Transition Metal Dichalcogenides for the Application of Pollution Reduction: A Review
Abstract
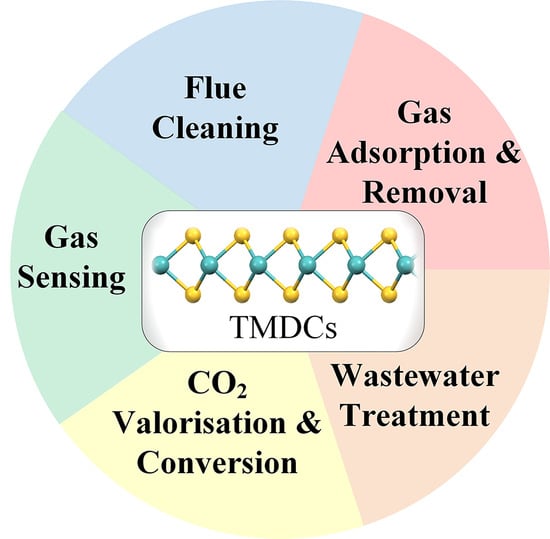
:1. Introduction
2. Gas Adsorption and Removal
2.1. Adsorption of Carbon Monoxide (CO)
2.2. Adsorption of Water Vapor (H2O)
2.3. Adsorption of Carbon Dioxide (CO2)
2.4. Sulphur Content Removal
2.5. Nitrogen Oxide (NOx) Removal
3. Gas Sensing for Pollution Reduction
3.1. NOx Detection
3.2. Ammonia Detection
3.3. Volatile Organic Compound (VOC) Detection
3.4. Detection of Sulphur Gases and Other Gases
4. TMDC Materials for Wastewater Treatment
4.1. Adsorption for Wastewater Treatment
4.2. Membrane Technology in Wastewater Treatment
4.3. Photocatalyst Technology in Wastewater Treatment
5. Fuel Cleaning
5.1. Fuel Hydrodesulfurization
5.2. Fuel Hydrodeoxygenation
6. CO2 Valorization and Conversion
6.1. Conversion of CO2 to Syngas and Other Gases
6.2. Direct Conversion of CO2 to High-Value Chemicals
7. Challenges and Future Prospects
- (i)
- (ii)
- (iii)
- High preparation/fabrication cost and poor uniformity of the materials in large-scale production [288]. More effort is required in sustainable synthesis and fabrication of TMDC-based technologies.
- (iv)
- The larger surface area offered by the TMDCs can enhance the adsorption capability, but also cause higher effects of environmental disturbance [283]. Precise defect engineering to improve material performances can also be carried out [225,267]. Optimization and data analytics on the design dilemma and defect engineering should be highlighted and properly studied.
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Radisavljevic, B.; Radenovic, A.; Brivio, J.; Giacometti, V.; Kis, A. Single-layer MoS2 transistors. Nat. Nanotechnol. 2011. [Google Scholar] [CrossRef]
- Yin, Z.; Li, H.; Li, H.; Jiang, L.; Shi, Y.; Sun, Y.; Lu, G.; Zhang, Q.; Chen, X.; Zhang, H. Single-layer MoS2 phototransistors. ACS Nano 2012. [Google Scholar] [CrossRef] [Green Version]
- Mak, K.F.; McGill, K.L.; Park, J.; McEuen, P.L. The valley hall effect in MoS2 transistors. Science 2014. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Jia, X.; Zhang, Q.; Wang, X. Metallic transition-metal dichalcogenide nanocatalysts for energy conversion. Chem 2018, 4, 1510–1537. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.-P.; Wu, X.; Huang, K.-J.; Xing, L.-L.; Zhang, Y.-Y.; Liu, L. Two-dimensional transition metal diseleniums for energy storage application: A review of recent developments. CrystEngComm 2017, 19, 404–418. [Google Scholar] [CrossRef]
- Lu, J.M.; Zheliuk, O.; Leermakers, I.; Yuan, N.F.Q.; Zeitler, U.; Law, K.T.; Ye, J.T. Evidence for two-dimensional Ising superconductivity in gated MoS2. Science 2015. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Zhang, Y.W. Thermal properties of transition-metal dichalcogenide. Chin. Phys. B 2018, 27, 034402. [Google Scholar] [CrossRef]
- Kappera, R.; Voiry, D.; Yalcin, S.E.; Branch, B.; Gupta, G.; Mohite, A.D.; Chhowalla, M. Phase-engineered low-resistance contacts for ultrathin MoS2 transistors. Nat. Mater. 2014. [Google Scholar] [CrossRef]
- Kolobov, A.V.; Tominaga, J. Two-Dimensional Transition-Metal Dichalcogenides; Springer International Publishing: Cham, Switzerland, 2016; ISBN 978-3-319-31449-5. [Google Scholar]
- Wang, Q.H.; Kalantar-Zadeh, K.; Kis, A.; Coleman, J.N.; Strano, M.S. Electronics and optoelectronics of two-dimensional transition metal dichalcogenides. Nat. Nanotechnol. 2012, 7, 699–712. [Google Scholar] [CrossRef]
- Santosh, K.C.; Longo, R.C.; Addou, R.; Wallace, R.M.; Cho, K. Impact of intrinsic atomic defects on the electronic structure of MoS2 monolayers. Nanotechnology 2014, 25, 375703. [Google Scholar] [CrossRef]
- Feng, S.; Lin, Z.; Gan, X.; Lv, R.; Terrones, M. Doping two-dimensional materials: Ultra-sensitive sensors, band gap tuning and ferromagnetic monolayers. Nanoscale Horizons 2017. [Google Scholar] [CrossRef]
- Ghorbani-Asl, M.; Borini, S.; Kuc, A.; Heine, T. Strain-dependent modulation of conductivity in single-layer transition-metal dichalcogenides. Phys. Rev. B Condens. Matter Mater. Phys. 2013. [Google Scholar] [CrossRef] [Green Version]
- Su, X.; Ju, W.; Zhang, R.; Guo, C.; Zheng, J.; Yong, Y.; Li, X. Bandgap engineering of MoS2/MX2 (MX2 = WS2, MoSe2 and WSe2) heterobilayers subjected to biaxial strain and normal compressive strain. RSC Adv. 2016. [Google Scholar] [CrossRef]
- Gusakova, J.; Wang, X.; Shiau, L.L.; Krivosheeva, A.; Shaposhnikov, V.; Borisenko, V.; Gusakov, V.; Tay, B.K. Electronic properties of bulk and monolayer TMDs: Theoretical study within DFT framework (GVJ-2e method). Phys. Status Solidi Appl. Mater. Sci. 2017. [Google Scholar] [CrossRef]
- Mak, K.F.; Lee, C.; Hone, J.; Shan, J.; Heinz, T.F. Atomically thin MoS2: A new direct-gap semiconductor. Phys. Rev. Lett. 2010. [Google Scholar] [CrossRef] [Green Version]
- Acerce, M.; Voiry, D.; Chhowalla, M. Metallic 1T phase MoS2 nanosheets as supercapacitor electrode materials. Nat. Nanotechnol. 2015. [Google Scholar] [CrossRef]
- Li, H.; Tan, Y.; Liu, P.; Guo, C.; Luo, M.; Han, J.; Lin, T.; Huang, F.; Chen, M. Atomic-sized pores enhanced electrocatalysis of TaS2 nanosheets for hydrogen evolution. Adv. Mater. 2016. [Google Scholar] [CrossRef]
- Stephenson, T.; Li, Z.; Olsen, B.; Mitlin, D. Lithium ion battery applications of molybdenum disulfide (MoS2) nanocomposites. Energy Environ. Sci. 2014. [Google Scholar] [CrossRef]
- Chang, Y.H.; Lin, C.T.; Chen, T.Y.; Hsu, C.L.; Lee, Y.H.; Zhang, W.; Wei, K.H.; Li, L.J. Highly efficient electrocatalytic hydrogen production by MoSx grown on graphene-protected 3D Ni foams. Adv. Mater. 2013. [Google Scholar] [CrossRef]
- Kannan, P.K.; Late, D.J.; Morgan, H.; Rout, C.S. Recent developments in 2D layered inorganic nanomaterials for sensing. Nanoscale 2015. [Google Scholar] [CrossRef]
- Sun, Y.; Gao, S.; Lei, F.; Xie, Y. Atomically-thin two-dimensional sheets for understanding active sites in catalysis. Chem. Soc. Rev. 2015, 44, 623–636. [Google Scholar] [CrossRef]
- Sang, Y.; Zhao, Z.; Zhao, M.; Hao, P.; Leng, Y.; Liu, H. From UV to near-infrared, WS2 nanosheet: A novel photocatalyst for full solar light spectrum photodegradation. Adv. Mater. 2015. [Google Scholar] [CrossRef]
- Atkin, P.; Daeneke, T.; Wang, Y.; Carey, B.J.; Berean, K.J.; Clark, R.M.; Ou, J.Z.; Trinchi, A.; Cole, I.S.; Kalantar-Zadeh, K. 2D WS2/carbon dot hybrids with enhanced photocatalytic activity. J. Mater. Chem. A 2016. [Google Scholar] [CrossRef]
- Di, J.; Xia, J.; Ge, Y.; Xu, L.; Xu, H.; Chen, J.; He, M.; Li, H. Facile fabrication and enhanced visible light photocatalytic activity of few-layer MoS2 coupled BiOBr microspheres. Dalt. Trans. 2014. [Google Scholar] [CrossRef]
- Jaramillo, T.F.; Jørgensen, K.P.; Bonde, J.; Nielsen, J.H.; Horch, S.; Chorkendorff, I. Identification of active edge sites for electrochemical H2 evolution from MoS2 nanocatalysts. Science 2007. [Google Scholar] [CrossRef] [Green Version]
- Mohanty, B.; Ghorbani-Asl, M.; Kretschmer, S.; Ghosh, A.; Guha, P.; Panda, S.K.; Jena, B.; Krasheninnikov, A.V.; Jena, B.K. MoS2 quantum dots as efficient catalyst materials for the oxygen evolution reaction. ACS Catal. 2018. [Google Scholar] [CrossRef]
- Ye, G.; Gong, Y.; Lin, J.; Li, B.; He, Y.; Pantelides, S.T.; Zhou, W.; Vajtai, R.; Ajayan, P.M. Defects engineered monolayer MoS2 for improved hydrogen evolution reaction. Nano Lett. 2016. [Google Scholar] [CrossRef]
- Chan, K.; Tsai, C.; Hansen, H.A.; Nørskov, J.K. Molybdenum sulfides and selenides as possible electrocatalysts for CO2 reduction. ChemCatChem 2014. [Google Scholar] [CrossRef]
- Mohajerin, T.J.; Helz, G.R.; Johannesson, K.H. Tungsten-molybdenum fractionation in estuarine environments. Geochim. Cosmochim. Acta 2016. [Google Scholar] [CrossRef] [Green Version]
- Ren, Z.; Zhou, T.; Hollings, P.; White, N.C.; Wang, F.; Yuan, F. Trace element geochemistry of molybdenite from the Shapinggou super-large porphyry Mo deposit, China. Ore Geol. Rev. 2018. [Google Scholar] [CrossRef]
- Golden, J.; McMillan, M.; Downs, R.T.; Hystad, G.; Goldstein, I.; Stein, H.J.; Zimmerman, A.; Sverjensky, D.A.; Armstrong, J.T.; Hazen, R.M. Rhenium variations in molybdenite (MoS2): Evidence for progressive subsurface oxidation. Earth Planet. Sci. Lett. 2013. [Google Scholar] [CrossRef]
- Pumera, M.; Sofer, Z.; Ambrosi, A. Layered transition metal dichalcogenides for electrochemical energy generation and storage. J. Mater. Chem. A 2014. [Google Scholar] [CrossRef]
- Eftekhari, A. Tungsten dichalcogenides (WS2, WSe2, and WTe2): Materials chemistry and applications. J. Mater. Chem. A 2017, 5, 18299–18325. [Google Scholar] [CrossRef]
- Zhan, Y.; Liu, Z.; Najmaei, S.; Ajayan, P.M.; Lou, J. Large-area vapor-phase growth and characterization of MoS2 atomic layers on a SiO2 substrate. Small 2012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, Q.; Li, C.; Wang, J.; Niu, J.; Gong, Y.; Zhang, Z.; Fang, Q.; Zhang, Y.; Shi, J.; Liao, L.; et al. Metallic vanadium disulfide nanosheets as a platform material for multifunctional electrode applications. Nano Lett. 2017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilson, J.A.; Yoffe, A.D. The transition metal dichalcogenides discussion and interpretation of the observed optical, electrical and structural properties. Adv. Phys. 1969. [Google Scholar] [CrossRef]
- Ubaldini, A.; Jacimovic, J.; Ubrig, N.; Giannini, E. Chloride-driven chemical vapor transport method for crystal growth of transition metal dichalcogenides. Cryst. Growth Des. 2013. [Google Scholar] [CrossRef]
- Zhang, X.; Lou, F.; Li, C.; Zhang, X.; Jia, N.; Yu, T.; He, J.; Zhang, B.; Xia, H.; Wang, S.; et al. Flux method growth of bulk MoS2 single crystals and their application as a saturable absorber. CrystEngComm 2015, 17, 4026–4032. [Google Scholar] [CrossRef]
- Ye, L.; Xu, H.; Zhang, D.; Chen, S. Synthesis of bilayer MoS2 nanosheets by a facile hydrothermal method and their methyl orange adsorption capacity. Mater. Res. Bull. 2014. [Google Scholar] [CrossRef]
- Kalosi, A.; Demydenko, M.; Bodik, M.; Hagara, J.; Kotlar, M.; Kostiuk, D.; Halahovets, Y.; Vegso, K.; Marin Roldan, A.; Maurya, G.S.; et al. Tailored langmuir–schaefer deposition of few-layer MoS2 nanosheet films for electronic applications. Langmuir 2019, 35, 9802–9808. [Google Scholar] [CrossRef]
- Huang, X.; Zeng, Z.; Zhang, H. Metal dichalcogenide nanosheets: Preparation, properties and applications. Chem. Soc. Rev. 2013, 42, 1934–1946. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wu, J.; Yin, Z.; Zhang, H. Preparation and applications of mechanically exfoliated single-layer and multilayer MoS2 and WSe2 nanosheets. Acc. Chem. Res. 2014, 47, 1067–1075. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, G.; Lotya, M.; Cucinotta, C.S.; Sanvito, S.; Bergin, S.D.; Menzel, R.; Shaffer, M.S.P.; Coleman, J.N. Solvent exfoliation of transition metal dichalcogenides: Dispersibility of exfoliated nanosheets varies only weakly between compounds. ACS Nano 2012. [Google Scholar] [CrossRef] [PubMed]
- Bissett, M.A.; Worrall, S.D.; Kinloch, I.A.; Dryfe, R.A.W. Comparison of two-dimensional transition metal dichalcogenides for electrochemical supercapacitors. Electrochim. Acta 2016. [Google Scholar] [CrossRef]
- Yue, Q.; Shao, Z.; Chang, S.; Li, J. Adsorption of gas molecules on monolayer MoS2 and effect of applied electric field. Nanoscale Res. Lett. 2013. [Google Scholar] [CrossRef] [Green Version]
- Kumar, R.; Goel, N.; Hojamberdiev, M.; Kumar, M. Transition metal dichalcogenides-based flexible gas sensors. Sensors Actuators A Phys. 2020, 303, 111875. [Google Scholar] [CrossRef]
- Elsevier. Welcome to Scopus Preview. Available online: https://www.scopus.com/home.uri (accessed on 4 May 2020).
- Blumenthal, I. Carbon monoxide poisoning. J. R. Soc. Med. 2001, 94, 270–272. [Google Scholar] [CrossRef]
- Li, Z.; He, J.; Wang, H.; Wang, B.; Ma, X. Enhanced methanation stability of nano-sized MoS2 catalysts by adding Al2O3. Front. Chem. Sci. Eng. 2015. [Google Scholar] [CrossRef]
- Almeida, K.; Peña, P.; Rawal, T.B.; Coley, W.C.; Akhavi, A.A.; Wurch, M.; Yamaguchi, K.; Le, D.; Rahman, T.S.; Bartels, L. A single layer of MoS2 activates gold for room temperature CO oxidation on an inert silica substrate. J. Phys. Chem. C 2019. [Google Scholar] [CrossRef]
- Lolla, S.; Luo, X. Tuning the catalytic properties of monolayer MoS2 through doping and sulfur vacancies. Appl. Surf. Sci. 2020. [Google Scholar] [CrossRef] [Green Version]
- Ma, D.; Tang, Y.; Yang, G.; Zeng, J.; He, C.; Lu, Z. CO catalytic oxidation on iron-embedded monolayer MoS2. Appl. Surf. Sci. 2015. [Google Scholar] [CrossRef]
- Lu, X.; Guo, L.; Wang, P.; Cui, M.; Kanghong, D.; Peng, W. Theoretical investigation of the adsorption of gas molecules on WSe2 monolayers decorated with Pt, Au nanoclusters. Appl. Surf. Sci. 2020. [Google Scholar] [CrossRef]
- Cui, H.; Zhang, G.; Zhang, X.; Tang, J. Rh-doped MoSe2 as a toxic gas scavenger: A first-principles study. Nanoscale Adv. 2019. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.; Zijlstra, B.; Filot, I.A.W.; Pestman, R.; Hensen, E.J.M. Mechanism of carbon monoxide dissociation on a cobalt Fischer–Tropsch catalyst. ChemCatChem 2018. [Google Scholar] [CrossRef] [Green Version]
- American Chemical Society (ACS). It’s Water Vapor, Not the CO2. Available online: https://www.acs.org/content/acs/en/climatescience/climatesciencenarratives/its-water-vapor-not-the-co2.html (accessed on 2 May 2020).
- Kong, D.; Wang, H.; Cha, J.J.; Pasta, M.; Koski, K.J.; Yao, J.; Cui, Y. Synthesis of MoS2 and MoSe2 films with vertically aligned layers. Nano Lett. 2013. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Kong, D.; Johanes, P.; Cha, J.J.; Zheng, G.; Yan, K.; Liu, N.; Cui, Y. MoSe2 and WSe2 nanofilms with vertically aligned molecular layers on curved and rough surfaces. Nano Lett. 2013. [Google Scholar] [CrossRef]
- Hinnemann, B.; Moses, P.G.; Bonde, J.; Jørgensen, K.P.; Nielsen, J.H.; Horch, S.; Chorkendorff, I.; Nørskov, J.K. Biomimetic hydrogen evolution: MoS2 nanoparticles as catalyst for hydrogen evolution. J. Am. Chem. Soc. 2005. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.; Groom, D.J.; Wang, X.; Yang, Z.; Gummalla, M.; Ball, S.C.; Myers, D.J.; Ferreira, P.J. Degradation mechanisms of platinum nanoparticle catalysts in proton exchange membrane fuel cells: The role of particle size. Chem. Mater. 2014. [Google Scholar] [CrossRef]
- Zhao, Y.; Hwang, J.; Tang, M.T.; Chun, H.; Wang, X.; Zhao, H.; Chan, K.; Han, B.; Gao, P.; Li, H. Ultrastable molybdenum disulfide-based electrocatalyst for hydrogen evolution in acidic media. J. Power Sources 2020. [Google Scholar] [CrossRef]
- Rong, H.; Zhang, H.; Xiao, S.; Li, C.; Hu, C. Optimizing energy consumption for data centers. Renew. Sustain. Energy Rev. 2016, 58, 674–691. [Google Scholar] [CrossRef]
- Li, B.B.; Qiao, S.Z.; Zheng, X.R.; Yang, X.J.; Cui, Z.D.; Zhu, S.L.; Li, Z.Y.; Liang, Y.Q. Pd coated MoS2 nanoflowers for highly efficient hydrogen evolution reaction under irradiation. J. Power Sources 2015. [Google Scholar] [CrossRef]
- Jian, J.; Li, Y.; Bi, H.; Wang, X.; Wu, X.; Qin, W. Aluminum decoration on MoS2 ultrathin nanosheets for highly efficient hydrogen evolution. ACS Sustain. Chem. Eng. 2020. [Google Scholar] [CrossRef]
- Bar-Ziv, R.; Ranjan, P.; Lavie, A.; Jain, A.; Garai, S.; Bar Hen, A.; Popovitz-Biro, R.; Tenne, R.; Arenal, R.; Ramasubramaniam, A.; et al. Au-MoS2 hybrids as hydrogen evolution electrocatalysts. ACS Appl. Energy Mater. 2019. [Google Scholar] [CrossRef] [Green Version]
- Chia, X.; Sutrisnoh, N.A.A.; Pumera, M. Tunable Pt-MoSx hybrid catalysts for hydrogen evolution. ACS Appl. Mater. Interfaces 2018. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, P.K.; Sarkar, S.; Mandler, D. Ionic strength induced electrodeposition of two-dimensional layered MoS2 nanosheets. Appl. Mater. Today 2017. [Google Scholar] [CrossRef]
- Cao, J.; Zhou, J.; Zhang, Y.; Liu, X. A Clean and facile synthesis strategy of MoS2 nanosheets grown on multi-wall CNTs for enhanced hydrogen evolution reaction performance. Sci. Rep. 2017. [Google Scholar] [CrossRef] [Green Version]
- Chua, X.J.; Pumera, M. The effect of varying solvents for MoS2 treatment on its catalytic efficiencies for HER and ORR. Phys. Chem. Chem. Phys. 2017. [Google Scholar] [CrossRef]
- Ren, J.; Zong, H.; Sun, Y.; Gong, S.; Feng, Y.; Wang, Z.; Hu, L.; Yu, K.; Yu, K. 2D organ-like molybdenum carbide (MXene) coupled with MoS2 nanoflowers enhances the catalytic activity in the hydrogen evolution reaction. CrystEngComm 2020. [Google Scholar] [CrossRef]
- Wang, Y.; Jian, C.; He, X.; Liu, W. Self-supported molybdenum selenide nanosheets grown on urchin-like cobalt selenide nanowires array for efficient hydrogen evolution. Int. J. Hydrogen Energy 2020. [Google Scholar] [CrossRef]
- Seok, J.; Lee, J.H.; Cho, S.; Ji, B.; Kim, H.W.; Kwon, M.; Kim, D.; Kim, Y.M.; Oh, S.H.; Kim, S.W.; et al. Active hydrogen evolution through lattice distortion in metallic MoTe2. 2D Mater. 2017. [Google Scholar] [CrossRef] [Green Version]
- Pan, Y.; Zheng, F.; Wang, X.; Qin, H.; Liu, E.; Sha, J.; Zhao, N.; Zhang, P.; Ma, L. Enhanced electrochemical hydrogen evolution performance of WS2 nanosheets by Te doping. J. Catal. 2020. [Google Scholar] [CrossRef]
- Kadam, S.R.; Enyashin, A.N.; Houben, L.; Bar-Ziv, R.; Bar-Sadan, M. Ni-WSe2 nanostructures as efficient catalysts for electrochemical hydrogen evolution reaction (HER) in acidic and alkaline media. J. Mater. Chem. A 2020. [Google Scholar] [CrossRef]
- Rahman, F.A.; Aziz, M.M.A.; Saidur, R.; Bakar, W.A.W.A.; Hainin, M.; Putrajaya, R.; Hassan, N.A. Pollution to solution: Capture and sequestration of carbon dioxide (CO2) and its utilization as a renewable energy source for a sustainable future. Renew. Sustain. Energy Rev. 2017, 71, 112–126. [Google Scholar] [CrossRef]
- Shen, Y.; Wang, H.; Zhang, X.; Zhang, Y. MoS2 nanosheets functionalized composite mixed matrix membrane for enhanced CO2 capture via surface drop-coating method. ACS Appl. Mater. Interfaces 2016. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Qin, G.; Ma, Y.; Wang, W.; Li, P.; Du, A.; Li, Z. Electric field controlled CO2 capture and CO2/N2 separation on MoS2 monolayers. Nanoscale 2017. [Google Scholar] [CrossRef]
- Aguilar, N.; Aparicio, S. Theoretical insights into CO2 adsorption by MoS2 nanomaterials. J. Phys. Chem. C 2019. [Google Scholar] [CrossRef]
- Shi, G.; Yu, L.; Ba, X.; Zhang, X.; Zhou, J.; Yu, Y. Copper nanoparticle interspersed MoS2 nanoflowers with enhanced efficiency for CO2 electrochemical reduction to fuel. Dalt. Trans. 2017. [Google Scholar] [CrossRef]
- Kim, R.; Kim, J.; Do, J.Y.; Seo, M.W.; Kang, M. Carbon dioxide photoreduction on the Bi2S3/MoS2 catalyst. Catalysts 2019, 998. [Google Scholar] [CrossRef] [Green Version]
- Hu, X.; Yang, H.; Gao, M.; Tian, H.; Li, Y.; Liang, Z.; Jian, X. Insights into the photoassisted electrocatalytic reduction of CO2 over a two-dimensional MoS2 nanostructure loaded on SnO2 nanoparticles. ChemElectroChem 2019. [Google Scholar] [CrossRef]
- Yu, L.; Xie, Y.; Zhou, J.; Li, Y.; Yu, Y.; Ren, Z. Robust and selective electrochemical reduction of CO2: The case of integrated 3D TiO2@MoS2 architectures and Ti-S bonding effects. J. Mater. Chem. A 2018. [Google Scholar] [CrossRef]
- Ai, W.; Kou, L.; Hu, X.; Wang, Y.; Krasheninnikov, A.V.; Sun, L.; Shen, X. Enhanced sensitivity of MoSe2 monolayer for gas adsorption induced by electric field. J. Phys. Condens. Matter 2019. [Google Scholar] [CrossRef] [PubMed]
- Rathi, K.; Pal, K. Ruthenium-decorated tungsten disulfide quantum dots for a CO2 gas sensor. Nanotechnology 2020. [Google Scholar] [CrossRef] [PubMed]
- Myllyvirta, L. Global Air Pollution Map: Ranking the World’s Worst SO2 and NO2 Emission Hotspots. Available online: https://storage.googleapis.com/planet4-africa-stateless/2019/03/625c2655-ranking-so2-and-no2-hotspots_19-march-2019.pdf (accessed on 2 May 2020).
- Aas, W.; Mortier, A.; Bowersox, V.; Cherian, R.; Faluvegi, G.; Fagerli, H.; Hand, J.; Klimont, Z.; Galy-Lacaux, C.; Lehmann, C.M.B.; et al. Global and regional trends of atmospheric sulfur. Sci. Rep. 2019. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Ma, S.; Jiang, X.; Yang, Z.; Jiang, W.; Wang, H. The adsorption of acidic gaseous pollutants on metal and nonmetallic surface studied by first-principles calculation: A review. Chinese Chem. Lett. 2019. [Google Scholar] [CrossRef]
- Gui, Y.; Chen, J.; Wang, W.; Zhu, Y.; Tang, C.; Xu, L. Adsorption mechanism of hydrogen sulfide and sulfur dioxide on Au–MoS2 monolayer. Superlattices Microstruct. 2019. [Google Scholar] [CrossRef]
- Qian, H.; Lu, W.; Wei, X.; Chen, W.; Deng, J. H2S and SO2 adsorption on Pt-MoS2 adsorbent for partial discharge elimination: A DFT study. Results Phys. 2019. [Google Scholar] [CrossRef]
- Yang, Y.; Ashraf, M.A.; Jermsittiparsert, K.; Jiang, L.; Zhang, D. Enhancing the adsorption performance and sensing capability of Ti-doped MoSe2 and MoS2 monolayers by applying electric field. Appl. Surf. Sci. 2020. [Google Scholar] [CrossRef]
- Ma, S.; Su, L.; Jin, L.; Su, J.; Jin, Y. A first-principles insight into Pd-doped MoSe2 monolayer: A toxic gas scavenger. Phys. Lett. A 2019. [Google Scholar] [CrossRef]
- Abbasi, A.; Sardroodi, J.J. A novel strategy for SOx removal by N-doped TiO2/WSe2 nanocomposite as a highly efficient molecule sensor investigated by van der Waals corrected DFT. Comput. Theor. Chem. 2017. [Google Scholar] [CrossRef]
- Ni, J.; Wang, W.; Quintana, M.; Jia, F.; Song, S. Adsorption of small gas molecules on strained monolayer WSe2 doped with Pd, Ag, Au, and Pt: A computational investigation. Appl. Surf. Sci. 2020. [Google Scholar] [CrossRef]
- Dan, M.; Xiang, J.; Wu, F.; Yu, S.; Cai, Q.; Ye, L.; Ye, Y.; Zhou, Y. Rich active-edge-site MoS2 anchored on reduction sites in metal sulfide heterostructure: Toward robust visible light photocatalytic hydrogen sulphide splitting. Appl. Catal. B Environ. 2019. [Google Scholar] [CrossRef]
- Li, Y.; Yu, S.; Doronkin, D.E.; Wei, S.; Dan, M.; Wu, F.; Ye, L.; Grunwaldt, J.D.; Zhou, Y. Highly dispersed PdS preferably anchored on In2S3 of MnS/In2S3 composite for effective and stable hydrogen production from H2S. J. Catal. 2019. [Google Scholar] [CrossRef] [Green Version]
- Yuan, Y.; Wang, W.; Shi, Y.; Song, L.; Ma, C.; Hu, Y. The influence of highly dispersed Cu2O-anchored MoS2 hybrids on reducing smoke toxicity and fire hazards for rigid polyurethane foam. J. Hazard. Mater. 2020. [Google Scholar] [CrossRef] [PubMed]
- Wen, M.Q.; Xiong, T.; Zang, Z.G.; Wei, W.; Tang, X.S.; Dong, F. Synthesis of MoS2/g-C3N4 nanocomposites with enhanced visible-light photocatalytic activity for the removal of nitric oxide (NO). Opt. Express 2016. [Google Scholar] [CrossRef] [PubMed]
- Xiong, T.; Wen, M.; Dong, F.; Yu, J.; Han, L.; Lei, B.; Zhang, Y.; Tang, X.; Zang, Z. Three dimensional Z-scheme (BiO)2CO3/MoS2 with enhanced visible light photocatalytic NO removal. Appl. Catal. B Environ. 2016. [Google Scholar] [CrossRef]
- Hu, J.; Chen, D.; Li, N.; Xu, Q.; Li, H.; He, J.; Lu, J. In situ fabrication of Bi2O2CO3/MoS2 on carbon nanofibers for efficient photocatalytic removal of NO under visible-light irradiation. Appl. Catal. B Environ. 2017. [Google Scholar] [CrossRef]
- Zhu, J.; Zhang, H.; Tong, Y.; Zhao, L.; Zhang, Y.; Qiu, Y.; Lin, X. First-principles investigations of metal (V, Nb, Ta)-doped monolayer MoS2: Structural stability, electronic properties and adsorption of gas molecules. Appl. Surf. Sci. 2017. [Google Scholar] [CrossRef]
- Ovcharenko, R.; Dedkov, Y.; Voloshina, E. Adsorption of NO2 on WSe2: DFT and photoelectron spectroscopy studies. J. Phys. Condens. Matter 2016. [Google Scholar] [CrossRef] [Green Version]
- Ala-Mantila, S.; Heinonen, J.; Junnila, S. Relationship between urbanization, direct and indirect greenhouse gas emissions, and expenditures: A multivariate analysis. Ecol. Econ. 2014. [Google Scholar] [CrossRef]
- Denmead, O.T.; Chen, D.; Griffith, D.W.T.; Loh, Z.M.; Bai, M.; Naylor, T. Emissions of the indirect greenhouse gases NH3 and NOx from Australian beef cattle feedlots. Aust. J. Exp. Agric. 2008, 48, 213. [Google Scholar] [CrossRef]
- Tai, A.P.K.; Martin, M.V.; Heald, C.L. Threat to future global food security from climate change and ozone air pollution. Nat. Clim. Chang. 2014. [Google Scholar] [CrossRef] [Green Version]
- Tjoelker, M.G.; Volin, J.C.; Oleksyn, J.; Reich, P.B. Interaction of ozone pollution and light effects on photosynthesis in a forest canopy experiment. Plant. Cell Environ. 1995. [Google Scholar] [CrossRef]
- Hulin, M.; Simoni, M.; Viegi, G.; Annesi-Maesano, I. Respiratory health and indoor air pollutants based on quantitative exposure assessments. Eur. Respir. J. 2012, 40, 1033–1045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ajugwo, A.O. Negative effects of gas flaring: The Nigerian experience. J. Environ. Pollut. Hum. Health 2013. [Google Scholar] [CrossRef]
- Collins, F.; Orpen, D.; Fay, C.; Foley, C.; Smeaton, A.F.; Diamond, D. Web-based monitoring of year-length deployments of autonomous gas sensing platforms on landfill sites. In Proceedings of the 2011 IEEE Sensors, Limerick, Ireland, 28–31 October 2011; pp. 1620–1623. [Google Scholar]
- Rossi, M.; Brunelli, D.; Adami, A.; Lorenzelli, L.; Menna, F.; Remondino, F. Gas-drone: Portable gas sensing system on UAVs for gas leakage localization. In Proceedings of the 2014 IEEE Sensors, Valencia, Spain, 2–5 November 2014; pp. 1431–1434. [Google Scholar]
- Li, L.; Fu, C.; Lou, Z.; Chen, S.; Han, W.; Jiang, K.; Chen, D.; Shen, G. Flexible planar concentric circular micro-supercapacitor arrays for wearable gas sensing application. Nano Energy 2017. [Google Scholar] [CrossRef]
- Suh, J.H.; Cho, I.; Kang, K.; Kweon, S.J.; Lee, M.; Yoo, H.J.; Park, I. Fully integrated and portable semiconductor-type multi-gas sensing module for IoT applications. Sensors Actuators B Chem. 2018. [Google Scholar] [CrossRef]
- Cho, B.; Hahm, M.G.; Choi, M.; Yoon, J.; Kim, A.R.; Lee, Y.J.; Park, S.G.; Kwon, J.D.; Kim, C.S.; Song, M.; et al. Charge-transfer-based gas sensing using atomic-layer MoS2. Sci. Rep. 2015. [Google Scholar] [CrossRef]
- Liu, L.; Ikram, M.; Ma, L.; Zhang, X.; Lv, H.; Ullah, M.; Khan, M.; Yu, H.; Shi, K. Edge-exposed MoS2 nanospheres assembled with SnS2 nanosheet to boost NO2 gas sensing at room temperature. J. Hazard. Mater. 2020. [Google Scholar] [CrossRef]
- Pham, T.; Li, G.; Bekyarova, E.; Itkis, M.E.; Mulchandani, A. MoS2-based optoelectronic gas sensor with sub-parts-per-billion limit of NO2 gas detection. ACS Nano 2019. [Google Scholar] [CrossRef]
- Guo, S.; Yang, D.; Zhang, S.; Dong, Q.; Li, B.; Tran, N.; Li, Z.; Xiong, Y.; Zaghloul, M.E. Development of a cloud-based epidermal MoSe2 device for hazardous gas sensing. Adv. Funct. Mater. 2019. [Google Scholar] [CrossRef]
- Zhang, Q.; Shen, Z.; Cao, J.; Zhang, R.; Zhang, L.; Huang, R.J.; Zheng, C.; Wang, L.; Liu, S.; Xu, H.; et al. Variations in PM2.5, TSP, BC, and trace gases (NO2, SO2, and O3) between haze and non-haze episodes in winter over Xi’an, China. Atmos. Environ. 2015. [Google Scholar] [CrossRef]
- Pijolat, C.; Pupier, C.; Sauvan, M.; Tournier, G.; Lalauze, R. Gas detection for automotive pollution control. Sensors Actuators B Chem. 1999. [Google Scholar] [CrossRef]
- Yamazoe, N.; Miura, N. Environmental gas sensing. Sensors Actuators B Chem. 1994. [Google Scholar] [CrossRef]
- Long, H.; Harley-Trochimczyk, A.; Pham, T.; Tang, Z.; Shi, T.; Zettl, A.; Carraro, C.; Worsley, M.A.; Maboudian, R. High surface area MoS2/graphene hybrid aerogel for ultrasensitive NO2 detection. Adv. Funct. Mater. 2016. [Google Scholar] [CrossRef] [Green Version]
- Liu, B.; Chen, L.; Liu, G.; Abbas, A.N.; Fathi, M.; Zhou, C. High-performance chemical sensing using Schottky-contacted chemical vapor deposition grown monolayer MoS2 transistors. ACS Nano 2014. [Google Scholar] [CrossRef]
- Yan, W.; Worsley, M.A.; Pham, T.; Zettl, A.; Carraro, C.; Maboudian, R. Effects of ambient humidity and temperature on the NO2 sensing characteristics of WS2/graphene aerogel. Appl. Surf. Sci. 2018. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, G.; Zhu, X.; Guo, Y. Ultrasensitive NO2 gas sensing based on rGO/MoS2 nanocomposite film at low temperature. Sensors Actuators B Chem. 2017. [Google Scholar] [CrossRef]
- Mirõ, P.; Ghorbani-Asl, M.; Heine, T. Two dimensional materials beyond MoS2: Noble-transition-metal dichalcogenides. Angew. Chemie Int. Ed. 2014. [Google Scholar] [CrossRef]
- Sajjad, M.; Montes, E.; Singh, N.; Schwingenschlögl, U. Superior gas sensing properties of monolayer PtSe2. Adv. Mater. Interfaces 2017. [Google Scholar] [CrossRef]
- Yim, C.; Lee, K.; McEvoy, N.; O’Brien, M.; Riazimehr, S.; Berner, N.C.; Cullen, C.P.; Kotakoski, J.; Meyer, J.C.; Lemme, M.C.; et al. High-performance hybrid electronic devices from layered PtSe2 films grown at low temperature. ACS Nano 2016. [Google Scholar] [CrossRef] [Green Version]
- Wu, E.; Xie, Y.; Yuan, B.; Zhang, H.; Hu, X.; Liu, J.; Zhang, D. Ultrasensitive and fully reversible NO2 gas sensing based on p-type MoTe2 under ultraviolet illumination. ACS Sensors 2018. [Google Scholar] [CrossRef] [PubMed]
- Ko, K.Y.; Song, J.G.; Kim, Y.; Choi, T.; Shin, S.; Lee, C.W.; Lee, K.; Koo, J.; Lee, H.; Kim, J.; et al. Improvement of gas-sensing performance of large-area tungsten disulfide nanosheets by surface functionalization. ACS Nano 2016. [Google Scholar] [CrossRef] [PubMed]
- Cho, B.; Kim, A.R.; Park, Y.; Yoon, J.; Lee, Y.J.; Lee, S.; Yoo, T.J.; Kang, C.G.; Lee, B.H.; Ko, H.C.; et al. Bifunctional sensing characteristics of chemical vapor deposition synthesized atomic-layered MoS2. ACS Appl. Mater. Interfaces 2015. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Deng, J.; Li, Y.; Yuan, H.; Xu, M. ZnO nanocrystal-coated MoS2 nanosheets with enhanced ultraviolet light gas sensitive activity studied by surface photovoltage technique. Ceram. Int. 2020. [Google Scholar] [CrossRef]
- Baek, J.; Yin, D.; Liu, N.; Omkaram, I.; Jung, C.; Im, H.; Hong, S.; Kim, S.M.; Hong, Y.K.; Hur, J.; et al. A highly sensitive chemical gas detecting transistor based on highly crystalline CVD-grown MoSe2 films. Nano Res. 2017. [Google Scholar] [CrossRef]
- Perrozzi, F.; Emamjomeh, S.M.; Paolucci, V.; Taglieri, G.; Ottaviano, L.; Cantalini, C. Thermal stability of WS2 flakes and gas sensing properties of WS2/WO3 composite to H2, NH3 and NO2. Sensors Actuators B Chem. 2017. [Google Scholar] [CrossRef]
- Han, S.; Bian, H.; Feng, Y.; Liu, A.; Li, X.; Zeng, F.; Zhang, X. Analysis of the relationship between O3, NO and NO2 in Tianjin, China. Aerosol Air Qual. Res. 2011. [Google Scholar] [CrossRef] [Green Version]
- Jones, A.E.; Weller, R.; Wolff, E.W.; Jacobi, H.W. Speciation and rate of photochemical NO and NO2 production in Antarctic snow. Geophys. Res. Lett. 2000. [Google Scholar] [CrossRef] [Green Version]
- Environmental Protection Agency (EPA). Nitrogen Oxides (NOx), Why and How They Are Controlled; Technical Bulletin No. EPA-456/F-99-006R; EPA: Research Triangle Park, NC, USA, 1999.
- Zhao, S.; Xue, J.; Kang, W. Gas adsorption on MoS2 monolayer from first-principles calculations. Chem. Phys. Lett. 2014. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Yin, Z.; He, Q.; Li, H.; Huang, X.; Lu, G.; Fam, D.W.H.; Tok, A.I.Y.; Zhang, Q.; Zhang, H. Fabrication of single- and multilayer MoS2 film-based field-effect transistors for sensing NO at room temperature. Small 2012. [Google Scholar] [CrossRef]
- Ramu, S.; Chandrakalavathi, T.; Murali, G.; Kumar, K.S.; Sudharani, A.; Ramanadha, M.; Peta, K.R.; Jeyalakshmi, R.; Vijayalakshmi, R.P. UV enhanced NO gas sensing properties of the MoS2 monolayer gas sensor. Mater. Res. Express 2019. [Google Scholar] [CrossRef]
- Burman, D.; Ghosh, R.; Santra, S.; Kumar Ray, S.; Kumar Guha, P. Role of vacancy sites and UV-ozone treatment on few layered MoS2 nanoflakes for toxic gas detection. Nanotechnology 2017. [Google Scholar] [CrossRef]
- Medina, H.; Li, J.G.; Su, T.Y.; Lan, Y.W.; Lee, S.H.; Chen, C.W.; Chen, Y.Z.; Manikandan, A.; Tsai, S.H.; Navabi, A.; et al. Wafer-scale growth of WSe2 monolayers toward phase-engineered hybrid WOx/WSe2 films with sub-ppb NOx gas sensing by a low-temperature plasma-assisted selenization process. Chem. Mater. 2017. [Google Scholar] [CrossRef]
- Committee on the Environment and Natural Resources Air Quality Research Subcommittee. Atmospheric Ammonia: Sources and Fate. A review of Ongoing Federal Research and Future Needs; NOAA Aeronomy Laboratory: Boulder, CO, USA, 2000. Available online: https://www.esrl.noaa.gov/csl/aqrsd/reports/ammonia.pdf (accessed on 2 May 2020).
- Pinder, R.W.; Gilliland, A.B.; Dennis, R.L. Environmental impact of atmospheric NH3 emissions under present and future conditions in the eastern United States. Geophys. Res. Lett. 2008. [Google Scholar] [CrossRef]
- Donham, K.J.; Cumro, D.; Reynolds, S. Synergistic effects of dust and ammonia on the occupational health effects of poultry production workers. J. Agromedicine 2002. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Yang, D.; Li, B.; Dong, Q.; Li, Z.; Zaghloul, M.E. An artificial intelligent flexible gas sensor based on ultra-large area MoSe2 nanosheet. In Proceedings of the 2019 IEEE 62nd International Midwest Symposium on Circuits and Systems (MWSCAS), Dallas, TX, USA, 4–7 August 2019; pp. 884–887. [Google Scholar]
- Luo, H.; Cao, Y.; Zhou, J.; Feng, J.; Cao, J.; Guo, H. Adsorption of NO2, NH3 on monolayer MoS2 doped with Al, Si, and P: A first-principles study. Chem. Phys. Lett. 2016. [Google Scholar] [CrossRef]
- Cho, B.; Yoon, J.; Lim, S.K.; Kim, A.R.; Kim, D.H.; Park, S.G.; Kwon, J.D.; Lee, Y.J.; Lee, K.H.; Lee, B.H.; et al. Chemical sensing of 2D graphene/MoS2 heterostructure device. ACS Appl. Mater. Interfaces 2015. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Xie, Y.; Chen, J.; Yu, Y.; Zheng, S.; Zhang, R.; Li, Q.; Chen, X.; Sun, C.; Zhang, H.; et al. Highly sensitive MoTe2 chemical sensor with fast recovery rate through gate biasing. 2D Mater. 2017. [Google Scholar] [CrossRef]
- Qin, Z.; Zeng, D.; Zhang, J.; Wu, C.; Wen, Y.; Shan, B.; Xie, C. Effect of layer number on recovery rate of WS2 nanosheets for ammonia detection at room temperature. Appl. Surf. Sci. 2017. [Google Scholar] [CrossRef]
- Late, D.J.; Doneux, T.; Bougouma, M. Single-layer MoSe2 based NH3 gas sensor. Appl. Phys. Lett. 2014. [Google Scholar] [CrossRef]
- Zhang, D.; Jiang, C.; Li, P.; Sun, Y. Layer-by-layer self-assembly of Co3O4 nanorod-decorated MoS2 nanosheet-based nanocomposite toward high-performance ammonia detection. ACS Appl. Mater. Interfaces 2017. [Google Scholar] [CrossRef] [PubMed]
- Abun, A.; Huang, B.R.; Saravanan, A.; Kathiravan, D.; Hong, P. Da Effect of PMMA on the surface of exfoliated MoS2 nanosheets and their highly enhanced ammonia gas sensing properties at room temperature. J. Alloys Compd. 2020. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, J.; Torad, N.L.; Xia, W.; Aslam, M.A.; Kaneti, Y.V.; Hou, Z.; Ding, Z.; Da, B.; Fatehmulla, A.; et al. Rational design of nanoporous MoS2/VS2 heteroarchitecture for ultrahigh performance ammonia sensors. Small 2020. [Google Scholar] [CrossRef]
- Durmusoglu, E.; Taspinar, F.; Karademir, A. Health risk assessment of BTEX emissions in the landfill environment. J. Hazard. Mater. 2010. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.; Jing, S.; Wang, H.; Ma, Y.; Li, L.; Song, W.; Kan, H. VOC characteristics and inhalation health risks in newly renovated residences in Shanghai, China. Sci. Total Environ. 2017. [Google Scholar] [CrossRef]
- Cetin, E.; Odabasi, M.; Seyfioglu, R. Ambient volatile organic compound (VOC) concentrations around a petrochemical complex and a petroleum refinery. Sci. Total Environ. 2003. [Google Scholar] [CrossRef]
- Malherbe, L.; Mandin, C. VOC emissions during outdoor ship painting and health-risk assessment. Atmos. Environ. 2007. [Google Scholar] [CrossRef]
- Chen, C.; Tsow, F.; Campbell, K.D.; Iglesias, R.; Forzani, E.; Tao, N. A wireless hybrid chemical sensor for detection of environmental volatile organic compounds. IEEE Sens. J. 2013. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.; Shao, K.; Wang, T. Detection of volatile organic compounds (VOCs) from exhaled breath as noninvasive methods for cancer diagnosis. Anal. Bioanal. Chem. 2016. [Google Scholar] [CrossRef]
- Barzegar, M.; Berahman, M.; Asgari, R. First-principles study of molecule adsorption on Ni-decorated monolayer MoS2. J. Comput. Electron. 2019. [Google Scholar] [CrossRef]
- Kim, J.S.; Yoo, H.W.; Choi, H.O.; Jung, H.T. Tunable volatile organic compounds sensor by using thiolated ligand conjugation on MoS2. Nano Lett. 2014. [Google Scholar] [CrossRef] [PubMed]
- Tomer, V.K.; Malik, R.; Chaudhary, V.; Mishra, Y.K.; Kienle, L.; Ahuja, R.; Lin, L. Superior visible light photocatalysis and low-operating temperature VOCs sensor using cubic Ag(0)-MoS2 loaded g-CN 3D porous hybrid. Appl. Mater. Today 2019. [Google Scholar] [CrossRef]
- Zhao, C.; Gan, X.; Yuan, Q.; Hu, S.; Fang, L.; Zhao, J. High-performance volatile organic compounds microsensor based on few-layer MoS2-coated photonic crystal cavity. Adv. Opt. Mater. 2018. [Google Scholar] [CrossRef]
- Chen, D.; Tang, J.; Zhang, X.; Li, Y.; Liu, H. Detecting decompositions of sulfur hexafluoride using MoS2 monolayer as gas sensor. IEEE Sens. J. 2019. [Google Scholar] [CrossRef]
- Chen, D.; Zhang, X.; Tang, J.; Cui, Z.; Cui, H.; Pi, S. Theoretical study of monolayer PtSe2 as outstanding gas sensor to detect SF6 decompositions. IEEE Electron Device Lett. 2018. [Google Scholar] [CrossRef]
- Park, J.; Mun, J.; Shin, J.S.; Kang, S.W. Highly sensitive two dimensional MoS2 gas sensor decorated with Pt nanoparticles. R. Soc. Open Sci. 2018. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Jermsittiparsert, K.; Gao, W.; Zhang, D. Structural, electronic and magnetic properties of the Ni/Cu-embedded S-vacancy defective MoS2 monolayers and their effects on the adsorption of SOx and O3 molecules. Appl. Surf. Sci. 2020. [Google Scholar] [CrossRef]
- Ma, D.; Ju, W.; Li, T.; Zhang, X.; He, C.; Ma, B.; Lu, Z.; Yang, Z. The adsorption of CO and NO on the MoS2 monolayer doped with Au, Pt, Pd, or Ni: A first-principles study. Appl. Surf. Sci. 2016. [Google Scholar] [CrossRef]
- Shen, J.; Yang, Z.; Wang, Y.; Xu, L.C.; Liu, R.; Liu, X. The gas sensing performance of borophene/MoS2 heterostructure. Appl. Surf. Sci. 2020. [Google Scholar] [CrossRef]
- Sahoo, M.P.K.; Wang, J.; Zhang, Y.; Shimada, T.; Kitamura, T. Modulation of gas adsorption and magnetic properties of monolayer-MoS2 by antisite defect and strain. J. Phys. Chem. C 2016. [Google Scholar] [CrossRef]
- Ma, D.; Ma, B.; Lu, Z.; He, C.; Tang, Y.; Lu, Z.; Yang, Z. Interaction between H2O, N2, CO, NO, NO2 and N2O molecules and a defective WSe2 monolayer. Phys. Chem. Chem. Phys. 2017. [Google Scholar] [CrossRef]
- Jasmine, J.M.; Aadhityan, A.; Preferencial kala, C.; Thiruvadigal, D.J. A first-principles study of Cl2, PH3, AsH3, BBr3 and SF4 gas adsorption on MoS2 monolayer with S and Mo vacancy. Appl. Surf. Sci. 2019. [Google Scholar] [CrossRef]
- UNEP. Tackling Global Water Pollution. Available online: https://www.unenvironment.org/explore-topics/water/what-we-do/tackling-global-water-pollution (accessed on 2 May 2020).
- Malik, S.N.; Khan, S.M.; Ghosh, P.C.; Vaidya, A.N.; Kanade, G.; Mudliar, S.N. Treatment of pharmaceutical industrial wastewater by nano-catalyzed ozonation in a semi-batch reactor for improved biodegradability. Sci. Total Environ. 2019, 678, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.A.; Khan, K.A.; Islam, M. Water and wastewater treatment using nano-technology. In Chemistry of Phytopotentials: Health, Energy and Environmental Perspectives; Springer: Berlin/Heidelberg, Germany, 2012; pp. 315–318. ISBN 978-3-642-23394-4. [Google Scholar]
- Prince, R.C. Oil spill dispersants: Boon or bane? Environ. Sci. Technol. 2015, 49, 6376–6384. [Google Scholar] [CrossRef] [Green Version]
- Abiodun, O.I.; Jantan, A.; Omolara, A.E.; Dada, K.V.; Mohamed, N.A.; Arshad, H. State-of-the-art in artificial neural network applications: A survey. Heliyon 2018, 4, e00938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gusain, R.; Kumar, N.; Fosso-Kankeu, E.; Ray, S.S. Efficient removal of Pb(II) and Cd(II) from industrial mine water by a hierarchical MoS2/SH-MWCNT nanocomposite. ACS Omega 2019, 4, 13922–13935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Göktaş, R.K.; MacLeod, M. Remoteness from sources of persistent organic pollutants in the multi-media global environment. Environ. Pollut. 2016, 217, 33–41. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Wu, A.; Ciacchi, L.C.; Wei, G. Recent advances in nanoporous membranes for water purification. Nanomaterials 2018, 8, 65. [Google Scholar] [CrossRef] [Green Version]
- SDG Knowledge Platform. Sustainable Development Goals. Available online: https://sustainabledevelopment.un.org/?menu=1300 (accessed on 2 May 2020).
- Lu, H.; Wang, J.; Stoller, M.; Wang, T.; Bao, Y.; Hao, H. An overview of nanomaterials for water and wastewater treatment. Adv. Mater. Sci. Eng. 2016, 2016, 4964828. [Google Scholar] [CrossRef] [Green Version]
- Li, G.; Wang, Y.; Bi, J.; Huang, X.; Mao, Y.; Luo, L.; Hao, H. Partial oxidation strategy to synthesize WS2/WO3 heterostructure with enhanced adsorption performance for organic dyes: Synthesis, modelling, and mechanism. Nanomaterials 2020, 10, 278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Mi, B. Environmental applications of 2D molybdenum disulfide (MoS2) nanosheets. Environ. Sci. Technol. 2017, 51, 8229–8244. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Huang, H.; Wang, X.; Wu, K.; Tang, G.; Li, C. Hydrothermal synthesis of 3D hierarchical flower-like MoSe2 microspheres and their adsorption performances for methyl orange. Appl. Surf. Sci. 2016, 379, 296–303. [Google Scholar] [CrossRef]
- Fang, Y.; Huang, Q.; Liu, P.; Shi, J.; Xu, G. Easy-separative MoS2-glue sponges with high-efficient dye adsorption and excellent reusability for convenient water treatment. Colloids Surfaces A Physicochem. Eng. Asp. 2018, 540, 112–122. [Google Scholar] [CrossRef]
- Song, H.J.; You, S.; Jia, X.H.; Yang, J. MoS2 nanosheets decorated with magnetic Fe3O4 nanoparticles and their ultrafast adsorption for wastewater treatment. Ceram. Int. 2015, 41, 13896–13902. [Google Scholar] [CrossRef]
- Li, H.; Xie, F.; Li, W.; Fahlman, B.D.; Chen, M.; Li, W. Preparation and adsorption capacity of porous MoS2 nanosheets. RSC Adv. 2016, 6, 105222–105230. [Google Scholar] [CrossRef]
- Massey, A.T.; Gusain, R.; Kumari, S.; Khatri, O.P. Hierarchical microspheres of MoS2 nanosheets: Efficient and regenerative adsorbent for removal of water-soluble dyes. Ind. Eng. Chem. Res. 2016, 55, 7124–7131. [Google Scholar] [CrossRef]
- Krishna Kumar, A.S.; Jiang, S.J.; Warchoł, J.K. Synthesis and characterization of two-dimensional transition metal dichalcogenide magnetic MoS2@Fe3O4 nanoparticles for adsorption of Cr(VI)/Cr(III). ACS Omega 2017, 2, 6187–6200. [Google Scholar] [CrossRef] [PubMed]
- Wan, Z.; Li, D.; Jiao, Y.; Ouyang, X.; Chang, L.; Wang, X. Bifunctional MoS2 coated melamine-formaldehyde sponges for efficient oil–water separation and water-soluble dye removal. Appl. Mater. Today 2017, 9, 551–559. [Google Scholar] [CrossRef]
- Li, R.; Deng, H.; Zhang, X.; Wang, J.J.; Awasthi, M.K.; Wang, Q.; Xiao, R.; Zhou, B.; Du, J.; Zhang, Z. High-efficiency removal of Pb(II) and humate by a CeO2–MoS2 hybrid magnetic biochar. Bioresour. Technol. 2019, 273, 335–340. [Google Scholar] [CrossRef]
- Wan, Z.; Liu, Y.; Chen, S.; Song, K.; Peng, Y.; Zhao, N.; Ouyang, X.; Wang, X. Facile fabrication of a highly durable and flexible MoS2@RTV sponge for efficient oil-water separation. Colloids Surfaces A Physicochem. Eng. Asp. 2018, 546, 237–243. [Google Scholar] [CrossRef]
- Heiranian, M.; Farimani, A.B.; Aluru, N.R. Water desalination with a single-layer MoS2 nanopore. Nat. Commun. 2015, 6, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kou, J.; Yao, J.; Wu, L.; Zhou, X.; Lu, H.; Wu, F.; Fan, J. Nanoporous two-dimensional MoS2 membranes for fast saline solution purification. Phys. Chem. Chem. Phys. 2016. [Google Scholar] [CrossRef] [PubMed]
- Kozubek, R.; Tripathi, M.; Ghorbani-Asl, M.; Kretschmer, S.; Madauß, L.; Pollmann, E.; O’Brien, M.; McEvoy, N.; Ludacka, U.; Susi, T.; et al. Perforating freestanding molybdenum disulfide monolayers with highly charged ions. J. Phys. Chem. Lett. 2019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, J.; Tang, X.; He, Y.; Fan, Y.; Chen, J.; Hao, Y. Robust stable MoS2/GO filtration membrane for effective removal of dyes and salts from water with enhanced permeability. Desalination 2020, 480, 114328. [Google Scholar] [CrossRef]
- Gao, J.; Zhang, M.; Wang, J.; Liu, G.; Liu, H.; Jiang, Y. Bioinspired modification of layer-stacked molybdenum disulfide (MoS2) membranes for enhanced nanofiltration performance. ACS Omega 2019, 4, 4012–4022. [Google Scholar] [CrossRef] [Green Version]
- Chu, H.; Liu, X.; Liu, B.; Zhu, G.; Lei, W.; Du, H.; Liu, J.; Li, J.; Li, C.; Sun, C. Hexagonal 2H-MoSe2 broad spectrum active photocatalyst for Cr(VI) reduction. Sci. Rep. 2016, 6, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Mittal, H.; Khanuja, M. Nanosheets- and nanourchins-like nanostructures of MoSe2 for photocatalytic water purification: Kinetics and reusability study. Environ. Sci. Pollut. Res. 2019, 1–13. [Google Scholar] [CrossRef]
- Zhou, X.; Yao, J.; Yang, M.; Ma, J.; Zhou, Q.; Ou, E.; Zhang, Z.; Sun, X. Synthesis of MoSe2/SrTiO3 heterostructures with enhanced ultraviolet-light-driven and visible-light-driven photocatalytic properties. Nano 2018, 13. [Google Scholar] [CrossRef] [Green Version]
- Kridiotis, A.C.; Longwell, J.P.; Sarofim, A.F.; Bar-ziv, E. Application of a stochastic model of imperfect mixing to the combustion of fuel-lean COH2 mixtures in air. Chem. Eng. Sci. 1989. [Google Scholar] [CrossRef]
- Kim, J.H.; Ma, X.; Zhou, A.; Song, C. Ultra-deep desulfurization and denitrogenation of diesel fuel by selective adsorption over three different adsorbents: A study on adsorptive selectivity and mechanism. Catal. Today 2006, 111, 74–83. [Google Scholar] [CrossRef]
- Kalam, M.A.; Masjuki, H.H. Emissions and deposit characteristics of a small diesel engine when operated on preheated crude palm oil. Biomass Bioenergy 2004. [Google Scholar] [CrossRef]
- Ashraful, A.M.; Masjuki, H.H.; Kalam, M.A.; Rizwanul Fattah, I.M.; Imtenan, S.; Shahir, S.A.; Mobarak, H.M. Production and comparison of fuel properties, engine performance, and emission characteristics of biodiesel from various non-edible vegetable oils: A review. Energy Convers. Manag. 2014, 80, 202–228. [Google Scholar] [CrossRef]
- Song, C.; Ma, X. New design approaches to ultra-clean diesel fuels by deep desulfurization and deep dearomatization. Appl. Catal. B Environ. 2003, 41, 207–238. [Google Scholar] [CrossRef]
- Itthibenchapong, V.; Srifa, A.; Kaewmeesri, R.; Kidkhunthod, P.; Faungnawakij, K. Deoxygenation of palm kernel oil to jet fuel-like hydrocarbons using Ni-MoS2/γ-Al2O3 catalysts. Energy Convers. Manag. 2017. [Google Scholar] [CrossRef]
- Jiang, L.; Kronbak, J.; Christensen, L.P. The costs and benefits of sulphur reduction measures: Sulphur scrubbers versus marine gas oil. Transp. Res. Part D Transp. Environ. 2014. [Google Scholar] [CrossRef]
- Ushakov, S.; Valland, H.; Nielsen, J.B.; Hennie, E. Effects of high sulphur content in marine fuels on particulate matter emission characteristics. J. Mar. Eng. Technol. 2013. [Google Scholar] [CrossRef]
- Jones, J.; Dupont, V.; Brydson, R.; Fullerton, D.; Nasri, N.; Ross, A.; Westwood, A.V. Sulphur poisoning and regeneration of precious metal catalysed methane combustion. Catal. Today 2003, 81, 589–601. [Google Scholar] [CrossRef]
- Paul, J.F.; Payen, E. Vacancy formation on MoS2 hydrodesulfurization catalyst: DFT study of the mechanism. J. Phys. Chem. B 2003. [Google Scholar] [CrossRef]
- Grimblot, J. Genesis, architecture and nature of sites of Co(Ni)-MoS2 supported hydroprocessing catalysts. Catal. Today 1998. [Google Scholar] [CrossRef]
- Liaw, S.J.; Raje, A.; Chary, K.V.R.; Davis, B.H. Catalytic hydrotreatment of Illinois No. 6 coal-derived naphtha: The removal of individual nitrogen and sulfur compounds over MoS2 and Mo2N. Appl. Catal. A Gen. 1995. [Google Scholar] [CrossRef]
- Mahmoudabadi, Z.S.; Rashidi, A.; Tavasoli, A. Synthesis of MoS2 quantum dots as a nanocatalyst for hydrodesulfurization of naphtha: Experimental and DFT study. J. Environ. Chem. Eng. 2020. [Google Scholar] [CrossRef]
- Olivas, A.; Zepeda, T.A.; Villalpando, I.; Fuentes, S. Performance of unsupported Ni(Co,Fe)/MoS2 catalysts in hydrotreating reactions. Catal. Commun. 2008. [Google Scholar] [CrossRef]
- Liu, L.H.; Liu, D.; Liu, B.; Li, G.C.; Liu, Y.Q.; Liu, C.G. Relation between the morphology of MoS2 in NiMo catalyst and its selectivity for dibenzothiophene hydrodesulfurization. Ranliao Huaxue Xuebao J. Fuel Chem. Technol. 2011. [Google Scholar] [CrossRef]
- Rangarajan, S.; Mavrikakis, M. On the preferred active sites of promoted MoS2 for hydrodesulfurization with minimal organonitrogen inhibition. ACS Catal. 2017. [Google Scholar] [CrossRef]
- Tye, C.T.; Smith, K.J. Hydrodesulfurization of dibenzothiophene over exfoliated MoS2 catalyst. Catal. Today 2006. [Google Scholar] [CrossRef]
- Abbasi, A.; Karimi, A.; Aghabozorg, H.; Sadighi, S.; Aval, M.A. Cobalt-promoted MoS2 nanosheets: A promising novel diesel hydrodesulfurization catalyst. Int. J. Chem. Kinet. 2020. [Google Scholar] [CrossRef]
- Moses, P.G.; Hinnemann, B.; Topsøe, H.; Nørskov, J.K. The hydrogenation and direct desulfurization reaction pathway in thiophene hydrodesulfurization over MoS2 catalysts at realistic conditions: A density functional study. J. Catal. 2007. [Google Scholar] [CrossRef]
- Kaluža, L.; Zdražil, M.; Žilková, N.; Čejka, J. High activity of highly loaded MoS2 hydrodesulfurization catalysts supported on organised mesoporous alumina. Catal. Commun. 2002. [Google Scholar] [CrossRef]
- Liu, N.; Wang, X.; Xu, W.; Hu, H.; Liang, J.; Qiu, J. Microwave-assisted synthesis of MoS2/graphene nanocomposites for efficient hydrodesulfurization. Fuel 2014. [Google Scholar] [CrossRef]
- Yang, L.; Wang, X.z.; Liu, Y.; Yu, Z.f.; Liang, J.j.; Chen, B.b.; Shi, C.; Tian, S.; Li, X.; Qiu, J.-S. Monolayer MoS2 anchored on reduced graphene oxide nanosheets for efficient hydrodesulfurization. Appl. Catal. B Environ. 2017. [Google Scholar] [CrossRef]
- Liu, G.; Robertson, A.W.; Li, M.M.J.; Kuo, W.C.H.; Darby, M.T.; Muhieddine, M.H.; Lin, Y.C.; Suenaga, K.; Stamatakis, M.; Warner, J.H.; et al. MoS2 monolayer catalyst doped with isolated Co atoms for the hydrodeoxygenation reaction. Nat. Chem. 2017. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Bai, X.; Ling, C.; Zhou, Q.; Yuan, S.; Chen, Q.; Wang, J. Forming atom–vacancy interface on the MoS2 catalyst for efficient hydrodeoxygenation reactions. Small Methods 2019. [Google Scholar] [CrossRef]
- Wu, K.; Wang, W.; Guo, H.; Yang, Y.; Huang, Y.; Li, W.; Li, C. Engineering co nanoparticles supported on defect MoS2–x for mild deoxygenation of lignin-derived phenols to arenes. ACS Energy Lett. 2020. [Google Scholar] [CrossRef]
- Varakin, A.N.; Mozhaev, A.V.; Pimerzin, A.A.; Nikulshin, P.A. Toward HYD/DEC selectivity control in hydrodeoxygenation over supported and unsupported Co(Ni)-MoS2 catalysts. A key to effective dual-bed catalyst reactor for co-hydroprocessing of diesel and vegetable oil. Catal. Today 2019. [Google Scholar] [CrossRef]
- Alvarez-Galvan, M.; Campos-Martin, J.; Fierro, J. Transition metal phosphides for the catalytic hydrodeoxygenation of waste oils into green diesel. Catalysts 2019, 9, 293. [Google Scholar] [CrossRef] [Green Version]
- Li, N.; Wei, L.; Bibi, R.; Chen, L.; Liu, J.; Zhang, L.; Zheng, Y.; Zhou, J. Catalytic hydrogenation of alkali lignin into bio-oil using flower-like hierarchical MoS2-based composite catalysts. Fuel 2016. [Google Scholar] [CrossRef]
- Zhou, X.; Zhou, J.; Yu, Y.; Ma, J.; Sun, X.; Hu, L. Catalytic hydrogenation of alkali lignin into bio-oil via nano-lamellar MoSe2-based composite catalysts. Nano 2017. [Google Scholar] [CrossRef]
- Porsin, A.A.; Vlasova, E.N.; Bukhtiyarova, G.A.; Nuzhdin, A.L.; Bukhtiyarov, V.I. Sulfide catalysts for production of motor fuels from fatty acid triglycerides. Russ. J. Appl. Chem. 2018, 91, 1905–1911. [Google Scholar] [CrossRef]
- Zhang, Y.; Meng, Z.; Shi, Q.; Gao, H.; Liu, Y.; Wang, Y.; Rao, D.; Deng, K.; Lu, R. Nanoporous MoS2 monolayer as a promising membrane for purifying hydrogen and enriching methane. J. Phys. Condens. Matter 2017. [Google Scholar] [CrossRef]
- Yu, L.; Mishra, I.K.; Xie, Y.; Zhou, H.; Sun, J.; Zhou, J.; Ni, Y.; Luo, D.; Yu, F.; Yu, Y.; et al. Ternary Ni2(1−x)Mo2xP nanowire arrays toward efficient and stable hydrogen evolution electrocatalysis under large-current-density. Nano Energy 2018. [Google Scholar] [CrossRef]
- Mohamed, M.; Yusup, S.; Loy, A.C.M. Effect of empty fruit bunch in calcium oxide for cyclic CO2 capture. Chem. Eng. Technol. 2019. [Google Scholar] [CrossRef]
- Yamamoto, A.; Shinkai, T.; Loy, A.C.M.; Mohamed, M.; Balean, F.H.; Yusup, S.; Quitain, A.T.; Kida, T. Application of a solid electrolyte CO2 sensor to the performance evaluation of CO2 capture materials. Sensors Actuators B Chem. 2020. [Google Scholar] [CrossRef]
- Holloway, S. Underground sequestration of carbon dioxide—A viable greenhouse gas mitigation option. Energy 2005, 30, 2318–2333. [Google Scholar] [CrossRef]
- Song, Y.; Peng, R.; Hensley, D.K.; Bonnesen, P.V.; Liang, L.; Wu, Z.; Meyer, H.M.; Chi, M.; Ma, C.; Sumpter, B.G.; et al. High-selectivity electrochemical conversion of CO2 to ethanol using a copper nanoparticle/N-doped graphene electrode. ChemistrySelect 2016. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Zhang, R.; Fu, J.; Geng, S.; Cheng, J.J.; Sun, Y. Pyrolysis kinetic and product analysis of different microalgal biomass by distributed activation energy model and pyrolysis-gas chromatography-mass spectrometry. Bioresour. Technol. 2014. [Google Scholar] [CrossRef] [PubMed]
- Manenti, F. syngas. In Computer Aided Chemical Engineering; Elsevier BV: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Marlin, D.S.; Sarron, E.; Sigurbjörnsson, Ó. Process advantages of direct CO2 to methanol synthesis. Front. Chem. 2018. [Google Scholar] [CrossRef] [PubMed]
- Melo Bravo, P.; Debecker, D.P. Combining CO2 capture and catalytic conversion to methane. Waste Dispos. Sustain. Energy 2019. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, D.L.T.; Kim, Y.; Hwang, Y.J.; Won, D.H. Progress in development of electrocatalyst for CO2 conversion to selective CO production. Carbon Energy 2020. [Google Scholar] [CrossRef] [Green Version]
- Ali, N.; Bilal, M.; Nazir, M.S.; Khan, A.; Ali, F.; Iqbal, H.M.N. Thermochemical and electrochemical aspects of carbon dioxide methanation: A sustainable approach to generate fuel via waste to energy theme. Sci. Total Environ. 2020. [Google Scholar] [CrossRef]
- Wang, Z.; Roberts, R.R.; Naterer, G.F.; Gabriel, K.S. Comparison of thermochemical, electrolytic, photoelectrolytic and photochemical solar-to-hydrogen production technologies. Int. J. Hydrogen Energy 2012, 37, 16287–16301. [Google Scholar] [CrossRef]
- Zhang, J.-H.; Zhou, Y.-G. Nano-impact electrochemistry: Analysis of single bioentities. TrAC Trends Anal. Chem. 2020, 123, 115768. [Google Scholar] [CrossRef]
- Abdullah, H.; Khan, M.M.R.; Ong, H.R.; Yaakob, Z. Modified TiO2 photocatalyst for CO2 photocatalytic reduction: An overview. J. CO2 Util. 2017, 22, 15–32. [Google Scholar] [CrossRef]
- Xie, S.; Zhang, Q.; Liu, G.; Wang, Y. Photocatalytic and photoelectrocatalytic reduction of CO2 using heterogeneous catalysts with controlled nanostructures. Chem. Commun. 2016. [Google Scholar] [CrossRef] [PubMed]
- Kisch, H. Semiconductor photocatalysis-mechanistic and synthetic aspects. Angew. Chemie Int. Ed. 2013, 52, 812–847. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Zhang, L.; Yu, J.; Wageh, S.; Al-Ghamdi, A.A.; Jaroniec, M. Direct Z-scheme photocatalysts: Principles, synthesis, and applications. Mater. Today 2018, 21, 1042–1063. [Google Scholar] [CrossRef]
- Geioushy, R.A.; El-Sheikh, S.M.; Hegazy, I.M.; Shawky, A.; El-Sherbiny, S.; Kandil, A.H.T. Insights into two-dimensional MoS2 sheets for enhanced CO2 photoreduction to C1 and C2 hydrocarbon products. Mater. Res. Bull. 2019. [Google Scholar] [CrossRef]
- Beheshti, M.; Kakooei, S.; Ismail, M.C.; Shahrestani, S. Investigation of CO2 electrochemical reduction to syngas on Zn/Ni-based electrocatalysts using the cyclic voltammetry method. Electrochim. Acta 2020. [Google Scholar] [CrossRef]
- Debe, M.K. Electrocatalyst approaches and challenges for automotive fuel cells. Nature 2012, 486, 43–51. [Google Scholar] [CrossRef]
- Zinola, C.F.; Martins, M.E.; Tejera, E.P.; Neves, N.P. Electrocatalysis: Fundamentals and applications. Int. J. Electrochem. 2012. [Google Scholar] [CrossRef] [Green Version]
- Asadi, M.; Kumar, B.; Behranginia, A.; Rosen, B.A.; Baskin, A.; Repnin, N.; Pisasale, D.; Phillips, P.; Zhu, W.; Haasch, R.; et al. Robust carbon dioxide reduction on molybdenum disulphide edges. Nat. Commun. 2014. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Zhang, Z.; Zhang, L.; Luo, Z.; Shen, J.; Lin, H.; Long, J.; Wu, J.C.S.; Fu, X.; Wang, X.; et al. Visible-light driven overall conversion of CO2 and H2O to CH4 and O2 on 3D-SiC@2D-MoS2 Heterostructure. J. Am. Chem. Soc. 2018. [Google Scholar] [CrossRef] [PubMed]
- Asadi, M.; Kim, K.; Liu, C.; Addepalli, A.V.; Abbasi, P.; Yasaei, P.; Phillips, P.; Behranginia, A.; Cerrato, J.M.; Haasch, R.; et al. Nanostructured transition metal dichalcogenide electrocatalysts for CO2 reduction in ionic liquid. Science 2016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.I.; Yu, H.; Rhee, C.K.; Sohn, Y. Electrochemical hydrogen evolution and CO2 reduction over hierarchical MoSxSe2−x hybrid nanostructures. Appl. Surf. Sci. 2019. [Google Scholar] [CrossRef]
- Xie, Y.; Li, X.; Wang, Y.; Li, B.; Yang, L.; Zhao, N.; Liu, M.; Wang, X.; Yu, Y.; Liu, J.M. Reaction mechanisms for reduction of CO2 to CO on monolayer MoS2. Appl. Surf. Sci. 2020. [Google Scholar] [CrossRef]
- Abbasi, P.; Asadi, M.; Liu, C.; Sharifi-Asl, S.; Sayahpour, B.; Behranginia, A.; Zapol, P.; Shahbazian-Yassar, R.; Curtiss, L.A.; Salehi-Khojin, A. Tailoring the edge structure of molybdenum disulfide toward electrocatalytic reduction of carbon dioxide. ACS Nano 2017. [Google Scholar] [CrossRef] [PubMed]
- Asadi, M.; Motevaselian, M.H.; Moradzadeh, A.; Majidi, L.; Esmaeilirad, M.; Sun, T.V.; Liu, C.; Bose, R.; Abbasi, P.; Zapol, P.; et al. Highly efficient solar-driven carbon dioxide reduction on molybdenum disulfide catalyst using choline chloride-based electrolyte. Adv. Energy Mater. 2019. [Google Scholar] [CrossRef]
- Liu, X.; Yang, H.; He, J.; Liu, H.; Song, L.; Li, L.; Luo, J. Highly active, durable ultrathin MoTe2 layers for the electroreduction of CO2 to CH4. Small 2018. [Google Scholar] [CrossRef]
- Primo, A.; He, J.; Jurca, B.; Cojocaru, B.; Bucur, C.; Parvulescu, V.I.; Garcia, H. CO2 methanation catalyzed by oriented MoS2 nanoplatelets supported on few layers graphene. Appl. Catal. B Environ. 2019. [Google Scholar] [CrossRef]
- Jung, H.; Cho, K.M.; Kim, K.H.; Yoo, H.W.; Al-Saggaf, A.; Gereige, I.; Jung, H.T. Highly efficient and stable CO2 reduction photocatalyst with a hierarchical structure of mesoporous TiO2 on 3D graphene with few-layered MoS2. ACS Sustain. Chem. Eng. 2018. [Google Scholar] [CrossRef]
- Qin, H.; Guo, R.T.; Liu, X.Y.; Pan, W.G.; Wang, Z.Y.; Shi, X.; Tang, J.Y.; Huang, C.Y. Z-scheme MoS2/g-C3N4 heterojunction for efficient visible light photocatalytic CO2 reduction. Dalt. Trans. 2018. [Google Scholar] [CrossRef]
- Jia, P.y.; Guo, R.t.; Pan, W.g.; Huang, C.y.; Tang, J.y.; Liu, X.y.; Qin, H.; Xu, Q.Y. The MoS2/TiO2 heterojunction composites with enhanced activity for CO2 photocatalytic reduction under visible light irradiation. Colloids Surfaces A Physicochem. Eng. Asp. 2019. [Google Scholar] [CrossRef]
- Kanan, M.W.; Nocera, D.G. In situ formation of an oxygen-evolving catalyst in neutral water containing phosphate and Co2+. Science 2008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reece, S.Y.; Hamel, J.A.; Sung, K.; Jarvi, T.D.; Esswein, A.J.; Pijpers, J.J.H.; Nocera, D.G. Wireless solar water splitting using silicon-based semiconductors and earth-abundant catalysts. Science 2011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Q.; Lei, Y.; Wang, D.; Li, Y. Defect engineering in earth-abundant electrocatalysts for CO2 and N2 reduction. Energy Environ. Sci. 2019, 12, 1730–1750. [Google Scholar] [CrossRef]
- Ji, Y.; Nørskov, J.K.; Chan, K. Scaling relations on basal plane vacancies of transition metal dichalcogenides for CO2 reduction. J. Phys. Chem. C 2019. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Fortes, M.; Schöneberger, J.C.; Boulamanti, A.; Tzimas, E. Methanol synthesis using captured CO2 as raw material: Techno-economic and environmental assessment. Appl. Energy 2016. [Google Scholar] [CrossRef]
- Pérez-Fortes, M.; Bocin-Dumitriu, A.; Tzimas, E. CO2 utilization pathways: Techno-economic assessment and market opportunities. Energy Procedia 2014, 63, 7968–7975. [Google Scholar] [CrossRef]
- Zheng, Y.; Yin, X.; Jiang, Y.; Bai, J.; Tang, Y.; Shen, Y.; Zhang, M. Nano Ag-decorated MoS2 nanosheets from 1T to 2H phase conversion for photocatalytically reducing CO2 to methanol. Energy Technol. 2019. [Google Scholar] [CrossRef]
- Bolivar, H.; Izquierdo, S.; Tremont, R.; Cabrera, C.R. Methanol oxidation at Pt/MoOx/MoSe2 thin film electrodes prepared with exfoliated MoSe2. J. Appl. Electrochem. 2003. [Google Scholar] [CrossRef]
- Tu, W.; Li, Y.; Kuai, L.; Zhou, Y.; Xu, Q.; Li, H.; Wang, X.; Xiao, M.; Zou, Z. Construction of unique two-dimensional MoS2-TiO2 hybrid nanojunctions: MoS2 as a promising cost-effective cocatalyst toward improved photocatalytic reduction of CO2 to methanol. Nanoscale 2017. [Google Scholar] [CrossRef]
- Xu, F.; Zhu, B.; Cheng, B.; Yu, J.; Xu, J. 1D/2D TiO2/MoS2 hybrid nanostructures for enhanced photocatalytic CO2 reduction. Adv. Opt. Mater. 2018. [Google Scholar] [CrossRef] [Green Version]
- Francis, S.A.; Velazquez, J.M.; Ferrer, I.M.; Torelli, D.A.; Guevarra, D.; McDowell, M.T.; Sun, K.; Zhou, X.; Saadi, F.H.; John, J.; et al. Reduction of aqueous CO2 to 1-propanol at MoS2 electrodes. Chem. Mater. 2018. [Google Scholar] [CrossRef] [Green Version]
- Dai, W.; Yu, J.; Luo, S.; Hu, X.; Yang, L.; Zhang, S.; Li, B.; Luo, X.; Zou, J. WS2 quantum dots seeding in Bi2S3 nanotubes: A novel Vis-NIR light sensitive photocatalyst with low-resistance junction interface for CO2 reduction. Chem. Eng. J. 2020. [Google Scholar] [CrossRef]
- Dai, W.; Yu, J.; Deng, Y.; Hu, X.; Wang, T.; Luo, X. Facile synthesis of MoS2/Bi2 WO6 nanocomposites for enhanced CO2 photoreduction activity under visible light irradiation. Appl. Surf. Sci. 2017. [Google Scholar] [CrossRef]
- Peng, H.; Lu, J.; Wu, C.; Yang, Z.; Chen, H.; Song, W.; Li, P.; Yin, H. Co-doped MoS2 NPs with matched energy band and low overpotential high efficiently convert CO2 to methanol. Appl. Surf. Sci. 2015. [Google Scholar] [CrossRef]
- Biswas, M.R.U.D.; Ali, A.; Cho, K.Y.; Oh, W.C. Novel synthesis of WSe2-Graphene-TiO2 ternary nanocomposite via ultrasonic technics for high photocatalytic reduction of CO2 into CH3OH. Ultrason. Sonochem. 2018. [Google Scholar] [CrossRef] [PubMed]
- Reyes Valle, C.; Villanueva Perales, A.L.; Vidal-Barrero, F.; Gómez-Barea, A. Techno-economic assessment of biomass-to-ethanol by indirect fluidized bed gasification: Impact of reforming technologies and comparison with entrained flow gasification. Appl. Energy 2013. [Google Scholar] [CrossRef]
- Villanueva Perales, A.L.; Reyes Valle, C.; Ollero, P.; Gómez-Barea, A. Technoeconomic assessment of ethanol production via thermochemical conversion of biomass by entrained flow gasification. Energy 2011. [Google Scholar] [CrossRef]
- Phillips, S.D. Technoeconomic analysis of a lignocellulosic biomass indirect gasification process to make ethanol via mixed alcohols synthesis. Ind. Eng. Chem. Res. 2007, 46, 8887–8897. [Google Scholar] [CrossRef]
- Kwok, K.M.; Ong, S.W.D.; Chen, L.; Zeng, H.C. Constrained growth of MoS2 nanosheets within a mesoporous silica shell and its effects on defect sites and catalyst stability for H2S decomposition. ACS Catal. 2018. [Google Scholar] [CrossRef]
- Xi, J.; Zhao, T.; Wang, D.; Shuai, Z. Tunable electronic properties of two-dimensional transition metal dichalcogenide alloys: A first-principles prediction. J. Phys. Chem. Lett. 2014. [Google Scholar] [CrossRef]
- Ahmed, S.; Yi, J. Two-dimensional transition metal dichalcogenides and their charge carrier mobilities in field-effect transistors. Nano Micro Lett. 2017. [Google Scholar] [CrossRef]
- Cates, E.L. Photocatalytic water treatment: So where are we going with this? Environ. Sci. Technol. 2017, 51, 757–758. [Google Scholar] [CrossRef]
- Hodges, B.C.; Cates, E.L.; Kim, J.H. Challenges and prospects of advanced oxidation water treatment processes using catalytic nanomaterials. Nat. Nanotechnol. 2018. [Google Scholar] [CrossRef]
- Chen, X.; Liu, C.; Mao, S. Environmental analysis with 2D transition-metal dichalcogenide-based field-effect transistors. Nano Micro Lett. 2020. [Google Scholar] [CrossRef] [Green Version]
- Ngan, S.L.; How, B.S.; Teng, S.Y.; Leong, W.D.; Loy, A.C.M.; Yatim, P.; Promentilla, M.A.B.; Lam, H.L. A hybrid approach to prioritize risk mitigation strategies for biomass polygeneration systems. Renew. Sustain. Energy Rev. 2020. [Google Scholar] [CrossRef]
- Vondra, M.; Touš, M.; Teng, S.Y. Digestate evaporation treatment in biogas plants: A Techno-economic assessment by Monte Carlo, neural networks and decision trees. J. Clean. Prod. 2019, 117870. [Google Scholar] [CrossRef] [Green Version]
- Teng, S.Y.; How, B.S.; Leong, W.D.; Teoh, J.H.; Siang Cheah, A.C.; Motavasel, Z.; Lam, H.L. Principal component analysis-aided statistical process optimisation (PASPO) for process improvement in industrial refineries. J. Clean. Prod. 2019, 225, 359–375. [Google Scholar] [CrossRef] [Green Version]
- Xu, C.; Gao, S.; Li, M. A novel PCA-based microstructure descriptor for heterogeneous material design. Comput. Mater. Sci. 2017. [Google Scholar] [CrossRef]
- Teng, S.Y.; Loy, A.C.M.; Leong, W.D.; How, B.S.; Chin, B.L.F.; Máša, V. Catalytic thermal degradation of Chlorella vulgaris: Evolving deep neural networks for optimization. Bioresour. Technol. 2019, 292, 121971. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ge, L.; Yuan, H.; Min, Y.; Li, L.; Chen, S.; Xu, L.; Goddard, W.A. Predicted optimal bifunctional electrocatalysts for the hydrogen evolution reaction and the oxygen evolution reaction using chalcogenide heterostructures based on machine learning analysis of in silico quantum mechanics based high throughput screening. J. Phys. Chem. Lett. 2020. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Hu, R.; Xie, X.; Wang, Z.; Jiang, P.; Zheng, C.; Gao, X.; Wu, T. Adopting big data to accelerate discovery of 2D TMDCs materials via CVR method for the potential application in urban airborne Hg0 sensor. Energy Procedia 2018, 152, 847–852. [Google Scholar] [CrossRef]
- Bassman, L.; Rajak, P.; Kalia, R.K.; Nakano, A.; Sha, F.; Aykol, M.; Huck, P.; Persson, K.; Sun, J.; Singh, D.J.; et al. Efficient discovery of optimal N-layered TMDC hetero-structures. MRS Adv. 2018, 3, 397–402. [Google Scholar] [CrossRef] [Green Version]











| Material | Target Gas | LOD | Condition | Remarks |
|---|---|---|---|---|
| MoS2/graphene hybrid aerogel [120] | NO2 | 50 ppb | RT to 200 °C | Response and recovery time < 1 min. |
| WS2 with AgNW functionalization [128] | NO2 | 25 ppm | 100 °C | 667% response compared to pristine 4L WS2 sensor. |
| MoS2 [121] | NO2 | 20 ppb | RT | Sensitivity of 194%/ppm |
| MoS2 [129] | NO2 | 120 ppb | RT, 100 °C | Peak to valley sensitivity > 50%. |
| MoS2 [115] | NO2 | 25 ppb | RT (with red light) | Sensitivity of 3300%/ppm. |
| rGO/MoS2 nanocomposite [123] | NO2 | 5.7 ppb | 60 °C | Over 55% sensing response for NO2 at 8 ppm |
| ZnO/MoS2 [130] | NO2 | 200 ppb | RT (with monochromatic light) | Sensitivity at 29.3%/ppm, response time of 4.3 min, recovery time of 1.2 min. |
| SnS2/MoS2 [114] | NO2 | 25.9 ppm | RT | Response time of 2 s and recovery time of 28.2 s. |
| MoSe2 [131] | NO2 | 300 ppm | RT | Response time of 20 min and recovery time of 30 min. |
| MoSe2 [116] | NO2 | 10 ppm | RT (with UV light) | Response time <200 s. |
| WS2/WO3 composite film [132] | NO2 | 100 ppb | 150 °C | Response time of 70 s and recover time of 120 s. |
| WS2/graphene aerogel [122] | NO2 | 10-15 ppb | RT | Response time of 70 s, recovery time of 300 s. (2 ppm) |
| MoTe2 [127] | NO2 | 20 ppb | RT (with UV of 2.5 mW/cm2) | Response time of 120 s with response of 18%. |
| PtSe2 [126] | NO2 | 0.9 ppb | RT | Response time of 1 s and recovery time of 4 s |
| Material | Target Gas | LOD | Condition | Remarks |
|---|---|---|---|---|
| MoS2 deposited onto Si/SiO2 [137] | NO | 0.8 ppm | RT | 80% decreased response at 2 ppm. |
| MoS2 [138] | NO | 100 ppm | RT, 50 °C, 100 °C (with UV light) | Response and recovery time below 600 s. Response at 25.63%. |
| UV-ozone treated MoS2 [139] | NO | 20 ppm | 125 °C | Stability issue over 120 ppm. Poor recovery. |
| WOx/WSe2 hybrid [140] | NO | 0.3 ppb | RT | Response time of 250 s, S/N ratio > 10, sensitivity of 520%/ppm |
| Material | Target Gas | LOD | Condition | Remarks |
|---|---|---|---|---|
| MoSe2 [149] | NH3 | 50 ppm | RT | Response time of 2.5 min, recovery time of 9 min. |
| Graphene/MoS2 [146] | NH3 | 5 ppm | 150 °C | Response time < 10 min, Recovery time < 30 min. |
| WS2 [148] | NH3 | 50 ppm | RT | Response time of 200 s, recovery time of 232.3 s. |
| MoS2 [121] | NH3 | 1 ppm | RT | Response time 5–9 min, recovery time < 15 min. |
| UV-treated MoS2 [139] | NH3 | 100 ppm | RT | Response time of 7 min, Recovery time of 12 min. |
| MoTe2 [147] | NH3 | 2 ppm | RT | Over 95% recovery using gate biases of 0 V and 20 V. Response time 10 min, recovery time 20 min. |
| MoS2/Co3O4 [150] | NH3 | 0.1 ppm | RT | Response time is 98 s, recovery time is 100 s. |
| PMMA-MoS2 [151] | NH3 | 1 ppm | RT | Sensitivity of 54%, response time of 10 s, recovery time of 14 s. |
| MoS2/VS2 [152] | NH3 | 5 ppm | 40 °C | Recovery and response time both < 5 min. |
| Mechanism | Catalyst/Material | Details | Condition | Product |
|---|---|---|---|---|
| Electro-catalyst | 2D nanoflake WSe2 [255] | 50 vol% EMIM-BF4 in water | −0.764 V | CO |
| Electro-catalyst | Hierarchical MoSxSe(2 − x) hybrid nanostructures [256] | 0.1 M H2SO4 solution | −0.70V | H2 |
| Electro-catalyst | 1 Monolayer MoS2 [257] | - | - | CO |
| Electro-catalyst | 5% niobium (Nb)-doped vertically aligned MoS2 [258] | 50 vol% EMIM-BF4 in water | −0.8 V | CO |
| Electro-catalyst | molybdenum disulfide nanoflakes (MoS2 NFs) [259] | 2.0 M C5H14ClNO | −2.0 V | CO |
| Electro-catalyst | Ultrathin MoTe2 [260] | 0.1 M KHCO3 | –1.0 V | CH4 |
| Photo-catalyst | MoS2 nanoplatelet supported on few layer graphene [261] | Up to 60% conversion, 90% CH4 selectivity | React with H2, 250–500 °C | CH4, CO |
| Photo-catalyst | Marigold-like Si@MoS2[254] | Produced 323 μL∙g−1∙h−1 CH4, 23 μL∙g−1∙h−1 O2 | React with H2O | CH4, O2 |
| Photo-catalyst | Mesoporous TiO2 on 3D Graphene with Few-layered MoS2[262] | CO selectivity of 97% and yield of 93.22 µmol/g h | - | CO |
| Photo-catalyst | Z-scheme MoS2/g-C3N4 heterojunction [263] | Produced 58.59 μmol∙g−1 in 7 h. | 25 °C, 100 kPa | CO |
| Photo-catalyst | MoS2/TiO2 heterojunction [264] | Produced 268.97 μmol∙g−1 CO and 49.93 μmol∙g−1 CH4 | 25 °C, 100 kPa | CO, CH4 |
| Mechanism | Catalyst/Material | Details | Condition | Product |
|---|---|---|---|---|
| Photo-catalyst | Undecorated 2D-MoS2 [249] | Produced 27.4 μmol∙g−1∙h−1 methanol and 2.2 μmol∙g−1∙h−1 acetaldehyde | 0.5 M NaHCO3 | Methanol, acetaldehyde |
| Photo-catalyst | MoS2/Bi2WO6 nanocomposites [277] | Produced 36.7 μmol∙g−1 methanol and 36.6 μmol∙g−1 ethanol (in 4 h) | Deionized water | Methanol, ethanol |
| Photoelectro-catalyst | Co-doped MoS2 NPs [278] | Produced 35 mmol∙L−1 methanol (in 350 min) | −0.64 V | Methanol |
| Photo-catalyst | Nano Ag decorated MoS2 nanosheets [271] | Produced 365.08 μmol∙g−1∙h−1 | 20 mL isopropanol, | Methanol |
| Electro-catalysis | MoS2 electrodes [275] | 0.1 M potassium phosphate buffer | −0.59 V | 1-propanol |
| Photo-Catalyst | WSe2/Graphene/TiO2 nanocomposite [279] | Produced 6.326 μmol∙g−1∙h−1 methanol | Distilled water with Na2SO3 reagent | Methanol |
| Photo-catalyst | MoS2/TiO2[273] | Produced 10.6 μmol∙g−1∙h−1 methanol | Distilled Water and methanol | Methanol |
| Photo-catalyst | WS2 quantum dots doped Bi2S3 nanotubes [276] | Produced 38.2 μmol∙g−1∙h−1 methanol and 27.8 μmol∙g−1∙h−1 ethanol | Ultrapure water | Methanol, ethanol |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Teng, S.Y.; Loy, A.C.M.; How, B.S.; Leong, W.D.; Tao, X. Transition Metal Dichalcogenides for the Application of Pollution Reduction: A Review. Nanomaterials 2020, 10, 1012. https://doi.org/10.3390/nano10061012
Zhang X, Teng SY, Loy ACM, How BS, Leong WD, Tao X. Transition Metal Dichalcogenides for the Application of Pollution Reduction: A Review. Nanomaterials. 2020; 10(6):1012. https://doi.org/10.3390/nano10061012
Chicago/Turabian StyleZhang, Xixia, Sin Yong Teng, Adrian Chun Minh Loy, Bing Shen How, Wei Dong Leong, and Xutang Tao. 2020. "Transition Metal Dichalcogenides for the Application of Pollution Reduction: A Review" Nanomaterials 10, no. 6: 1012. https://doi.org/10.3390/nano10061012
APA StyleZhang, X., Teng, S. Y., Loy, A. C. M., How, B. S., Leong, W. D., & Tao, X. (2020). Transition Metal Dichalcogenides for the Application of Pollution Reduction: A Review. Nanomaterials, 10(6), 1012. https://doi.org/10.3390/nano10061012