Stability of Quantum-Dot Light Emitting Diodes with Alkali Metal Carbonates Blending in Mg Doped ZnO Electron Transport Layer
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Material Information of R-QDs
3.2. Thin-Film Analysis for X2CO3:MZO ETLs
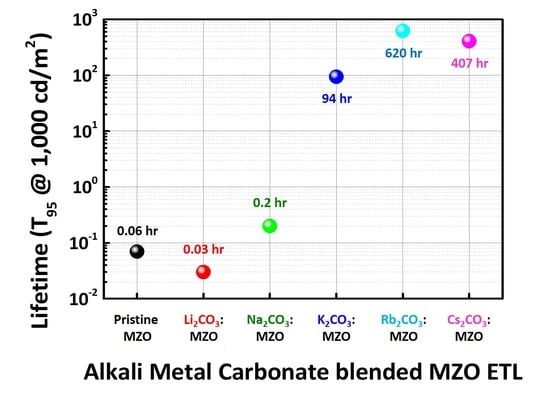
3.3. Device Performance and Operational Lifetime of Inverted R-QLEDs with X2CO3:MZO ETLs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Colvin, V.; Schlamp, M.C.; Allvisatos, A.P. Light-emitting diodes made from cadmium selenide nanocrystals and a semiconducting polymer. Nature 1994, 370, 354–357. [Google Scholar] [CrossRef]
- Kim, S.; Fisher, B.; Eisler, H.-J.; Bawendi, M. Type-II Quantum Dots: CdTe/CdSe(Core/Shell) and CdSe/ZnTe(Core/Shell) Heterostructures. J. Am. Chem. Soc. 2003, 125, 11466–11467. [Google Scholar] [CrossRef]
- Talapin, D.V.; Mekis, I.; Götzinger, S.; Kornowski, A.; Benson, O.; Weller, H. CdSe/CdS/ZnS and CdSe/ZnSe/ZnS Core-Shell-Shell Nanocrystals. J. Phys. Chem. B 2004, 108, 18826–18831. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, J.; Jiang, C.; Bohnenberger, T.; Basché, T.; Mews, A. Electroluminescence from Isolated CdSe/ZnS Quantum Dots in Multilayered Light-emitting Diodes. J. Appl. Phys. 2004, 96, 3206–3210. [Google Scholar] [CrossRef]
- Dong, Y.; Caruge, J.-M.; Zhou, Z.; Hamilton, C.; Popovic, Z.; Ho, J.; Stenvenson, M.; Liu, G.; Bulovic, V.; Bawendi, M.; et al. Ultra-Bright, Highly Efficient, Low Roll-off Inverted Quantum-dot Light Emitting Devices (QLEDs). Dig. Tech. Pap. Soc. Inf. Disp. Int. Symp. 2015, 46, 270–273. [Google Scholar] [CrossRef]
- Song, J.; Wang, O.; Shen, H.; Ling, Q.; Li, Z.; Wang, L.; Zhang, X.; Li, L.S. Over 30% External Quantum Efficiency Light-Emitting Diodes by Engineering Quantum Dot-Assisted Energy Level Match for Hole Transport Layer. Adv. Funct. Mater. 2019, 29, 1970226. [Google Scholar] [CrossRef] [Green Version]
- Lin, J.; Park, Y.-S.; Wu, K.; Yun, H.J.; Klimov, V.I. Droop-Free Colloidal Quantum Dot Light-Emitting Diodes. Nano Lett. 2018, 18, 6645–6653. [Google Scholar]
- Shen, P.; Cao, F.; Wang, H.; Wei, B.; Wang, F.; Sun, X.W.; Tang, X. Solution-Processed Double-Junction Quantum-Dot Light-Emitting Diodes with an EQE of Over 40%. ACS Appl. Mater. Interfaces 2019, 11, 1065–1070. [Google Scholar] [CrossRef]
- Kwak, J.; Bae, W.K.; Lee, D.; Park, I.; Lim, J.; Park, M.; Cho, H.; Woo, J.; Yoon, D.Y.; Char, K.; et al. Bright and Efficient Full-Color Colloidal Quantum Dot Light-Emitting Diodes Using an Inverted Device Structure. Nano Lett. 2012, 12, 2362–2366. [Google Scholar] [CrossRef]
- Kathirgamanathan, P.; Kumaraverl, M.; Bramananthan, N.; Ravichandran, S. High Efficiency and Highly Saturated Red Emitting Inverted Quantum Dot Devices (QLEDs): Optimization of Their Efficiencies with Low Temperature Annealed Sol–gel Derived ZnO as the Electron Transporter and a Novel High Mobility Hole Transporter and Thermal Annealing of the Devices. J. Mater. Chem. C 2018, 6, 11622–11644. [Google Scholar]
- Wu, J.; Zhang, X.; Cia, J.; Lei, W.; Wang, B. Investigation on the Wetting Issues in Solution Processed Inverted Quantum Dot Light-Emitting Diodes. Org. Electron. 2018, 62, 434–440. [Google Scholar] [CrossRef]
- Wang, T.; Zhu, B.; Wang, S.; Yuan, Q.; Zhang, H.; Kang, Z.; Wang, R.; Zhang, H.; Ji, W. Influence of Shell Thickness on the Performance of NiO-Based All-Inorganic Quantum Dot Light-Emitting Diodes. ACS Appl. Mater. Interfaces 2018, 10, 14894–14900. [Google Scholar] [CrossRef]
- Zhong, Z.; Zou, J.; Jiang, C.; Lan, L.; Song, C.; He, Z.; Mu, L.; Wang, L.; Wang, J.; Peng, J.; et al. Improved Color Purity and Efficiency of Blue Quantum Dot Light-Emitting Diodes. Org. Electron. 2018, 58, 245–249. [Google Scholar] [CrossRef]
- Fu, Y.; Jiang, W.; Kim, D.; Lee, W.; Chae, H. Highly Efficient and Fully Solution-Processed Inverted Light-Emitting Diodes with Charge Control Interlayers. ACS Appl. Mater. Interfaces 2018, 10, 17295–17300. [Google Scholar] [CrossRef]
- Ding, K.; Fanh, Y.; Dong, S.; Chen, H.; Luo, B.; Jiang, K.; Gu, H.; Fan, L.; Liu, S.; Hu, B.; et al. 24.1% External Quantum Efficiency of Flexible Quantum Dot Light-Emitting Diodes by Light Extraction of Silver Nanowire Transparent Electrodes. Adv. Opt. Mater. 2018, 6, 1800347. [Google Scholar] [CrossRef]
- Li, Y.; Dai, X.; Chen, D.; Ye, Y.; Gao, Y.; Peng, X.; Jin, Y. Inverted Quantum Dot Light-Emitting Diodes with Conductive Interlayers of Zirconium Acetylacetonate. J. Mater. Chem. C 2019, 7, 3154–3159. [Google Scholar] [CrossRef]
- Liang, F.; Liu, Y.; Hu, Y.; Shi, Y.-L.; Liu, Y.-Q.; Wang, Z.-K.; Wang, X.-D.; Sun, B.-Q.; Liao, L.-S. Polymer as an Additive in the Emitting Layer for High-Performance Quantum Dot Light-Emitting Diodes. ACS Appl. Mater. Interfaces 2017, 9, 20239–20246. [Google Scholar] [CrossRef]
- Park, J.W.; Lim, J.T.; Oh, J.S.; Kim, S.H.; Viet, P.P.; Jhon, M.S.; Yeon, G.Y. Electron-injecting Properties of Rb2CO3-doped Alq3 Thin Films in Organic Light-emitting Diodes. J. Vac. Sci. Technol. A 2013, 31, 031101. [Google Scholar] [CrossRef]
- Chen, F.-C.; Wu, J.-L.; Yang, S.S.; Hsieh, K.-H.; Chen, W.-C. Cesium Carbonate as a Functional Interlayer for Polymer Photovoltaic Devices. J. Appl. Phys. 2008, 103, 103721. [Google Scholar] [CrossRef] [Green Version]
- Lim, J.T.; Park, J.W.; Kwon, J.W.; Yeom, G.Y.; Lhm, K.; Lee, K.J. Optoelectronic Characteristics of Organic Light-Emitting Diodes with a Rb2CO3-Mixed C60 Layer as an Electron Ohmic-Contact. J. Electrochem. Soc. 2013, 160, G1–G5. [Google Scholar] [CrossRef]
- Triana, M.A.; Chen, H.; Zhang, D.; Camargo, R.J.; Zhai, T.; Duhm, S.; Dong, Y. Bright Inverted Quantum-dot Light-emitting Diodes by All-solution Processing. J. Mater. Chem. C 2018, 6, 7487–7492. [Google Scholar] [CrossRef]
- Huang, J.; Xu, Z.; Yang, Y. Low-Work-Function Surface Formed by Solution-Processed and Thermally Deposited Nanoscale Layers of Cesium Carbonate. Adv. Funct. Mater. 2007, 17, 1966–1973. [Google Scholar] [CrossRef]
- Park, Y.; Noh, S.; Lee, D.; Kim, J.; Lee, C. Study of the Cesium Carbonate (Cs2CO3) Inter Layer Fabricated by Solution Process on P3HT:PCBM Solar Cells. Mol. Cryst. Liq. Cryst. 2011, 538, 20–27. [Google Scholar] [CrossRef]
- Chang, C.-H.; Hsu, M.-K.; Wu, S.-W.; Chen, M.-H.; Lin, H.-H.; Li, C.-S.; Pi, T.-W.; Chang, H.-H.; Chen, N.-P. Using Lithium Carbonate-based Electron Injection Structures in High-Performance Inverted Organic Light-Emitting Diodes. Phys. Chem. Chem. Phys. 2015, 17, 13123–13128. [Google Scholar] [CrossRef]
- Azmi, R.; Seo, G.; Ahn, T.K.; Jang, S.-Y. High-Efficiency Air-Stable Colloidal Quantum Dot Solar Cells Based on Potassium Doped ZnO Electron Accepting Layer. ACS Appl. Mater. Interfaces 2018, 10, 35244–35249. [Google Scholar] [CrossRef]
- Savva, A.; Choulis, S.A. Cesium-doped Zinc Oxide as Electron Selective Contact in Inverted Organic Photovoltaics. Appl. Phys. Lett. 2013, 102, 233301. [Google Scholar] [CrossRef]
- Pan, J.; Wei, C.; Wang, L.; Zhuang, J.; Huang, Q.; Su, W.; Cui, Z.; Nathan, A.; Lei, W.; Chen, J. Boosting the Efficiency of Inverted Quantum-dot Light-emitting Diodes by Balancing Charge Densities and Suppressing Exciton Quenching through Band Alignment. Nanoscale 2018, 10, 592–602. [Google Scholar] [CrossRef]
- Chen, G.; Liu, F.; Ling, Z.; Zhang, P.; Wei, B.; Zhu, W. Efficient Organic Light Emitting Diodes Using Solution-Processed Alkali Metal Carbonate Doped ZnO as Electron Injection Layer. Front. Chem. 2019, 7, 226. [Google Scholar] [CrossRef]
- Jeong, J.; Kim, H.; Yoon, Y.J.; Walker, B.; Song, S.; Heo, J.; Park, S.Y.; Kim, J.W.; Kim, G.-H.; Kim, J.Y. Formamidinium-based Planar Heterojunction Perovskite Solar Cells with Alkali Carbonate-doped Zinc Oxide Layer. RSC Adv. 2018, 8, 24110–24115. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.-M.; Yusoff, A.R.M.; Youn, J.-H.; Jang, J. Inverted Quantum-dot Light Emitting Diodes with Cesium Carbonate doped Aluminium-Zinc-Oxide as the Cathode Buffer Layer for High Brightness. J. Mater. Chem. C 2013, 1, 3924–3930. [Google Scholar] [CrossRef]
- Kim, H.-M.; Cho, S.; Kim, J.; Shin, H.; Jang, J. Li and Mg Co-Doped Zinc Oxide Electron Transporting Layer for Highly Efficient Quantum Dot Light-Emitting Diodes. ACS Appl. Mater. Interfaces 2018, 10, 24028–24036. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Kim, H.-M.; Kim, J.; Jang, J. Remarkable Lifetime Improvement of Quantum-dot Light Emitting Diodes by Incorporating Rubidium Carbonate in Metal-Oxide Electron Transport Layer. J. Mater. Chem. C 2019, 7, 10082–10091. [Google Scholar] [CrossRef]
- Moyen, E.; Jun, H.; Kim, H.-M.; Jang, J. Surface Engineering of Room Temperature-Grown Inorganic Perovskite Quantum Dots for Highly Efficient Inverted Light-Emitting Diodes. ACS Appl. Mater. Interfaces 2018, 10, 42647–42656. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-M.; Kim, J.; Cho, S.; Jang, J. Solution-Processed Metal-Oxide p−n Charge Generation Junction for High-Performance Inverted Quantum-Dot Light-Emitting Diodes. ACS Appl. Mater. Interfaces 2017, 9, 38678–38686. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-M.; Youn, J.-H.; Seo, G.-J.; Jang, J. Inverted Quantum-Dot Light-Emitting Diodes with Solution-Processed Aluminium–Zinc Oxide as a Cathode Buffer. J. Mater. Chem. C 2013, 1, 1567–1573. [Google Scholar] [CrossRef]
- Kim, J.; Kim, H.-M.; Jang, J. Low Work Function 2.81 eV Rb2CO3-Doped Polyethylenimine Ethoxylated for Inverted Organic Light-Emitting Diodes. ACS Appl. Mater. Interfaces 2018, 10, 18993–19001. [Google Scholar] [CrossRef] [PubMed]
- Andre, L.; Abanades, S. Investigation of Metal Oxides, Mixed Oxides, Perovskites and Alkalineearth Carbonates/Hydroxides as Suitable Candidate Materials for High-Temperature Thermochemical Energy Storage using Reversible Solid-Gas Reactions. Mater. Today Energy 2018, 10, 48–61. [Google Scholar] [CrossRef]
- The LibreTexts Libraries. Available online: https://chem.libretexts.org/Bookshelves/Inorganic_Chemistry/ (accessed on 2 December 2020).
- The Chemguide. Available online: https://www.chemguide.co.uk/inorganic/group1/ (accessed on 2 December 2020).
- Zhang, H.; Wang, F.; Kuang, Y.; Li, Z.; Lin, Q.; Shen, H.; Wang, H.; Guo, L.; Li, L.S. Se/S Ratio-Dependent Properties and Application of Gradient-Alloyed CdSe1−xSx Quantum Dots: Shell-free Structure, Non-blinking Photoluminescence with Single-Exponential Decay, and Efficient QLEDs. ACS Appl. Mater. Interfaces 2019, 11, 6238–6247. [Google Scholar] [CrossRef]
- Chen, S.; Cao, W.; Liu, T.; Tsang, S.-W.; Yang, Y.; Yan, X.; Qian, L. On the Degradation Mechanisms of Quantum-dot Light-emitting Diode. Nat. Commun. 2019, 10, 765. [Google Scholar] [CrossRef]
- Jiang, C.; Tang, R.; Wang, X.; Ju, H.; Chen, G.; Chen, T. Akali Metals Doping for High-Performance Planar Heterojunction Sb2S3 Solar Cells. Sol. RRL 2019, 3, 1800272. [Google Scholar] [CrossRef] [Green Version]
- Kirkwood, N.; Singh, B.; Mulvaney, P. Enhancing Quantum Dot LED Efficiency by Tuning Electron Mobility in the ZnO Electron Transport Layer. Adv. Mater. Interfaces 2016, 3, 1600868. [Google Scholar] [CrossRef]








| ETLs | Pristine MZO | Alkali Metal Carbonate Dopant in MZO | ||||
|---|---|---|---|---|---|---|
| Cs2CO3 | Rb2CO3 | K2CO3 | Na2CO3 | Li2CO3 | ||
| CB (eV) | 2.80 | 2.05 | 2.09 | 2.29 | 2.09 | 1.46 |
| WF (eV) | 3.10 | 2.08 | 2.28 | 2.57 | 2.61 | 2.57 |
| CB-WF (eV) | 0.30 | 0.03 | 0.19 | 0.28 | 0.52 | 1.11 |
| VB (eV) | 6.47 | 5.45 | 5.61 | 5.78 | 5.70 | 5.07 |
| Eg (eV) | 3.67 | 3.40 | 3.52 | 3.49 | 3.61 | 3.61 |
| Exciton Decay Time | QD Underlayer | |||||
|---|---|---|---|---|---|---|
| Glass | Alkali Metal Carbonate Blended MZO | |||||
| Li2CO3 | Na2CO3 | K2CO3 | Rb2CO3 | Cs2CO3 | ||
| τ1 (ns) | 13.9 | 13.0 | 12.9 | 11.0 | 11.8 | 14.0 |
| A1 (%) | 76.8 | 73.2 | 70.8 | 57.9 | 64.9 | 73.8 |
| τ2 (ns) | 30.7 | 26.0 | 23.9 | 21.1 | 22.1 | 28.3 |
| A2 (%) | 23.2 | 26.8 | 29.2 | 42.1 | 35.1 | 26.2 |
| τavr (ns) | 20.6 | 18.5 | 17.7 | 16.8 | 17.0 | 20.0 |
| Roughness | MZO | Alkali Metal Carbonate Blended MZO | ||||
|---|---|---|---|---|---|---|
| Li2CO3 | Na2CO3 | K2CO3 | Rb2CO3 | Cs2CO3 | ||
| Rpv (nm) | 13.8 | 17.8 | 18.5 | 37 | 55.7 | 13.4 |
| Rq (nm) | 2.5 | 2.5 | 1.8 | 1.9 | 3.0 | 1.5 |
| Ra (nm) | 1.7 | 2.0 | 1.5 | 1.5 | 1.9 | 1.2 |
| Thin-Films | Pristine MZO | Alkali Metal Carbonate Blended MZO | ||||
|---|---|---|---|---|---|---|
| Cs2CO3 | Rb2CO3 | K2CO3 | Na2CO3 | Li2CO3 | ||
| σ (×10−7 S/cm) | 0.7 | 15.7 | 4.3 | 3.0 | 1.2 | 0.3 |
| ETLs | VT (1) (V) | VD (1) (V) | CEmax | PEmax | Lmax | EQEmax | @ 1k cd/m2 | @ 10k cd/m2 | @ 1k cd/m2 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| (cd/A) | (lm/W) | (cd/m2) | (%) | CE (cd/A) | PE (lm/W) | CE (cd/A) | PE (lm/W) | T95 (h) | |||
| MZO | 2.8 | 5.3 | 9.3 | 8.5 | <30k | 7.2 | 6.4 | 3.7 | 3.4 | 1.3 | 0.06 |
| Li2CO3:MZO | 2.5 | 5.9 | 9.9 | 9.4 | <20k | 7.4 | 7.6 | 4.0 | 2.8 | 0.9 | 0.03 |
| Na2CO3:MZO | 2.0 | 3.4 | 16.3 | 20.5 | <70k | 13.2 | 16.0 | 14.6 | 11.4 | 6.6 | 0.2 |
| K2CO3:MZO | 2.2 | 3.3 | 13.0 | 11.2 | >150k | 11.5 | 11.3 | 10.7 | 12.9 | 8.6 | 94 |
| Rb2CO3:MZO | 2.4 | 3.7 | 12.2 | 8.3 | >150k | 9.6 | 9.6 | 8.2 | 11.0 | 6.7 | 620 |
| Cs2CO3:MZO | 2.5 | 4.0 | 11.0 | 5.2 | >150k | 8.1 | 6.4 | 5.0 | 8.1 | 4.2 | 407 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.-M.; Jeong, W.; Kim, J.H.; Jang, J. Stability of Quantum-Dot Light Emitting Diodes with Alkali Metal Carbonates Blending in Mg Doped ZnO Electron Transport Layer. Nanomaterials 2020, 10, 2423. https://doi.org/10.3390/nano10122423
Kim H-M, Jeong W, Kim JH, Jang J. Stability of Quantum-Dot Light Emitting Diodes with Alkali Metal Carbonates Blending in Mg Doped ZnO Electron Transport Layer. Nanomaterials. 2020; 10(12):2423. https://doi.org/10.3390/nano10122423
Chicago/Turabian StyleKim, Hyo-Min, Wonkyeong Jeong, Joo Hyun Kim, and Jin Jang. 2020. "Stability of Quantum-Dot Light Emitting Diodes with Alkali Metal Carbonates Blending in Mg Doped ZnO Electron Transport Layer" Nanomaterials 10, no. 12: 2423. https://doi.org/10.3390/nano10122423
APA StyleKim, H. -M., Jeong, W., Kim, J. H., & Jang, J. (2020). Stability of Quantum-Dot Light Emitting Diodes with Alkali Metal Carbonates Blending in Mg Doped ZnO Electron Transport Layer. Nanomaterials, 10(12), 2423. https://doi.org/10.3390/nano10122423