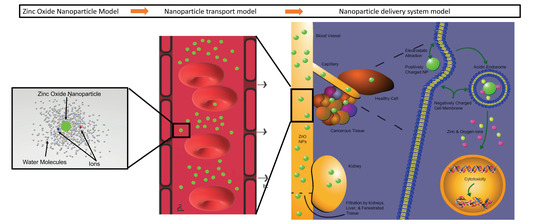
The Self-Adaptation Ability of Zinc Oxide Nanoparticles Enables Reliable Cancer Treatments
Abstract
:1. Introduction
- NS large enough to appropriately conduct NPs through the microvascular flow with little effective diffusion towards smaller vessels to prevent rapid clearance to healthy tissues [18] and promote longer-term dosages.
- Balanced interplay between NS, ZP, and SCD that generate strong long-range Coulombic forces, dampened by the salts in biological fluids, to pull NPs out of the circulatory system and through the interstitium, against contrary convection, to the vicinity of the cancerous cells themselves [19].
2. Materials and Methods
2.1. A Unique Complexation Surface Model for ZnO NPs
2.2. Biological Environment Models
2.3. ZnO NPs Circulatory Transport
2.4. NP-NP and NP-Cell Models for Short-Range Interactions
3. Results
3.1. Impact of the Biological Environment on the Behavior of ZnO NPs
3.2. Blood Circulation
3.3. Long-Range Interactions
3.4. Association Rates
3.5. Zinc and Oxygen Free Ions Released into the Cytoplasm
4. Discussion
4.1. General Consideration
4.2. Blood Circulation Time
4.3. Long-Range Targeting
4.4. Short-Range Targeting
4.5. NP Internalization and Cytotoxicity
5. Conclusions
- Geometrical considerations reveal that the electrostatic properties of the nanoparticle do not depend on the nanoparticle size for radii greater than 40 nm. In such a situation, the nanoparticle behavior is governed by their hydrodynamic properties.
- The nanoparticle charge is fully neutralized at a pH value of approximately equal to 8.2, more significant than the physiological and pathological values.
- Significantly lower values for zeta potential and surface charge density are present in intracellular fluids compared to those obtained for interstitial/blood plasma.
- Considerations of blood rheology and vessel permeation reveal a substantial increase in zinc oxide nanoparticle diffusivity with increasing capillarity and nanoparticle size. Thus, nanoparticle sizes injected into large vessels of the bloodstream are subject to higher diffusion than into small vessels. Small nanoparticle sizes may more rapidly infiltrate the micro-vasculature of the body. Whereas, large nanoparticle sizes increase the blood circulation time with decreased nanoparticle transfer into micro-vasculature of the body.
- Low hydraulic conductivities found in tissues generate a negligible difference in the behavior of the nanoparticles in skeletal, tumorous, and kidney capillaries.
- Long-range electrostatic interactions may interfere with the effective diffusivity of nanoparticles in the micro-vasculature. Positively charged vessels may limit uptake into tissues, while negatively charged vessels can result in a clearance inversely proportional to the square of the micro-vessel size.
- A negatively charged cancerous cell results in a positive cell-zinc oxide nanoparticle attraction energy, while a positively charged healthy cell results in repulsive energy. Large nanoparticle sizes are exponentially more attracted to a cancerous cell, but they are also subject to greater micro-vessel constraints.
- Considerations on nanoparticle-Cell association rates reveal a significant increase in the selectivity for cancerous cells versus healthy cells at large nanoparticle size and low pH.
- Large nanoparticle sizes prevent high level nanoparticle aggregation.
- Large nanoparticles need less energy at the nanoparticle-cell interface to endocytose, while more energetic nanoparticles need to be smaller for optimal uptake.
- Inside the cellular lysosome, nanoparticles dissolve, releasing free zinc and oxygen ions producing reactive-oxygen-species, reacting with the environment, escaping the lysosome, and inducing apoptotic mechanisms within the cancerous cell.
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Truskey, A.G.; Yuan, F.; Katz, D.F. Transport Phenomena in Biological Systems; Pearson/Prentice Hall: Upper Saddle River, NJ, USA, 2004; 793p. [Google Scholar]
- Rasmussen, J.W.; Martinez, E.; Louka, P.; Wingett, D.G. Zinc oxide nanoparticles for selective destruction of tumor cells and potential for drug delivery applications. Expert Opin. Drug Deliv. 2010, 7, 1063–1077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mudunkotuwa, I.A.; Rupasinghe, T.; Wu, C.M.; Grassian, V.H. Dissolution and Aggregation of Zinc Oxide Nanoparticles at Circumneutral pH: A Study of Size Effects in the Presence and Absence of Citric Acid; Langmuir: The ACS Journal of Surfaces and Colloids: Washington, DC, USA, 2012; pp. 396–403. [Google Scholar]
- Copur, M. Optimization of dissolution of Zn and Cd metals from Waelz sintering waste by in aqueous (NH4)(2)SO4 solution. Energy Educ. Sci. Technol. Part a-Energy Sci. Res. 2010, 25, 17–29. [Google Scholar]
- Odzak, N.; Kistler, D.; Behra, R.; Sigg, L. Dissolution of metal and metal oxide nanoparticles in aqueous media. Environ. Pollut. 2014, 191, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.N.; Zhang, M.; Xia, L.; Zhang, J.; Xing, G. The Toxic Effects and Mechanisms of CuO and ZnO Nanoparticles. Materials 2012, 5, 2850–2871. [Google Scholar] [CrossRef] [Green Version]
- Nel, A.E.; Mädler, L.; Velegol, D.; Xia, T.; Hoek, E.M.; Somasundaran, P.; Thompson, M. Understanding biophysicochemical interactions at the nano-bio interface Understanding biophysicochemical interactions at the nano-bio interface. Nat. Mater. 2009, 8, 543–557. [Google Scholar] [CrossRef]
- Auffan, M.; Rose, J.; Bottero, J.Y.; Lowry, G.V.; Jolivet, J.P.; Wiesner, M.R. Towards a definition of inorganic nanoparticles from an environmental, health and safety perspective. Nat. Nanotechnol. 2009, 4, 634–641. [Google Scholar] [CrossRef]
- Dimapilis EA, S.; Hsu, C.S.; Mendoza RM, O.; Lu, M.C. Zinc oxide nanoparticles for water disinfection. Sustain. Environ. Res. 2018, 28, 47–56. [Google Scholar] [CrossRef]
- Dimapilis EA, S.; Hsu, C.S.; Mendoza RM, O.; Lu, M.C. The Role of Reactive Oxygen Species (ROS) in the Biological Activities of Metallic Nanoparticles. Int. J. Mol. Sci. 2017, 18, 47–56. [Google Scholar]
- Fu, P.P.; Xia, Q.; Hwang, H.M.; Ray, P.C.; Yu, H. Mechanisms of nanotoxicity: Generation of reactive oxygen species. J. Food Drug Anal. 2014, 22, 64–75. [Google Scholar] [CrossRef] [Green Version]
- Hunter, R.J.; Ottewill, R.H. Zeta Potential in Colloid Science: Principles and Applications; Colloid Science: Academic Press: London, UK, 1981; 386p. [Google Scholar]
- Puttipipatkhachorn, S.; Nunthanid, J.; Yamamoto, K.; Peck, G.E. Drug physical state and drug-polymer interaction on drug release from chitosan matrix films. J. Control Release 2001, 75, 143–153. [Google Scholar] [CrossRef]
- Xu, S.; Wang, Z.L. One-dimensional ZnO nanostructures: Solution growth and functional properties. Nano Res. 2011, 4, 1013–1098. [Google Scholar] [CrossRef] [Green Version]
- Johanson, G.C.; Hellmer, L.U.; Anfalt, L. Uptake and Biodistribution of Nanoparticles; Swedish Chemicals Agency: Sundyberg, Sweden, 2016. [Google Scholar]
- Li, S.D.; Huang, L. Pharmacokinetics and biodistribution of nanoparticles. Mol. Pharm. 2008, 5, 496–504. [Google Scholar] [CrossRef] [PubMed]
- Barui, A.K.K.R.; Patra, C.R. Chapter 6—Biomedical Applications of Zinc Oxide Nanoparticles. In Inorganic Frameworks as Smart Nanomedicines; Grumezescu, A.M., Ed.; William Andrew: Norwich, NY, USA, 2018; p. 40. [Google Scholar]
- Gentile, F.; Ferrari, M.; Decuzzi, P. The transport of nanoparticles in blood vessels: The effect of vessel permeability and blood rheology. Ann. Biomed. Eng. 2008, 36, 254–261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohshima, H. Electrical Phenomena at Interfaces and Biointerfaces: Fundamentals and Applications in Nano-, Bio-, and Environmental Sciences; Wiley: Hoboken, NJ, USA, 2012; 850p. [Google Scholar]
- Yu, J.B.; Chung, H.E.; Choi, S.J. Effects of Physicochemical Properties of Zinc Oxide Nanoparticles on Cellular Uptake. J. Phys. Conf. Ser. 2011, 304, 012007. [Google Scholar] [CrossRef]
- Gao, J.H.; Shi, W.D.; Freund, L.B. Mechanics of receptor-mediated endocytosis. Proc. Natl. Acad. Sci. USA 2005, 102, 9469–9474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, S.; Li, J.; Lykotrafitis, G.; Bao, G.; Suresh, S. Size-Dependent Endocytosis of Nanoparticles. Adv. Mater. 2009, 21, 419–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Jong, W.H.; Hagens, W.I.; Krystek, P.; Burger, M.C.; Sips, A.J.; Geertsma, R.E. Particle size-dependent organ distribution of gold nanoparticles after intravenous administration. Biomaterials 2008, 29, 1912–1919. [Google Scholar] [CrossRef]
- Khan, M.K.; Nigavekar, S.S.; Minc, L.D.; Kariapper, M.S.; Nair, B.M.; Lesniak, W.G.; Balogh, L.P. In vivo biodistribution of dendrimers and dendrimer nanocomposites -- implications for cancer imaging and therapy. Technol. Cancer Res. Treat. 2005, 4, 603–613. [Google Scholar] [CrossRef]
- Medasani, B.; Ovanesyan, Z.; Thomas, D.G.; Sushko, M.L.; Marucho, M. Ionic asymmetry and solvent excluded volume effects on spherical electric double layers: A density functional approach. J. Chem. Phys. 2014, 140, 204510. [Google Scholar] [CrossRef] [Green Version]
- Ovanesyan, Z.; Aljzmi, A.; Almusaynid, M.; Khan, A.; Valderrama, E.; Nash, K.L.; Marucho, M. Ion-ion correlation, solvent excluded volume and pH effects on physicochemical properties of spherical oxide nanoparticles. J. Colloid Interface Sci. 2016, 462, 325–333. [Google Scholar] [CrossRef] [Green Version]
- Hunley, C.; Marucho, M. Electrical double layer properties of spherical oxide nanoparticles. Phys. Chem. Chem. Phys. 2017, 19, 5396–5404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, P.; Chun, J.; Thomas, D.G.; Schnieders, M.J.; Marucho, M.; Zhang, J.; Baker, N.A. Biomolecular electrostatics and solvation: A computational perspective. Q. Rev. Biophys. 2012, 45, 427–491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marucho, M. A java application to characterize biomolecules and nanomaterials in electrolyte aqueous solutions. Comput. Phys. Commun. 2019, 242, 104–119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mikelonis, M.A.; Youn, S.; Lawler, D.F. DLVO Approximation Methods for Predicting the Attachment of Silver Nanoparticles to Ceramic Membranes. Langmuir 2016, 32, 1723–1731. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Brackenbury, W.J. Membrane potential and cancer progression. Front. Physiol. 2013, 4. [Google Scholar] [CrossRef] [Green Version]
- Binggeli, R.; Cameron, I.L. Cellular-Potentials of Normal and Cancerous Fibroblasts and Hepatocytes. Cancer Res. 1980, 40, 1830–1835. [Google Scholar]
- Ohshima, H.; Healy, T.W.; White, L.R. Accurate Analytic Expressions for the Surface-Charge Density Surface-Potential Relationship and Double-Layer Potential Distribution for a Spherical Colloidal Particle. J. Colloid Interface Sci. 1982, 90, 17–26. [Google Scholar] [CrossRef]
- Pivovarov, S. Surface Structure and Site Density of the Oxide-Solution Interface. J. Colloid Interface Sci. 1997, 196, 321–323. [Google Scholar] [CrossRef]
- Dirkse, T.P. The Solubility Product Constant of Zno. J. Electrochem. Soc. 1986, 133, 1656–1657. [Google Scholar] [CrossRef]
- Al-Hilli, M.S.; Al-Mofarji, R.T.; Willander, M. Zinc oxide nanorod for intracellular pH sensing. Appl. Phys. Lett. 2006, 89. [Google Scholar] [CrossRef]
- Ovanesyan, Z.; Medasani, B.; Fenley, M.O.; Guerrero-García, G.I.; Olvera de la Cruz, M.; Marucho, M. Excluded volume and ion-ion correlation effects on the ionic atmosphere around B-DNA: Theory, simulations, and experiments. J. Chem. Phys. 2014, 141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krezel, A.; Maret, W. The biological inorganic chemistry of zinc ions. Arch. Biochem. Biophys. 2016, 611, 3–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plum, M.L.; Rink, L.; Haase, H. The Essential Toxin: Impact of Zinc on Human Health. Int. J. Environ. Res. Public Health 2010, 7, 1342–1365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. Sect. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- College, A.C. Fluid/Electrolyte Balance. Describes the Composition of the Electrolytes of Bodily Fluids and the Systems with Which the Body Monitors and Controls These Electrolytes. 2008. Available online: www.austincc.edu/apreview/EmphasisItems/Electrolytefluidbalance.html (accessed on 11 October 2017).
- Baxter, T.L.; Jain, R.K. Transport of Fluid and Macromolecules in Tumors. 1. Role of Interstitial Pressure and Convection. Microvasc. Res. 1989, 37, 77–104. [Google Scholar] [CrossRef]
- Kim, K.M.; Kim, T.H.; Kim, H.M.; Kim, H.J.; Gwak, G.H.; Paek, S.M.; Oh, J.M. Colloidal behaviors of ZnO nanoparticles in various aqueous media. Toxicol. Environ. Health Sci. 2012, 4, 121–131. [Google Scholar] [CrossRef]
- Zhang, Y.; Ram, M.K.; Stefanakos, E.K.; Goswami, D.Y. Synthesis, Characterization, and Applications of ZnO Nanowires. J. Nanomater. 2012, 2012, 624520. [Google Scholar] [CrossRef]
- Meisner, T.O.; Potthoff, A. Implications of the stability behavior of zinc oxide nanoparticles for toxicological studies. Int. Nano Lett. 2014, 4, 13. [Google Scholar] [CrossRef]
- Degen, A.; Kosec, M. Effect of pH and impurities on the surface charge of zinc oxide in aqueous solution. J. Eur. Ceram. Soc. 2000, 20, 667–673. [Google Scholar] [CrossRef]
- Omar, F.M.A.; Stoll, S. Stability of ZnO Nanoparticles in Solution. Influence of pH, Dissolution, Aggregation and Disaggregation Effects. J. Colloid Sci. Biotechnol. 2014, 3, 10. [Google Scholar]
- Sayes, C.M.; Berg, J.M.; Romoser, A.; Banerjee, N.; Zebda, R. The relationship between pH and zeta potential of similar to 30 nm metal oxide nanoparticle suspensions relevant to in vitro toxicological evaluations. Nanotoxicology 2009, 3, 276–283. [Google Scholar]
- Marsalek, R. Particle size and Zeta Potential of ZnO. APCBEE Procedia 2014, 9, 5. [Google Scholar] [CrossRef] [Green Version]
- Trefalt, G.; Ruiz-Cabello, F.J.M.; Borkovec, M. Interaction Forces, Heteroaggregation, and Deposition Involving Charged Colloidal Particles. J. Phys. Chem. B 2014, 118, 6346–6355. [Google Scholar] [CrossRef] [PubMed]
- Duan, P.X.; Li, Y.P. Physicochemical Characteristics of Nanoparticles Affect Circulation, Biodistribution, Cellular Internalization, and Trafficking. Small 2013, 9, 1521–1532. [Google Scholar] [CrossRef]
- Hanley, C.; Layne, J.; Punnoose, A.; Reddy, K.M.; Coombs, I.; Coombs, A.; Wingett, D. Preferential killing of cancer cells and activated human T cells using ZnO nanoparticles. Nanotechnology 2008, 19, 295103. [Google Scholar] [CrossRef] [Green Version]
- Heng, C.B.; Zhao, X.; Tan, C.E.; Khamis, N.; Assodani, A.; Xiong, S.; Loo, C.J.S. Evaluation of the cytotoxic and inflammatory potential of differentially shaped zinc oxide nanoparticles. Arch. Toxicol. 2011, 85, 1517–1528. [Google Scholar] [CrossRef]
- Voets, T.; Droogmans, G.; Nilius, B. Membrane currents and the resting membrane potential in cultured bovine pulmonary artery endothelial cells. J. Physiol. Lond. 1996, 497, 95–107. [Google Scholar] [CrossRef] [Green Version]
- Skutelsky, E.; Rudich, Z.; Danon, D. Surface charge properties of the luminal front of blood vessel walls: An electron microscopical analysis. Thromb. Res. 1975, 7, 623–634. [Google Scholar] [CrossRef]
- Srinivasan, S.; Sawyer, P.N. Role of Surface Charge of Blood Vessel Wall, Blood Cells, and Prosthetic Materials in Intravascular Thrombosis. J. Colloid Interface Sci. 1970, 32, 456–463. [Google Scholar] [CrossRef]
- Markosyan, A.A.; Chepurov, A.K. Effect of Zeta-Potential on Intravascular Blood Clotting. Bull. Exp. Biol. Med. USSR 1969, 67, 499–501. [Google Scholar]
- Wiig, H.; Tenstad, O.; Iversen, O.P.; Kalluri, R.; Bjerkvig, V. Interstitial fluid: The overlooked component of the tumor microenvironment? Fibrogenesis Tissue Repair 2010, 3, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.; Mccrate, M.J.; Lee, C.J.; Li, H. The role of surface charge on the uptake and biocompatibility of hydroxyapatite nanoparticles with osteoblast cells. Nanotechnology 2011, 22, 105708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bisht, G.; Rayamajhi, S. ZnO Nanoparticles: A Promising Anticancer Agent. Nanobiomedicine 2016, 3, 9. [Google Scholar] [CrossRef] [PubMed]
- Pattan, G.; Kaul, G. Health hazards associated with nanomaterials. Toxicol. Ind. Health 2014, 30, 499–519. [Google Scholar] [CrossRef]









| Ionic Species | Blood Plasma | Interstitial Fluid | Intracellular Fluid |
|---|---|---|---|
| Sodium | 151 | 142 | 11 |
| Potassium | 10 | 9 | 141 |
| Calcium | 0 | 2 | 0 |
| Magnesium | 0 | 1 | 19 |
| Chlorine | 110 | 118 | 0 |
| Carbonate | 14 | 30 | 12 |
| Hydrogen phosphate | 0 | 2 | 47 |
| Sulfate | 0 | 0 | 10 |
| Oxygen | 18.5 | 0.25 | 32 |
| Vessel | L (mm) | Re (mm) | U (mm/s) |
|---|---|---|---|
| Arteriole | 1.5–2 | 0.02–0.1 | 5 |
| Capillary | 0.5 | 0.005–0.01 | 0.1–1 |
| Venules | 1 | 0.02–0.05 | 0.5 |
| Organ | Lp × 10−8 (µm/s/Pa) | II (Re = 10 µm/Re = 70 µm) |
|---|---|---|
| Skeletal Muscle | 250 | 0.0010/0.00005 |
| Tumor [42] | 1500 | 0.0025/0.0001 |
| Glomerulus in Kidney | 15,000 | 0.0077/0.0042 |
| Organ | Inlet (mmHg) | Outlet (mmHg) | Interstitial (mmHg) |
|---|---|---|---|
| Skeletal Muscle | 30 | 15 | 4.7 |
| Tumor [42] | 30 | 15 | 22 |
| Glomerulus in Kidney | 35 | 15 | −1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taylor, Z.; Marucho, M. The Self-Adaptation Ability of Zinc Oxide Nanoparticles Enables Reliable Cancer Treatments. Nanomaterials 2020, 10, 269. https://doi.org/10.3390/nano10020269
Taylor Z, Marucho M. The Self-Adaptation Ability of Zinc Oxide Nanoparticles Enables Reliable Cancer Treatments. Nanomaterials. 2020; 10(2):269. https://doi.org/10.3390/nano10020269
Chicago/Turabian StyleTaylor, Zane, and Marcelo Marucho. 2020. "The Self-Adaptation Ability of Zinc Oxide Nanoparticles Enables Reliable Cancer Treatments" Nanomaterials 10, no. 2: 269. https://doi.org/10.3390/nano10020269
APA StyleTaylor, Z., & Marucho, M. (2020). The Self-Adaptation Ability of Zinc Oxide Nanoparticles Enables Reliable Cancer Treatments. Nanomaterials, 10(2), 269. https://doi.org/10.3390/nano10020269