Grafting Thin Layered Graphene Oxide onto the Surface of Nonwoven/PVDF-PAA Composite Membrane for Efficient Dye and Macromolecule Separations
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Graphene Oxide (GO)
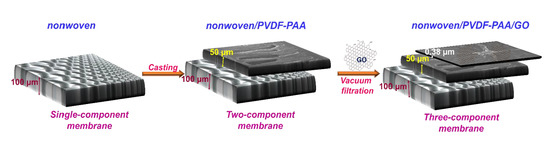
2.2. Synthesis of Nonwoven/PVDF-PAA Composite
2.3. Synthesis of Nonwoven/PVDF-PAA/GO Composite Membrane
2.4. Characterization
2.5. Filtration Analysis of Dyes And Proteins
3. Results
3.1. Morphological and Structural Properties of GO
3.2. Surface and Cross-Sectional Morphologies of GO Composite Membrane
3.3. Surface Charge and Contact Angle Measurements
3.4. Permeance and Rejection Performance of Dyes and FA Solutions
3.5. Permeance and Rejection Performance of BSA Solution
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ma, J.; Guo, X.; Ying, Y.; Liu, D.; Zhong, C. Composite ultrafiltration membrane tailored by MOF@ GO with highly improved water purification performance. Chem. Eng. J. 2017, 313, 890–898. [Google Scholar] [CrossRef] [Green Version]
- Lyu, J.; Wen, X.; Kumar, U.; You, Y.; Chen, V.; Joshi, R. Separation and purification using GO and r-GO membranes. RSC Adv. 2018, 8, 23130–23151. [Google Scholar] [CrossRef] [Green Version]
- Zahid, M.; Rashid, A.; Akram, S.; Rehan, Z.; Razzaq, W. A Comprehensive review on polymeric nano-composite membranes for water treatment. J. Membr. Sci. Technol. 2018, 8, 2. [Google Scholar] [CrossRef]
- Zhan, Y.; Wan, X.; He, S.; Yang, Q.; He, Y. Design of durable and efficient poly (arylene ether nitrile)/bioinspired polydopamine coated graphene oxide nanofibrous composite membrane for anionic dyes separation. Chem. Eng. J. 2018, 333, 132–145. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, A.; Colombi Ciacchi, L.; Wei, G. Recent advances in nanoporous membranes for water purification. Nanomaterials 2018, 8, 65. [Google Scholar] [CrossRef] [Green Version]
- Chen, B.; Jiang, H.; Liu, X.; Hu, X. Water transport confined in graphene oxide channels through the rarefied effect. Phys. Chem. Chem. Phys. 2018, 20, 9780–9786. [Google Scholar] [CrossRef]
- Ali, I.; Bamaga, O.; Gzara, L.; Bassyouni, M.; Abdel-Aziz, M.; Soliman, M.; Drioli, E.; Albeirutty, M. Assessment of blend PVDF membranes, and the effect of polymer concentration and blend composition. Membranes 2018, 8, 13. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; He, Y.; Fan, Y.; Zeng, G.; Zhang, L. Nature-inspired polyphenol chemistry to fabricate halloysite nanotubes decorated PVDF membrane for the removal of wastewater. Sep. Purif. Technol. 2019, 212, 326–336. [Google Scholar] [CrossRef]
- Li, Z.; Rana, D.; Matsuura, T.; Lan, C.Q. The performance of polyvinylidene fluoride - polytetrafluoroethylene nanocomposite distillation membranes: An experimental and numerical study. Sep. Purif. Technol. 2019, 226, 192–208. [Google Scholar] [CrossRef]
- An, A.K.; Guo, J.; Lee, E.-J.; Jeong, S.; Zhao, Y.; Wang, Z.; Leiknes, T. PDMS/PVDF hybrid electrospun membrane with superhydrophobic property and drop impact dynamics for dyeing wastewater treatment using membrane distillation. J. Membr. Sci. 2017, 525, 57–67. [Google Scholar] [CrossRef] [Green Version]
- Roy, S.; Bhalani, D.V.; Jewrajka, S.K. Surface segregation of segmented amphiphilic copolymer of poly (dimethylsiloxane) and poly (ethylene glycol) on poly (vinylidene fluoride) blend membrane for oil–water emulsion separation. Sep. Purif. Technol. 2020, 232, 115940. [Google Scholar] [CrossRef]
- Lin, H.-T.; Venault, A.; Huang, H.Q.; Lee, K.-R.; Chang, Y. Introducing a PEGylated diblock copolymer into PVDF hollow-fibers for reducing their fouling propensity. J. Taiwan Inst. Chem. Eng. 2018, 87, 252–263. [Google Scholar] [CrossRef]
- Li, M.; Li, J.; Zhou, M.; Xian, Y.; Shui, Y.; Wu, M.; Yao, Y. Super-hydrophilic electrospun PVDF/PVA-blended nanofiber membrane for microfiltration with ultrahigh water flux. J. Appl. Polym. Sci. 2020, 137, 48416. [Google Scholar] [CrossRef]
- Chen, L.; Wu, Y.; Li, Y.; Zhang, X.; Qian, J. pH-responsive poly(vinylidene fluoride) membranes containing a novel poly(vinylidene fluoride)-poly(acrylic acid) block copolymer blending material. Mater. Lett. 2018, 210, 124–127. [Google Scholar] [CrossRef]
- Zhou, A.; Jia, R.; Wang, Y.; Sun, S.; Xin, X.; Wang, M.; Zhao, Q.; Zhu, H. Abatement of sulfadiazine in water under a modified ultrafiltration membrane (PVDF-PVP-TiO2-dopamine) filtration-photocatalysis system. Sep. Purif. Technol. 2020, 234, 116099. [Google Scholar] [CrossRef]
- Ismail, N.; Salleh, W.; Ismail, A.; Hasbullah, H.; Yusof, N.; Aziz, F.; Jaafar, J. Hydrophilic polymer-based membrane for oily wastewater treatment: A review. Sep. Purif. Technol. 2020, 233, 116007. [Google Scholar] [CrossRef]
- Gonzales, R.R.; Park, M.J.; Tijing, L.; Han, D.S.; Phuntsho, S.; Shon, H.K. Modification of nanofiber support layer for thin film composite forward osmosis membranes via layer-by-layer polyelectrolyte deposition. Membranes 2018, 8, 70. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Huang, G.; An, C.; Feng, R.; Wu, Y.; Huang, C. Plasma-induced PAA-ZnO coated PVDF membrane for oily wastewater treatment: Preparation, optimization, and characterization through Taguchi OA design and synchrotron-based X-ray analysis. J. Membr. Sci. 2019, 582, 70–82. [Google Scholar] [CrossRef]
- Cruz, N.K.O.; Semblante, G.U.; Senoro, D.B.; You, S.-J.; Lu, S.-C. Dye degradation and antifouling properties of polyvinylidene fluoride/titanium oxide membrane prepared by sol–gel method. J. Taiwan Inst. Chem. Eng. 2014, 45, 192–201. [Google Scholar] [CrossRef]
- Baskoro, F.; Wong, C.-B.; Kumar, S.R.; Chang, C.-W.; Chen, C.-H.; Chen, D.W.; Lue, S.J. Graphene oxide-cation interaction: Inter-layer spacing and zeta potential changes in response to various salt solutions. J. Membr. Sci. 2018, 554, 253–263. [Google Scholar] [CrossRef]
- Saleem, H.; Trabzon, L.; Kilic, A.; Zaidi, S.J. Recent advances in nanofibrous membranes: Production and application in water treatment and desalination. Desalination 2020, 478, 114178. [Google Scholar] [CrossRef]
- He, Y.; Chen, X.; Bi, S.; Shi, C.; Chen, L.; Li, L. Structure and pH-sensitive properties of poly (vinylidene fluoride) membrane changed by blending poly (acrylic acid) microgels. Polym. Adv. Technol. 2013, 24, 934–944. [Google Scholar] [CrossRef]
- Wang, N.C.; Fang, L.F.; Wang, J.; Zhang, P.; Wang, W.B.; Lin, C.E.; Xiao, L.; Chen, C.; Zhao, B.; Abdallah, H. pH-dependent property of carboxyl-based ultrafiltration membranes fabricated from poly (vinyl chloride-r-acrylic acid). J. Appl. Polym. Sci. 2018, 136, 47068. [Google Scholar] [CrossRef]
- Li, X.; Wang, M.; Wang, C.; Cheng, C.; Wang, X. Facile immobilization of Ag nanocluster on nanofibrous membrane for oil/water separation. ACS Appl. Mater. Interfaces 2014, 6, 15272–15282. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-C.; Kumar, S.R.; Shih, C.-M.; Hung, W.-S.; An, Q.-F.; Hsu, H.-C.; Huang, S.-H.; Lue, S.J. High permeance nanofiltration thin film composites with a polyelectrolyte complex top layer containing graphene oxide nanosheets. J. Membr. Sci. 2017, 540, 391–400. [Google Scholar] [CrossRef]
- Senusi, F.; Shahadat, M.; Ismail, S.; Hamid, S.A. Recent advancement in membrane technology for water purification. In Modern Age Environmental Problems and Their Remediation; Oves, M., Zain, K., Mohammad, M.I., Ismail, I., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 147–167. [Google Scholar] [CrossRef]
- Gorrasi, G.; Bugatti, V.; Milone, C.; Mastronardo, E.; Piperopoulos, E.; Iemmo, L.; Di Bartolomeo, A. Effect of temperature and morphology on the electrical properties of PET/conductive nanofillers composites. Compos. Part B Eng. 2018, 135, 149–154. [Google Scholar] [CrossRef]
- Bugatti, V.; Viscusi, G.; Di Bartolomeo, A.; Iemmo, L.; Zampino, D.C.; Vittoria, V.; Gorrasi, G. Ionic liquid as dispersing agent of LDH-carbon nanotubes into a biodegradable vinyl alcohol polymer. Polymers 2020, 12, 495. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.R.; Wang, J.-J.; Wu, Y.-S.; Yang, C.-C.; Lue, S.J. Synergistic role of graphene oxide-magnetite nanofillers contribution on ionic conductivity and permeability for polybenzimidazole membrane electrolytes. J. Power Sour. 2020, 445, 227293. [Google Scholar] [CrossRef]
- Zhang, Y.; Chung, T.-S. Graphene oxide membranes for nanofiltration. Curr. Opin. Chem. Eng. 2017, 16, 9–15. [Google Scholar] [CrossRef]
- Xue, S.; Ji, C.; Kowal, M.D.; Molas, J.C.; Lin, C.-W.; McVerry, B.T.; Turner, C.L.; Mak, W.H.; Anderson, M.; Muni, M. Nanostructured graphene oxide composite membranes with ultrapermeability and mechanical robustness. Nano Lett. 2020, 20, 2209–2218. [Google Scholar] [CrossRef]
- Wang, Y.; He, Z.; Gupta, K.M.; Shi, Q.; Lu, R. Molecular dynamics study on water desalination through functionalized nanoporous graphene. Carbon 2017, 116, 120–127. [Google Scholar] [CrossRef]
- Ghaffar, A.; Zhang, L.; Zhu, X.; Chen, B. Porous PVdF/GO nanofibrous membranes for selective separation and recycling of charged organic dyes from water. Environ. Sci. Technol. 2018, 52, 4265–4274. [Google Scholar] [CrossRef]
- Du, J.; Tian, Y.; Li, N.; Zhang, J.; Zuo, W. Enhanced antifouling performance of ZnS/GO/PVDF hybrid membrane by improving hydrophilicity and photocatalysis. Polym. Adv. Technol. 2019, 30, 351–359. [Google Scholar] [CrossRef]
- Athanasekou, C.; Sapalidis, A.; Katris, I.; Savopoulou, E.; Beltsios, K.; Tsoufis, T.; Kaltzoglou, A.; Falaras, P.; Bounos, G.; Antoniou, M. Mixed matrix PVDF/graphene and composite-skin PVDF/graphene oxide membranes applied in membrane distillation. Polym. Eng. Sci. 2019, 59, E262–E278. [Google Scholar] [CrossRef]
- Ai, J.; Yang, L.; Liao, G.; Xia, H.; Xiao, F. Applications of graphene oxide blended poly (vinylidene fluoride) membranes for the treatment of organic matters and its membrane fouling investigation. Appl. Surf. Sci. 2018, 455, 502–512. [Google Scholar] [CrossRef]
- Jang, W.; Yun, J.; Jeon, K.; Byun, H. PVdF/graphene oxide hybrid membranes via electrospinning for water treatment applications. RSC Adv. 2015, 5, 46711–46717. [Google Scholar] [CrossRef]
- Chen, B.; Zhang, Y.; Zhang, J.; Zhu, L.; Zhao, H. PEGylated polyvinylidene fluoride membranes via grafting from a graphene oxide additive for improving permeability and antifouling properties. RSC Adv. 2019, 9, 18688–18696. [Google Scholar] [CrossRef] [Green Version]
- Lue, S.J.; Pai, Y.-L.; Shih, C.-M.; Wu, M.-C.; Lai, S.-M. Novel bilayer well-aligned Nafion/graphene oxide composite membranes prepared using spin coating method for direct liquid fuel cells. J. Membr. Sci. 2015, 493, 212–223. [Google Scholar] [CrossRef]
- Chang, W.-T.; Chao, Y.-H.; Li, C.-W.; Lin, K.-L.; Wang, J.-J.; Kumar, S.R.; Lue, S.J. Graphene oxide synthesis using microwave-assisted vs. modified Hummer’s methods: Efficient fillers for improved ionic conductivity and suppressed methanol permeability in alkaline methanol fuel cell electrolytes. J. Power Sour. 2019, 414, 86–95. [Google Scholar] [CrossRef]
- Amin, I.N.H.M.; Nizam, M.H.M. Assessment of membrane fouling indices during removal of reactive dye from batik wastewater. J. Water Reuse Desalin. 2016, 6, 505–514. [Google Scholar] [CrossRef] [Green Version]
- Song, S.; Ma, Y.; Shen, H.; Zhang, M.; Zhang, Z. Removal and recycling of ppm levels of methylene blue from an aqueous solution with graphene oxide. RSC Adv. 2015, 5, 27922–27932. [Google Scholar] [CrossRef]
- Kadota, K.; Semba, K.; Shakudo, R.; Sato, H.; Deki, Y.; Shirakawa, Y.; Tozuka, Y. Inhibition of photodegradation of highly dispersed folic acid nanoparticles by the antioxidant effect of transglycosylated rutin. J. Agric. Food Chem. 2016, 64, 3062–3069. [Google Scholar] [CrossRef] [PubMed]
- Otter, D. MILK | Physical and Chemical Properties. In Encyclopedia of Food Sciences and Nutrition, 2nd ed.; Caballero, B., Ed.; Academic Press: Oxford, UK, 2003; pp. 3957–3963. [Google Scholar]
- Yang, M.; Zhao, C.; Zhang, S.; Li, P.; Hou, D. Preparation of graphene oxide modified poly (m-phenylene isophthalamide) nanofiltration membrane with improved water flux and antifouling property. Appl. Surf. Sci. 2017, 394, 149–159. [Google Scholar] [CrossRef]
- Kumar, S.R.; Kim, J.G.; Viswanathan, C.; Kim, W.B.; Selvan, R.K.; Ponpandian, N. Facile synthesis of monodispersed 3D hierarchical Fe3O4 nanostructures decorated r-GO as the negative electrodes for Li-ion batteries. Mater. Res. Bull. 2018, 97, 272–280. [Google Scholar] [CrossRef]
- Van Tran, T.T.; Kumar, S.R.; Lue, S.J. Separation mechanisms of binary dye mixtures using a PVDF ultrafiltration membrane: Donnan effect and intermolecular interaction. J. Membr. Sci. 2019, 575, 38–49. [Google Scholar] [CrossRef]
- Vi, T.T.T.; Kumar, S.R.; Rout, B.; Liu, C.-H.; Wong, C.-B.; Chang, C.-W.; Chen, C.-H.; Chen, D.W.; Lue, S.J. The preparation of graphene oxide-silver nanocomposites: The effect of silver loads on Gram-positive and Gram-negative antibacterial activities. Nanomaterials 2018, 8, 163. [Google Scholar] [CrossRef] [Green Version]
- Zhao, C.; Xu, X.; Chen, J.; Yang, F. Effect of graphene oxide concentration on the morphologies and antifouling properties of PVDF ultrafiltration membranes. J. Environ. Chem. Eng. 2013, 1, 349–354. [Google Scholar] [CrossRef]
- Susilowati, A.; Aspiyanto, A.; Maryati, Y. Characteristic of folic acid monomer and distribution af both fermented broccoli (Brassica oleracea L.) and spinach (Amaranthus sp.) for forticant of natural nano folate. Proc. AIP Conf. Proc. 2018, 2049, 030006. [Google Scholar]
- Du, J.; Hoag, S.W. Characterization of excipient and tableting factors that influence folic acid dissolution, friability, and breaking strength of oil-and water-soluble multivitamin with minerals tablets. Drug Dev. Ind. Pharm. 2003, 29, 1137–1147. [Google Scholar] [CrossRef]
- Mulder, J. Basic Principles of Membrane Technology; Springer Science & Business Media: Berlin, Germany, 2012. [Google Scholar]
- Li, W.; Wu, W.; Li, Z. Controlling interlayer spacing of graphene oxide membranes by external pressure regulation. ACS Nano 2018, 12, 9309–9317. [Google Scholar] [CrossRef]
- Nguyen, H.H.; Ryu, J.; Che, J.-H.; Kang, T.S.; Lee, J.K.; Song, C.W.; Ko, S. Robust size control of bovine serum albumin (BSA) nanoparticles by intermittent addition of a desolvating agent and the particle formation mechanism. Food Chem. 2013, 141, 695–701. [Google Scholar]
- Galisteo-González, F.; Molina-Bolívar, J. Systematic study on the preparation of BSA nanoparticles. Coll. Surf. B Biointerfaces 2014, 123, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Su, Y.; Zhao, X.; Zhang, R.; Zhao, J.; Fan, X.; Jiang, Z. Surface fluorination of polyamide nanofiltration membrane for enhanced antifouling property. J. Membr. Sci. 2014, 455, 15–23. [Google Scholar] [CrossRef]
- Tarhini, M.; Benlyamani, I.; Hamdani, S.; Agusti, G.; Fessi, H.; Greige-Gerges, H.; Bentaher, A.; Elaissari, A. Protein-based nanoparticle preparation via nanoprecipitation method. Materials 2018, 11, 394. [Google Scholar] [CrossRef] [Green Version]
- Vatanpour, V.; Khorshidi, S. Surface modification of polyvinylidene fluoride membranes with ZIF-8 nanoparticles layer using interfacial method for BSA separation and dye removal. Mater. Chem. Phys. 2020, 241, 122400. [Google Scholar] [CrossRef]
- Lee, J.; Chae, H.-R.; Won, Y.J.; Lee, K.; Lee, C.-H.; Lee, H.H.; Kim, I.-C.; Lee, J.-M. Graphene oxide nanoplatelets composite membrane with hydrophilic and antifouling properties for wastewater treatment. J. Membr. Sci. 2013, 448, 223–230. [Google Scholar] [CrossRef]
- Krishnan, S.; Weinman, C.J.; Ober, C.K. Advances in polymers for anti-biofouling surfaces. J. Mater. Chem. 2008, 18, 3405–3413. [Google Scholar] [CrossRef]
- Ayyaru, S.; Ahn, Y.-H. Application of sulfonic acid group functionalized graphene oxide to improve hydrophilicity, permeability, and antifouling of PVDF nanocomposite ultrafiltration membranes. J. Membr. Sci. 2017, 525, 210–219. [Google Scholar] [CrossRef]
- González Flecha, F.L.; Levi, V. Determination of the molecular size of BSA by fluorescence anisotropy. Biochem. Mol. Biol. Educ. 2003, 31, 319–322. [Google Scholar] [CrossRef]









| Samples | Molecular Weight (Da) | Molecular Structure | Concen-Tration (ppm) | pH | Surface Charge (mV) |
|---|---|---|---|---|---|
| Rhodamine-B | 479.02 |  | 10 | 4.09 | −8.6 ± 1.1 |
| Methyl orange | 327.33 |  | 10 | 5.64 | −31.6 ± 1.5 |
| Folic acid | 441.4 |  | 500 | 3.86 | −45 ± 1.1 |
| Bovine serum albumin | 66 K |  | 500 | 6.20 | −30 ± 1.0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baskoro, F.; Rajesh Kumar, S.; Jessie Lue, S. Grafting Thin Layered Graphene Oxide onto the Surface of Nonwoven/PVDF-PAA Composite Membrane for Efficient Dye and Macromolecule Separations. Nanomaterials 2020, 10, 792. https://doi.org/10.3390/nano10040792
Baskoro F, Rajesh Kumar S, Jessie Lue S. Grafting Thin Layered Graphene Oxide onto the Surface of Nonwoven/PVDF-PAA Composite Membrane for Efficient Dye and Macromolecule Separations. Nanomaterials. 2020; 10(4):792. https://doi.org/10.3390/nano10040792
Chicago/Turabian StyleBaskoro, Febri, Selvaraj Rajesh Kumar, and Shingjiang Jessie Lue. 2020. "Grafting Thin Layered Graphene Oxide onto the Surface of Nonwoven/PVDF-PAA Composite Membrane for Efficient Dye and Macromolecule Separations" Nanomaterials 10, no. 4: 792. https://doi.org/10.3390/nano10040792
APA StyleBaskoro, F., Rajesh Kumar, S., & Jessie Lue, S. (2020). Grafting Thin Layered Graphene Oxide onto the Surface of Nonwoven/PVDF-PAA Composite Membrane for Efficient Dye and Macromolecule Separations. Nanomaterials, 10(4), 792. https://doi.org/10.3390/nano10040792