti, H.S.; Mishra, B.K. Sugar derived surfactants. In Design and Selection of Performance Surfactants: Annual Surfactants Review; Karsa, D.R., Ed.; Sheffield Academic Press: Sheffield, UK, 1999; Volume 2, pp. 1–40. ISBN 1-85075-993-6. [Google Scholar]
- Fanun, M. Microemulsions with Mixed Nonionic Surfactants and Flavour Oil. J. Surfactants Deterg. 2010, 13, 321–328. [Google Scholar] [CrossRef]
- Weete, J.D.; Betageri, S.; Griffith, G.L. Improvement of lecithin as an emulsifier for water-in-oil emulsions by thermalization. J. Am. Oil. Chem. Soc. 1994, 71, 731–737. [Google Scholar] [CrossRef]
- Xuan, X.-Y.; Cheng, Y.-L.; Acosta, E. Lecithin-linker microemulsion gelatin gels for extended drug delivery. Pharmaceutics 2012, 4, 104–129. [Google Scholar] [CrossRef]
- Zhou, H.; Yue, Y.; Liu, G.; Li, Y.; Zhang, J.; Gong, Q.; Yan, Z.; Duan, M. Preparation and characterization of a lecithin nanoemulsion as a topical delivery system. Nanoscale Res. Lett. 2009, 5, 224–230. [Google Scholar] [CrossRef] [Green Version]
- Surabhi, K.; Katare, O.P.; Sushma, D. Lecithinised Microemulsions for Topical Delivery of Tretinoin. Int. J. Drug Dev. Res. 2010, 2, 711–719. [Google Scholar]
- Golwala, P.; Rathod, S.; Patil, R.; Joshi, A.; Ray, D.; Aswal, V.K.; Bahadur, P.; Tiwari, S. Effect of cosurfactant addition on phase behavior and microstructure of a water dilutable microemulsion. Colloids Surf. B Biointerfaces 2020, 186, 110736. [Google Scholar] [CrossRef]
- Zhong, F.; Xu, W.; Fu, T.; Li, Y. Preparation and Characterization of Functional Compounds Encapsulated Microemulsion with Nonionic Surfactants. J. Food Drug Anal. 2012, 20, 203–207. [Google Scholar] [CrossRef]
- Kogan, A.; Garti, N. Microemulsions as transdermal drug delivery vehicles. Adv. Colloid Interface Sci. 2006, 123‒126, 369–385. [Google Scholar] [CrossRef]
- Kumar, A.; Kushwaha, V.; Sharma, P.K. Pharmaceutical microemulsion: Formulation, characterization and drug delivery across skin. Int. J. Drug Dev. Res. 2014, 6, 1–21. [Google Scholar]
- Abd Sisak, M.A.; Daik, R.; Ramli, S. Study on the effect of oil phase and co-surfactant on microemulsion systems. Malaysian J. Anal. Sci. 2017, 21, 1409–1416. [Google Scholar] [CrossRef]
- Xavier-Junior, F.H.; Vauthier, C.; Morais, A.R.V.; Alencar, E.N.; Egito, E.S.T. Microemulsion systems containing bioactive natural oils: An overview on the state of the art. Drug Dev. Ind. Pharm. 2017, 43, 700–714. [Google Scholar] [CrossRef] [PubMed]
- Djekic, L.; Primorac, M. The influence of cosurfactants and oils on the formation of pharmaceutical microemulsions based on PEG-8 caprylic/capric glycerides. Int. J. Pharm. 2008, 352, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Hortolomei, M.; Popovici, I.; Ochiuz, L. Influence of formulation factors on the physico-chemical characteristics of dermal microemulsions prepared with sucrose esters. Farmacia 2012, 60, 484–492. [Google Scholar]
- Scomoroscenco, C.; Cinteza, L.O.; Teodorescu, M.; Gifu, I.C.; Ianchis, R.; Nistor, C.L.; Petcu, C.; Ninciuleanu, C.M.; Alexandrescu, E.; Mihaescu, C.I. Preparation and Characterization of Vegetable Oil-Based Microemulsions. Proceedings 2019, 29, 74. [Google Scholar] [CrossRef] [Green Version]
- Pascoa, H.; Diniz, D.G.A.; Florentino, I.F.; Costa, E.A.; Bara, M.T.F. Microemulsion based on Pterodon emarginatus oil and its anti-inflammatory potential. Braz. J. Pharm. Sci. 2015, 51, 117–125. [Google Scholar] [CrossRef]
- Chaiyana, W.; Leelapornpisid, P.; Phongpradist, R.; Kiattisin, K. Enhancement of antioxidant and skin moisturizing effects of olive oil by incorporation into microemulsions. Nanomater. Nanotechnol. 2016, 6, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Lin, T.-K.; Zhong, L.; Santiago, J.L. Anti-Inflammatory and Skin Barrier Repair Effects of Topical Application of Some Plant Oils. Int. J. Mol. Sci. 2017, 19, 70. [Google Scholar] [CrossRef] [Green Version]
- Butnariu, M.; Sarac, I. Essential Oils from Plants. J. Biotech. Biomed. Sci. 2018, 1, 35–43. [Google Scholar] [CrossRef] [Green Version]
- Tongnuanchan, P.; Benjakul, S. Essential Oils: Extraction, Bioactivities, and Their Uses for Food Preservation. J. Food Sci. 2014, 79, 1231–1249. [Google Scholar] [CrossRef]
- Pandit, J.; Aqil, M.; Sultana, Y. Terpenes and Essential Oils as Skin Penetration Enhancers. In Percutaneous Penetration Enhancers Chemical Methods in Penetration Enhancement: Modification of Stratum Corneum; Dragicevic, N., Maibach, H.I., Eds.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 173–193. [Google Scholar] [CrossRef]
- Fernández-Peña, L.; Gutiérrez-Muro, S.; Guzmán, E.; Lucia, A.; Ortega, F.; Rubio, R.G. Oil-In-Water Microemulsions for Thymol Solubilization. Colloids Interfaces 2019, 3, 64. [Google Scholar] [CrossRef] [Green Version]
- Edris, A.E.; Rawlinson-Malone, C.F.R. Preferential solubilization behaviours and stability of some phenolic-bearing essential oils formulated in different microemulsion systems. Int. J. Cosmet. Sci. 2012, 34, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Liu, T.; Ma, H.; Tian, Y.; Li, L.; Li, Z.; Gao, M.; Zhang, J.; Tang, Z. Preparation of Essential Oil-Based Microemulsions for Improving the Solubility, pH Stability, Photostability, and Skin Permeation of Quercetin. AAPS PharmSciTech 2017, 18, 3097–3104. [Google Scholar] [CrossRef] [PubMed]
- Zu, Y.; Yu, H.; Liang, L.; Fu, Y.; Efferth, T.; Liu, X.; Wu, N. Activities of ten essential oils towards Propionibacterium acnes and PC-3, A-549 and MCF-7 cancer cells. Molecules 2010, 15, 3200–3210. [Google Scholar] [CrossRef] [PubMed]
- Orchard, A.; van Vuuren, S. Commercial Essential Oils as Potential Antimicrobials to Treat Skin Diseases. Evid. Based Complement. Alternat. Med. 2017, 2017, 1–92. [Google Scholar] [CrossRef] [Green Version]
- Kim, B.Y.; Shin, S. Antimicrobial and Improvement Effects of Tea Tree and Lavender Oils on Acne Lesions. J. Converg. Inf. Technol. 2013, 8, 339–345. [Google Scholar]
- Kim, K.Y.; Jang, H.H.; Lee, S.N.; Kim, Y.-S.; An, S. Effects of the myrtle essential oil on the acne skin—clinical trials for Korean women. Biomed. Dermatol. 2018, 2, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Ma, Q.; Davidson, P.M.; Zhong, Q. Antimicrobial properties of microemulsions formulated with essential oils, soybean oil, and Tween 80. Int. J. Food Microbiol. 2016, 226, 20–25. [Google Scholar] [CrossRef] [Green Version]
- Kaur, G.; Mehta, S.K. Developments of Polysorbate (Tween) based microemulsions: Preclinical drug delivery, toxicity and antimicrobial applications. Int. J. Pharm. 2017, 529, 134–160. [Google Scholar] [CrossRef] [PubMed]
- Moghimipour, E.; Salimi, A.; Eftekhari, S. Design and characterization of microemulsion systems for naproxen. Adv. Pharm. Bull. 2013, 3, 63–71. [Google Scholar] [CrossRef]
- Hessien, M.; Singh, N.; Kim, C.; Prouzet, E. Stability and tunability of O/W nanoemulsions prepared by phase inversion composition. Langmuir 2011, 27, 2299–2307. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Ma, X.-H.; Yao, G.-L.; Zhang, W.-T.; Zha, Y. Microemulsion-based anthocyanin systems: Effect of surfactants, cosurfactants, and its stability. Int. J. Food Prop. 2018, 21, 1152–1165. [Google Scholar] [CrossRef]
- Chiappisi, L.; Noirez, L.; Gradzielski, M. A journey through the phase diagram of a pharmaceutically relevant microemulsion system. J. Colloid Interface Sci. 2016, 473, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.W.; Gao, P. Emulsions and Microemulsions for Topical and Transdermal Drug Delivery. In Handbook of Non-Invasive Drug Delivery Systems, 1st ed.; Kulkarni, V.S., Ed.; William Andrew (Elsevier): Oxford, UK, 2010; pp. 59–94. [Google Scholar] [CrossRef]
- Date, A.A.; Naik, B.; Nagarsenker, M.S. Novel Drug Delivery Systems: Potential in Improving Topical Delivery of Antiacne Agents. Skin Pharmacol. Physiol. 2006, 19, 2–16. [Google Scholar] [CrossRef]
- Oxley, K.S.; Jackson, J.B. Acne (Vulgaris and Rosacea). In xPharm: The Comprehensive Pharmacology Reference; Enna, S.J., Bylund, D.B., Eds.; Elsevier: Oxford, UK, 2007; pp. 1–5. [Google Scholar] [CrossRef]
- Shukla, T.; Upmanyu, N.; Agrawal, M.; Saraf, S.; Saraf, S.; Alexander, A. Biomedical applications of microemulsion through dermal and transdermal route. Biomed. Pharmacother. 2018, 108, 1477–1494. [Google Scholar] [CrossRef]
- Mortazavi, S.A.; Pishrochi, S.; Jafari Azar, Z. Formulation and In-vitro Evaluation of Tretinoin Microemulsion as a Potential Carrier for Dermal Drug Delivery. Iran. J. Pharm. Res. 2013, 12, 599–609. [Google Scholar]
- Gupta, R.; Badhe, Y.; Rai, B.; Mitragotri, S. Molecular mechanism of the skin permeation enhancing effect of ethanol: A molecular dynamics study. RSC Adv. 2020, 10, 12234–12248. [Google Scholar] [CrossRef] [Green Version]
- Carrer, V.; Alonso, C.; Pont, M.; Zanuy, M.; Córdoba, M.; Espinosa, S.; Barba, C.; Oliver, M.A.; Martí, M.; Coderch, L. Effect of propylene glycol on the skin penetration of drugs. Arch. Dermatol. Res. 2020, 312, 337–352. [Google Scholar] [CrossRef]
- Praça, F.G.; Viegas, J.S.R.; Peh, H.Y.; Garbin, T.N.; Medina, W.S.G.; Lopes Badra Bentley, M.V. Microemulsion co-delivering vitamin A and vitamin E as a new platform for topical treatment of acute skin inflammation. Mater. Sci. Eng. C 2020, 110, 110639. [Google Scholar] [CrossRef]
- Hathout, R.M.; Mansour, S.; Mortada, N.D.; Geneidi, A.S.; Guy, R.H. Uptake of microemulsion components into the stratum corneum and their molecular effects on skin barrier function. Mol. Pharm. 2010, 7, 1266–1273. [Google Scholar] [CrossRef]
- Osborne, D.W.; Musakhanian, J. Skin Penetration and Permeation Properties of Transcutol®-Neat or Diluted Mixtures. AAPS PharmSciTech 2018, 19, 3512–3533. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, C.; Jain, P.; Sharma, H.; Lam, S.; Sonti, S. Investigating the effect of transcutol on the physical properties of an O/W cream. J. Disper. Sci. Technol. 2020, 41, 600–606. [Google Scholar] [CrossRef]
- Björklund, S.; Pham, Q.D.; Jensen, L.B.; Knudsen, N.Ø.; Nielsen, L.D.; Ekelund, K.; Ruzgas, T.; Engblom, J.; Sparr, E. The effects of polar excipients transcutol and dexpanthenol on molecular mobility, permeability, and electrical impedance of the skin barrier. J. Colloid Interface Sci. 2016, 479, 207–220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Changez, M.; Chander, J.; Dinda, A.K. Transdermal permeation of tetracaine hydrochloride by lecithin microemulsion: In vivo. Colloids Surf. B Biointerfaces 2006, 48, 58–66. [Google Scholar] [CrossRef]
- Patzelt, A.; Lademann, J.; Richter, H.; Darvin, M.E.; Schanzer, S.; Thiede, G.; Sterry, W.; Vergou, T.; Hauser, M. In vivo investigations on the penetration of various oils and their influence on the skin barrier. Skin Res. Technol. 2012, 18, 364–369. [Google Scholar] [CrossRef]
- Chaiyana, W.; Leelapornpisid, P.; Jakmunee, J.; Korsamphan, C. Antioxidant and Moisturizing Effect of Camellia assamica Seed Oil and Its Development into Microemulsion. Cosmetics 2018, 5, 40. [Google Scholar] [CrossRef] [Green Version]
- Agrahari, V. Novel drug delivery systems, devices, and fabrication methods. Drug Deliv. Transl. Res. 2018, 8, 303–306. [Google Scholar] [CrossRef] [Green Version]
- Rangarajan, M.; Zatz, J.L. Effect of formulation on the topical delivery of alpha-tocopherol. J. Cosmet. Sci. 2003, 54, 161–174. [Google Scholar]
- Keen, M.A.; Hassan, I. Vitamin E in dermatology. Indian Dermatol. Online J. 2016, 7, 311–315. [Google Scholar] [CrossRef]
- Fabre, G.; Bayach, I.; Berka, K.; Paloncýová, M.; Starok, M.; Rossi, C.; Duroux, J.-L.; Otyepka, M.; Trouillas, P. Synergism of antioxidant action of vitamins E, C and quercetin is related to formation of molecular associations in biomembranes. Chem. Commun. 2015, 51, 7713–7716. [Google Scholar] [CrossRef]
- Rozman, B.; Gasperlin, M. Stability of vitamins C and E in topical microemulsions for combined antioxidant therapy. Drug Deliv. 2007, 14, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Bugaj, A.M. Intradermal Delivery of Active Cosmeceutical Ingredients. In Novel Delivery Systems for Transdermal and Intradermal Drug Delivery, 1st ed.; Donnelly, R.F., Singh, T.R.R., Eds.; John Wiley & Sons: Chichester, UK, 2015; pp. 209–242. [Google Scholar] [CrossRef]
- Caritá, A.C.; Fonseca-Santos, B.; Shultz, J.D.; Michniak-Kohn, B.; Chorilli, M.; Leonardi, G.R. Vitamin C: One compound, several uses. Advances for delivery, efficiency and stability. Nanomedicine 2020, 24, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Telang, P.S. Vitamin C in dermatology. Indian Dermatol. Online J. 2013, 4, 143–146. [Google Scholar] [CrossRef] [PubMed]
- Pepe, D.; Phelps, J.; Lewis, K.; Dujack, J.; Scarlett, K.; Jahan, S.; Bonnier, E.; Milic-Pasetto, T.; Hass, M.A.; Lopes, L.B. Decylglucoside-based microemulsions for cutaneous localization of lycopene and ascorbic acid. Int. J. Pharm. 2012, 434, 420–428. [Google Scholar] [CrossRef] [PubMed]
- Ramli, S.; Chyi, K.T.; Zainuddin, N.; Mokhtar, W.N.A.W.; Abdul Rahman, I. The Influence of Surfactant/Co-Surfactant Hydrophilic-Lipophilic Balance on the Formation of Limonene-Based Microemulsion as Vitamin C Carrier. Sains Malays. 2019, 48, 1035–1042. [Google Scholar] [CrossRef]
- Moghimipour, E.; Salimi, A.; Leis, F. Preparation and evaluation of tretinoin microemulsion based on pseudo-ternary phase diagram. Adv. Pharm. Bull. 2012, 2, 141–147. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, D.; de Andrade, D.F.; de Oliveira, E.G.; Beck, R.C.R. Liquid chromatography method to assay tretinoin in skin layers: Validation and application in skin penetration/retention studies. Heliyon 2020, 6, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Ramli, S.; Ross, B.P.; Gentle, I.R. Formulation and physical characterization of microemulsions containing isotretinoin. In Proceedings of the 2009 International Conference on Biomedical and Pharmaceutical Engineering, Singapore, 2–4 December 2009. [Google Scholar] [CrossRef]
- Patel, M.R.; Patel, R.B.; Parikh, J.R.; Patel, B.G. Improving the isotretinoin photostability by incorporating in microemulsion matrix. ISRN Pharm. 2011, 2011, 1–6. [Google Scholar] [CrossRef]
- Patel, M.R.; Patel, R.B.; Parikh, J.R.; Patel, B.G. Novel isotretinoin microemulsion-based gel for targeted topical therapy of acne: Formulation consideration, skin retention and skin irritation studies. Appl. Nanosci. 2016, 6, 539–553. [Google Scholar] [CrossRef] [Green Version]
- Gürbüz, A.; Özhan, G.; Güngör, S.; Erdal, M.S. Colloidal carriers of isotretinoin for topical acne treatment: Skin uptake, ATR-FTIR and in vitro cytotoxicity studies. Arch. Dermatol. Res. 2015, 307, 607–615. [Google Scholar] [CrossRef]
- Gürbüz, A.; Güngör, S.; Erdal, M.S. Development and In Vitro Characterization of Microemulsions of Isotretinoin. Acta Pharm. Sci. 2017, 55, 17–32. [Google Scholar] [CrossRef] [Green Version]
- Wani, A.; Sanghani, C.; Wani, S. Formulation, Characterization, and in Vitro Evaluation of Novel Microemulsion-Based Spray for Topical Delivery of Isotretinoin. Asian J. Pharm. Clin. Res. 2018, 11, 226–232. [Google Scholar] [CrossRef]
- Patel, M.R.; Patel, R.B.; Parikh, J.R.; Patel, B.G. Formulation consideration and skin retention study of microemulsion containing tazarotene for targeted therapy of acne. J. Pharm. Investig. 2016, 46, 55–66. [Google Scholar] [CrossRef]
- Gurram, A.K.; Deshpande, P.B.; Kar, S.S.; Nayak, U.Y.; Udupa, N.; Reddy, M.S. Role of Components in the Formation of Self-microemulsifying Drug Delivery Systems. Indian J. Pharm. Sci. 2015, 77, 249–257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das, S.S.; Singh, A.; Kar, S.; Ghosh, R.; Pal, M.; Fatima, M.; Singh, N.; Singh, S.K. Application of QbD Framework for Development of Self-Emulsifying Drug Delivery Systems. In Pharmaceutical Quality by Design: Principles and Applications; Beg, S., Hasnain, M.S., Eds.; Academic Press (Elsevier): London, UK, 2019; pp. 297–350. [Google Scholar] [CrossRef]
- Patel, M.R.; Patel, R.B.; Parikh, J.R.; Patel, B.G. Novel microemulsion-based gel formulation of tazarotene for therapy of acne. Pharm. Dev. Technol. 2016, 21, 921–932. [Google Scholar] [CrossRef]
- Zachar, C.L. Pharmaceutically Relevant Microemulsions with Potential Topical, Ophthalmic, and Parenteral Applications. Master’s Thesis, The University of Toledo, Toledo, Spain, 2010. [Google Scholar]
- Nasr, M.; Abdel-Hamid, S. Optimizing the dermal accumulation of a tazarotene microemulsion using skin deposition modeling. Drug Dev. Ind. Pharm. 2016, 42, 636–643. [Google Scholar] [CrossRef]
- Bhatia, G.; Zhou, Y.; Banga, A.K. Adapalene microemulsion for transfollicular drug delivery. J. Pharm. Sci. 2013, 102, 2622–2631. [Google Scholar] [CrossRef]
- Pajić, N.Z.B.; Todosijević, M.N.; Vuleta, G.M.; Cekić, N.D.; Dobričić, V.D.; Vučen, S.R.; Čalija, B.R.; Lukić, M.Ž.; Ilić, T.M.; Savić, S.D. Alkyl polyglucoside vs. ethoxylated surfactant-based microemulsions as vehicles for two poorly water-soluble drugs: Physicochemical characterization and in vivo skin performance. Acta Pharm. 2017, 67, 415–439. [Google Scholar] [CrossRef] [Green Version]
- Silva, A.E.; Barratt, G.; Chéron, M.; Egito, E.S.T. Development of oil-in-water microemulsions for the oral delivery of amphotericin B. Int. J. Pharm. 2013, 454, 641–648. [Google Scholar] [CrossRef] [Green Version]
- Pajić, N.B.; Ilić, T.; Nikolić, I.; Dobričić, V.; Pantelić, I.; Savić, S. Alkyl polyglucoside-based adapalene-loaded microemulsions for targeted dermal delivery: Structure, stability and comparative biopharmaceutical characterization with a conventional dosage form. J. Drug Deliv. Sci. Technol. 2019, 54, 101245. [Google Scholar] [CrossRef]
- Nielson, C.; Hsu, S.; Motaparthi, K. Topical Antibacterial Agents. In Comprehensive Dermatologic Drug Therapy, 4th ed.; Wolverton, S., Wu, J.J., Eds.; Elsevier: Philadelphia, PA, USA, 2020; pp. 474–476. ISBN 978-0-323-61211-1. [Google Scholar]
- Hamilton, R.J.; Duffy, N.A.; Stone, D. Tarascon Pocket Pharmacopoeia, 28th ed.; Jones & Bartlett Learning: Burlington, NJ, USA, 2014; pp. 152–153. ISBN 978-1-284-02760-2. [Google Scholar]
- Ochiuz, L.; Hortolomei, M. Development of Microemulsion Dermal Products Based on Avocado Oil for Topical Administration. In Properties and Uses of Microemulsions; Karunaratne, D.N., Pamunuwa, G., Ranatunga, U., Eds.; IntechOpen: Rijeka, Croatia, 2017; pp. 17–30. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Agarwal, S.P.; Ahuja, A.; Ali, J.; Choudhry, R.; Baboota, S. Preparation, characterization, and in vitro antimicrobial assessment of nanocarrier based formulation of nadifloxacin for acne treatment. Pharmazie 2011, 66, 111–114. [Google Scholar] [PubMed]
- Shinde, U.; Pokharkar, S.; Modani, S. Design and evaluation of microemulsion gel system of nadifloxacin. Indian J. Pharm. Sci. 2012, 74, 237–247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peira, E.; Carlotti, M.E.; Cavalli, R.; Trott, M. Azelaic acid sodium salt in the formulation of microemulsions for topical applications. J. Drug Deliv. Sci. Technol. 2006, 16, 375–379. [Google Scholar] [CrossRef]
- Salimi, A.; Zadeh, B.S.M.; Godazgari, S.; Rahdar, A. Development and Evaluation of Azelaic Acid-Loaded Microemulsion for Transfollicular Drug Delivery Through Guinea Pig Skin: A Mechanistic Study. Adv. Pharm. Bull. 2020, 10, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Rivero, A.L.; Whitfeld, M. An update on the treatment of rosacea. Aust. Prescr. 2018, 41, 20–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tirnaksiz, F.; Kayiş, A.; Çelebi, N.; Adişen, E.; Erel, A. Preparation and evaluation of topical microemulsion system containing metronidazole for remission in rosacea. Chem. Pharm. Bull. 2012, 60, 583–592. [Google Scholar] [CrossRef] [Green Version]
- Rao, M.; Sukre, G.; Aghav, S.; Kumar, M. Optimization of Metronidazole Emulgel. J. Pharm. 2013, 2013, 1–9. [Google Scholar] [CrossRef]
- Mahore, J.G.; Suryawanshi, S.D.; Shirolkar, S.V.; Deshkar, S.S. Enhancement of Percutaneous Delivery of Dapsone by Microemulsion Gel. J. Young Pharm. 2017, 9, 507–512. [Google Scholar] [CrossRef] [Green Version]
- Beheshti-Mall, L.; Shafaroodi, H.; Jafariazar, Z.; Afshar, M. A novel hydrogel-thickened microemulsion of dapsone for acne treatment: Development, characterization, physicochemical stability and ex vivo permeation studies. Marmara Pharm. J. 2018, 22, 267–276. [Google Scholar] [CrossRef]
- Das, S.; Lee, S.H.; Chia, V.D.; Chow, P.S.; Macbeath, C.; Liu, Y.; Shlieout, G. Development of microemulsion based topical ivermectin formulations: Pre-formulation and formulation studies. Colloids Surf. B Biointerfaces 2020, 189, 110823. [Google Scholar] [CrossRef]
- Boonme, P.; Boonthongchuay, C.; Wongpoowarak, W.; Amnuaikit, T. Evaluation of nicotinamide microemulsion on the skin penetration enhancement. Pharm. Dev. Technol. 2016, 21, 116–120. [Google Scholar] [CrossRef]
- Pansang, S.; Maphanta, S.; Tuntijarukorn, P.; Viyocha, J. Skin irritation test of a microemulsion containing essential oil isolated from Ocimum basilicum. Sci. Asia 2010, 36, 355–358. [Google Scholar] [CrossRef]
- Jantrawut, P.; Boonsermsukcharoen, K.; Thipnan, K.; Chaiwarit, T.; Hwang, K.-M.; Park, E.-S. Enhancement of Antibacterial Activity of Orange Oil in Pectin Thin Film by Microemulsion. Nanomaterials 2018, 8, 545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szumała, P.; Jungnickel, C.; Kozłowska-Tylingo, K.; Jacyna, B.; Cal, K. Transdermal transport of collagen and hyaluronic acid using water in oil microemulsion. Int. J. Pharm. 2019, 572. [Google Scholar] [CrossRef] [PubMed]
- Kohane, D.S.; Langer, R. Biocompatibility and drug delivery systems. Chem. Sci. 2010, 1, 441–446. [Google Scholar] [CrossRef]
- Nemes, D.; Ujhelyi, Z.; Arany, P.; Pető, A.; Fehér, P.; Váradi, J.; Fenyvesi, F.; Vecsernyés, M.; Bácskay, I. Biocompatibility investigation of different pharmaceutical excipients used in liquid dosage forms. Pharmazie 2018, 73, 16–18. [Google Scholar] [CrossRef] [PubMed]
- Peng, K.; Sottmann, T.; Stubenrauch, C. Gelled non-toxic microemulsions: Phase behavior & rheology. Soft Matter 2019, 15, 8361–8371. [Google Scholar] [CrossRef]







| No. | API | Mechanisms and Effects | Formulations | Ref. |
|---|---|---|---|---|
| 1. | Erythromycin -topical- | Antibacterial Bacterial resistance may occur | Solution with zinc acetate Gel with isotretinoin | [105,106,107,108] |
| 2. | Zinc acetate -topical- | Anti-inflammatory Antioxidant | Solution with erythromycin | [109] |
| 3. | Clindamycin phosphate -topical- | Antibacterial Bacterial resistance may occur | Gel, 1% Gel with tretinoin, 1.2%/0.025% Gel with nicotinamide 1%/4% Gel with benzoyl peroxide 1%/2.5% | [105] [110,111,112,113] |
| 4. | Nicotinamide -topical- | Anti-inflammatory Moisturizer | Gel 4%/5% alone or with clindamycin | [111,112] |
| 5. | Metronidazole -topical- | Antibacterial Anti-inflammatory | Cream, 1% Gel, 1%, 2% | [114,115] |
| 6. | Ivermectin -topical- | Anti-parasitic Anti-inflammatory | Cream, 1% | [116] |
| 7. | Doxycycline -systemic- | Antibacterial Bacterial resistance may occur | Capsules, 100 mg | [117] |
| 8. | Minocycline -systemic- -topical- | Antibacterial Hepatic affection, vestibular events, autoimmune skin disorders may occur Induce photosensitivity | Tablets, 100 mg Foam, 4% | [117,118] |
| 9. | Tetracycline -systemic- -topical- | Antibacterial Affected oral absorption when is administered with food Induce photosensitivity | Capsules, 250 mg Ointment, 3% | [119] [120,121] |
| 10. | Cotrimoxazole -systemic- | Antibacterial Off-label use Just in refractar cases | Tablets, 400 mg/80 mg, 800 mg/160 mg | [122] |
| 11. | Azelaic acid -topical- | Anti-inflammatory, antioxidant Increased erythema may occur Induce photosensitivity | Cream, 20% Gel, 15% Foam, 15% | [123] |
| 12. | Benzoyl peroxide -topical- | Antibacterial Erythema, burning sensations, dryness may occur Induce photosensitivity | Gel, alone or with clindamycin | [113] |
| 13. | Salicylic acid -topical- | Anti-inflammatory Keratoplastic Erythema, dryness may occur | Cosmeceuticals Associated with topical moisturizers Gel, 2% | [124,125,126] |
| 14. | Isotretinoin -systemic- -topical- | Equilibration of desquamation process, by targeting retinoic acid receptors (RAR) (α, β and γ) Anti-inflammatory Erythema, dryness may occur | Capsules 10–40 mg Gel, 0.05% and 0.1% with erythromycin 4% | [93] [127,128] |
| 15. | Tretinoin -topical- | Equilibration of desquamation process, by targeting retinoic acid receptors RAR (α, β and γ) Anti-inflammatory Erythema, dryness may occur | Cream Gel formulation with clindamycin Concentration domain 0.01–0.1% | [127,129] |
| 16. | Adapalene -topical- | Equilibration of desquamation process, by targeting retinoic acid receptors RAR (α, β and γ) Anti-inflammatory Erythema, dryness may occur | Gel with benzoyl peroxide 0.1/2.5% and 0.3/2.5% | [127,129] |
| 17. | Tazarotene -topical- | Equilibration of desquamation process, by targeting retinoic acid receptors RAR (α, β and γ), selectively on β and γ receptors Anti-inflammatory Erythema, dryness may occur | Cream Gel Foam, 0.1% | [127,130] |
| 18. | Trifarotene -topical- | Equilibration of desquamation process, by targeting RAR γ Anti-inflammatory, comedolytic | Cream, 0.005% | [131] |
| 19. | Cortexolone 17α-propionate -topical- | Antiandrogen Anti-inflammatory | Cream, 1% | [132] |
| 20. | Spironolactone -systemic- | Antiandrogen Only women recommended | Tablets, 25–200 mg | [133] |
| 21. | Cyproterone -systemic- | Antiandrogen Headache, edema, hepatic affection, menstrual cycle modification may occur Last line therapy | Tablets, 50–100 mg or combined with ethinylestradiol 2 mg/0.035 mg | [134] |
| 22. | Dapsone -systemic- -topical- | Antibacterial High tolerability | Tablets, 300 mg Gel, 5%, 7.5% | [135,136] |
| No. | Advantages of Microemulsions | Ref. |
|---|---|---|
| 1. | Thermodynamic stability, induced by the S/CoS mixture that provides a low interfacial tension due to a monolayer formed at the contact of oil particles with the water particles. | [175] |
| 2. | Spontaneous formation due to an easy preparation method, without energy consumption; economic manufacturing. | [176,177] |
| 3. | Incorporation of both hydrophilic and lipophilic compounds, resolving the solubility drawbacks for poor soluble drugs; MEs promotes their delivery at skin site in accord with a proper diffusion process. | [177,178] |
| 4. | The solubilization capacity for API and the superior bioavailability are proportional with a high concentration of the S/CoS mixture introduced in the system. | [179] |
| 5. | A high required concentration of surfactant, in association with a cosurfactant assure an easy passage through stratum corneum, acting as penetration enhancers; the barrier effect will be diminished. | [177,180] |
| 6. | The selected API in microemulsion formulation is protected against hydrolytic and oxidative processes, being incorporated as encapsulated particles in oil or water fine droplets; MEs have a superior stability with improvement in half life. | [177,179] |
| 7. | Incorporating APIs that are usually formulated for oral dosage forms and can be adapted in skin target. In this manner, it can be avoided the first pass liver effect and serious adverse reactions, promoting a localized action, without systemic effects. | [181] |
| No. | Advantages | Ref. |
|---|---|---|
| 1 | EOs can be selected as an oil phase in ME or combined with a second vegetable or synthetic oil. | [247] |
| 2 | EOs can be a good alternative to chemical solvents. | [248] |
| 3 | EOs act as penetration enhancers at skin site, increasing the localization of drugs. | [246] |
| 4 | EOs have low toxicity, being GRAS recognized. | [246] |
| 5 | EOs protect the API from degradation reactions, improving its stability; the microemulsification will protect EOs from degradation and volatilization. | [248,249] |
| 6 | EOs can exert self-anti-acne action on microbial species like P. acnes or S. aureus. | [250] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Talianu, M.-T.; Dinu-Pîrvu, C.-E.; Ghica, M.V.; Anuţa, V.; Jinga, V.; Popa, L. Foray into Concepts of Design and Evaluation of Microemulsions as a Modern Approach for Topical Applications in Acne Pathology. Nanomaterials 2020, 10, 2292. https://doi.org/10.3390/nano10112292
Talianu M-T, Dinu-Pîrvu C-E, Ghica MV, Anuţa V, Jinga V, Popa L. Foray into Concepts of Design and Evaluation of Microemulsions as a Modern Approach for Topical Applications in Acne Pathology. Nanomaterials. 2020; 10(11):2292. https://doi.org/10.3390/nano10112292
Chicago/Turabian StyleTalianu, Marina-Theodora, Cristina-Elena Dinu-Pîrvu, Mihaela Violeta Ghica, Valentina Anuţa, Viorel Jinga, and Lăcrămioara Popa. 2020. "Foray into Concepts of Design and Evaluation of Microemulsions as a Modern Approach for Topical Applications in Acne Pathology" Nanomaterials 10, no. 11: 2292. https://doi.org/10.3390/nano10112292
APA StyleTalianu, M. -T., Dinu-Pîrvu, C. -E., Ghica, M. V., Anuţa, V., Jinga, V., & Popa, L. (2020). Foray into Concepts of Design and Evaluation of Microemulsions as a Modern Approach for Topical Applications in Acne Pathology. Nanomaterials, 10(11), 2292. https://doi.org/10.3390/nano10112292