Synthesis of Novel 1T/2H-MoS2 from MoO3 Nanowires with Enhanced Photocatalytic Performance
Abstract
:1. Introduction
2. Experiment
2.1. Materials
2.2. Preparation
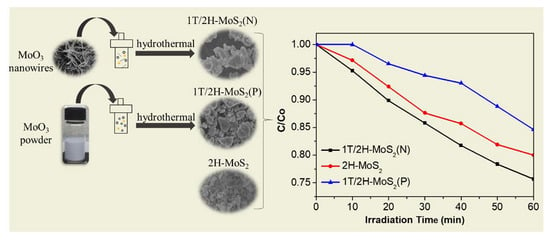
2.2.1. Preparation of 1T/2H-MoS2(N)
2.2.2. Preparation of 1T/2H-MoS2(P)
2.3. Characterization
2.4. Photodegradation
2.5. Trapping Experiment
3. Result and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Yang, Q.; Chen, Z.; Zhou, D.; Shen, W.; Naito, S. Effect of cobalt substitution on nanoporous nickel phosphate VSB-5 catalyst for the catalytic reduction of NO by H2. Catal. Today 2017, 297, 64–69. [Google Scholar] [CrossRef]
- Berkner, S.; Konradi, S.; Schönfeld, J. Antibiotic resistance and the environment—There and back again. EMBO Rep. 2014, 15, 740–744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Chen, Z.; Fang, J.; Yang, Q.; Yang, X.; Zhao, W.; Zhou, D.; Qian, X.; Liu, C.; Shao, J. Facile synthesis of TiO2 film on glass for the photocatalytic removal of rhodamine B and tetracycline hydrochloride. Mater. Express 2019, 9, 437–443. [Google Scholar] [CrossRef]
- Homem, V.; Santos, L. Degradation and removal methods of antibiotics from aqueous matrices—A review. J. Environ. Manag. 2011, 92, 2304–2347. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.; Chen, Z.; Yang, X.; Zhao, W.; Liu, C.; Sun, T.; Zhou, D.; Yang, Q.; Wei, G.; Fan, M. Perovskite cesium lead bromide quantum dots: A new efficient photocatalyst for degrading antibiotic residues in organic system. J. Clean. Prod. 2020, 249, 119335. [Google Scholar] [CrossRef]
- Yang, Q.; Chen, Z.; Yang, X.; Zhou, D.; Qian, X.; Zhang, J.; Zhang, D. Facile synthesis of Si3N4 nanowires with enhanced photocatalytic application. Mater. Lett. 2018, 212, 41–44. [Google Scholar] [CrossRef]
- Li, C.; Xu, Y.; Tu, W.; Chen, G.; Xu, R. Metal-free photocatalysts for various applications in energy conversion and environmental purification. Green Chem. 2017, 19, 882–899. [Google Scholar] [CrossRef]
- Ahmed, S.N.; Haider, W. Heterogeneous photocatalysis and its potential applications in water and wastewater treatment: A review. Nanotechnology 2018, 29, 342001. [Google Scholar] [CrossRef] [Green Version]
- Fei, J.; Li, J. Controlled preparation of porous TiO2-Ag Nanostructures through supramolecular assembly for plasmon-enhanced photocatalysis. Adv. Mater. 2014, 27, 314–319. [Google Scholar] [CrossRef]
- Zhao, W.; Yang, X.; Liu, C.; Qian, X.; Wen, Y.; Yang, Q.; Sun, T.; Chang, W.; Liu, X.; Chen, Z. Facile construction of all-solid-state Z-scheme g-C3N4/TiO2 thin film for the efficient visible-light degradation of organic pollutant. Nanomaterials 2020, 10, 600. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Chen, Z.; Zhou, D.; Zhao, W.; Qian, X.; Yang, Q.; Sun, T.; Shen, C. Ultra-low Au–Pt Co-decorated TiO2 nanotube arrays: Construction and its improved visible-light-induced photocatalytic properties. Sol. Energy Mater. Sol. Cells 2019, 201, 110065. [Google Scholar] [CrossRef]
- Zhao, X.; Feng, J.-R.; Liu, J.; Lu, J.; Shi, W.; Yang, G.; Wang, G.; Feng, P.; Cheng, P. Metal–organic framework-derived ZnO/ZnS heteronanostructures for efficient visible-light-driven photocatalytic hydrogen production. Adv. Sci. 2018, 5, 1700590. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Zhi, C.; Qian, Y.; Dong, X.; Zhang, J.; Qin, L. In-situ construction of all-solid-state Z-scheme g-C3N4/TiO2 nanotube arrays photocatalyst with enhanced visible-light-induced properties. Sol. Energy Mater. Sol. Cells 2016, 157, 399–405. [Google Scholar] [CrossRef]
- Zhou, D.; Yu, B.; Chen, Q.; Shi, H.; Zhang, Y.; Li, D.; Yang, X.; Zhao, W.; Liu, C.; Wei, G.; et al. Improved visible light photocatalytic activity on Z-scheme g-C3N4 decorated TiO2 nanotube arrays by a simple impregnation method. Mater. Res. Bull. 2020, 124, 110757. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, Y.; Chen, Z.; Qin, L.; Yang, L.; Zhu, L.; Tang, P.; Gao, T.; Huang, Y.; Sha, Z.; et al. Visible light induced photocatalysis on CdS quantum dots decorated TiO2 nanotube arrays. Appl. Catal. A Gen. 2015, 498, 159–166. [Google Scholar] [CrossRef]
- Xiang, Q.; Cheng, F.; Lang, D. Hierarchical layered WS2/graphene-modified CdS nanorods for efficient photocatalytic hydrogen evolution. ChemSusChem 2016, 9, 996–1002. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhao, L.-D. Promising thermoelectric bulk materials with 2D structures. Adv. Mater. 2017, 29, 14. [Google Scholar] [CrossRef]
- Seo, Y.-M.; Cho, H.-J.; Jang, H.-S.; Jang, W.; Lim, J.-Y.; Jang, Y.; Gu, T.; Choi, J.-Y.; Whang, D. 2D doping layer for flexible transparent conducting graphene electrodes with low sheet resistance and high stability. Adv. Electron. Mater. 2018, 4, 6. [Google Scholar] [CrossRef]
- Ago, H. Crystal Growth and Device Applications of Two-Dimensional Layered Materials; IEEE: New York, NY, USA, 2017; pp. 86–87. [Google Scholar]
- Li, M.-Y.; Chen, C.-H.; Shi, Y.; Li, L.-J. Heterostructures based on two-dimensional layered materials and their potential applications. Mater. Today 2016, 19, 322–335. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Chen, Z.; Fang, J.; Yang, Q.; Zhao, W.; Qian, X.; Zhou, D.; Liu, C.; Chen, M. Freestanding 3D MoS2 nanosheets/graphene aerogel heterostructure as a recyclable photocatalyst for efficiently degrading antibiotic residues. Mater. Lett. 2019, 252, 5–7. [Google Scholar] [CrossRef]
- Mao, J.; Wang, Y.; Zheng, Z.; Deng, D. The rise of two-dimensional MoS2 for catalysis. Front. Phys. 2018, 13, 19. [Google Scholar] [CrossRef]
- Castellanos-Gomez, A.; Barkelid, M.; Goossens, S.; Calado, V.E.; van der Zant, H.S.J.; Steele, G.A. Laser-thinning of MoS2: On demand generation of a single-layer semiconductor. Nano Lett. 2012, 12, 3187–3192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, X.; Chen, Z.; Fang, J.; Yang, Q.; Zhao, W.; Qian, X.; Liu, C.; Zhou, D.; Tao, S.; Liu, X. Efficient exfoliation to MoS2 nanosheets by salt-assisted refluxing and ultrasonication with photocatalytic application. Mater. Lett. 2019, 255, 126596. [Google Scholar] [CrossRef]
- Yu, Y.; Nam, G.-H.; He, Q.; Wu, X.-J.; Zhang, K.; Yang, Z.; Chen, J.; Ma, Q.; Zhao, M.; Liu, Z.; et al. High phase-purity 1T′-MoS2- and 1T′-MoSe2-layered crystals. Nat. Chem. 2018, 10, 638–643. [Google Scholar] [CrossRef] [PubMed]
- Geng, X.; Jiao, Y.; Han, Y.; Mukhopadhyay, A.; Yang, L.; Zhu, H. Freestanding metallic 1T MoS2 with dual ion diffusion paths as high rate anode for sodium-ion batteries. Adv. Funct. Mater. 2017, 27, 8. [Google Scholar] [CrossRef]
- Lei, Z.; Zhan, J.; Tang, L.; Zhang, Y.; Wang, Y. Recent development of metallic (1T) phase of molybdenum disulfide for energy conversion and storage. Adv. Energy Mater. 2018, 8, 29. [Google Scholar] [CrossRef]
- Shi, S.; Sun, Z.; Hu, Y.H. Synthesis, stabilization and applications of 2-dimensional 1T metallic MoS2. J. Mater. Chem. A 2018, 6, 23932–23977. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, X.; Bao, S.; Zhang, Z.; Fei, H.; Wu, Z. Phase engineering of a multiphasic 1T/2H MoS2 catalyst for highly efficient hydrogen evolution. J. Mater. Chem. A 2017, 5, 2681–2688. [Google Scholar] [CrossRef]
- Xuyen, N.T.; Ting, J.-M. Hybridized 1T/2H MoS2 having controlled 1T concentrations and its use in supercapacitors. Chem. A Eur. J. 2017, 23, 17348–17355. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, D.; Li, B.; Zhang, C.; Du, Z.; Yin, H.; Bi, X.; Yang, S. Ultrastable in-plane 1T-2H MoS2 heterostructures for enhanced hydrogen evolution reaction. Adv. Energy Mater. 2018, 8, 1801345. [Google Scholar] [CrossRef]
- Wen, P.; Guo, J.; Ren, L.; Wang, C.; Lan, Y.; Jiang, X. One-step hydrothermal preparation of 1D α-MoO3 nanobelt electrode material for supercapacitor. Nano 2019, 14, 59–66. [Google Scholar] [CrossRef]
- Qu, B.; Sun, Y.; Liu, L.; Li, C.; Yu, C.; Zhang, X.; Chen, Y. Ultrasmall Fe2O3 nanoparticles/MoS2 nanosheets composite as high-performance anode material for lithium ion batteries. Sci. Rep. 2017, 7, 42772. [Google Scholar] [CrossRef] [PubMed]
- Geng, X.; Sun, W.; Wu, W.; Chen, B.; Al-Hilo, A.; Benamara, M.; Zhu, H.; Watanabe, F.; Cui, J.; Chen, T.-P. Pure and stable metallic phase molybdenum disulfide nanosheets for hydrogen evolution reaction. Nat. Commun. 2016, 7, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minero, C.; Mariella, G.; Maurino, V.; Vione, A.D.; Pelizzetti, E. Photocatalytic transformation of organic compounds in the presence of inorganic ions. 2. Competitive reactions of phenol and alcohols on a titanium dioxide−fluoride system. Langmuir 2015, 16, 8964–8972. [Google Scholar] [CrossRef]
- Tian, Y.; Chang, B.; Lu, J.; Fu, J.; Xi, F.; Dong, X. Hydrothermal synthesis of graphitic carbon nitride–Bi2WO6 heterojunctions with enhanced visible light photocatalytic activities. Powder Technol. 2014, 267, 126–133. [Google Scholar] [CrossRef]
- Yu, J.; Wang, S.; Low, J.; Xiao, W. Enhanced photocatalytic performance of direct Z-scheme g-C3N4–TiO2 photocatalysts for the decomposition of formaldehyde in air. Phys. Chem. Chem. Phys. 2013, 15, 16883–16890. [Google Scholar] [CrossRef]








© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, W.; Liu, X.; Yang, X.; Liu, C.; Qian, X.; Sun, T.; Chang, W.; Zhang, J.; Chen, Z. Synthesis of Novel 1T/2H-MoS2 from MoO3 Nanowires with Enhanced Photocatalytic Performance. Nanomaterials 2020, 10, 1124. https://doi.org/10.3390/nano10061124
Zhao W, Liu X, Yang X, Liu C, Qian X, Sun T, Chang W, Zhang J, Chen Z. Synthesis of Novel 1T/2H-MoS2 from MoO3 Nanowires with Enhanced Photocatalytic Performance. Nanomaterials. 2020; 10(6):1124. https://doi.org/10.3390/nano10061124
Chicago/Turabian StyleZhao, Wan, Xin Liu, Xiuru Yang, Chunxi Liu, Xiaoxiao Qian, Tao Sun, Wenya Chang, Jingjing Zhang, and Zhi Chen. 2020. "Synthesis of Novel 1T/2H-MoS2 from MoO3 Nanowires with Enhanced Photocatalytic Performance" Nanomaterials 10, no. 6: 1124. https://doi.org/10.3390/nano10061124
APA StyleZhao, W., Liu, X., Yang, X., Liu, C., Qian, X., Sun, T., Chang, W., Zhang, J., & Chen, Z. (2020). Synthesis of Novel 1T/2H-MoS2 from MoO3 Nanowires with Enhanced Photocatalytic Performance. Nanomaterials, 10(6), 1124. https://doi.org/10.3390/nano10061124