A Facile Method for the Non-Covalent Amine Functionalization of Carbon-Based Surfaces for Use in Biosensor Development
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Graphene Device Manufacture
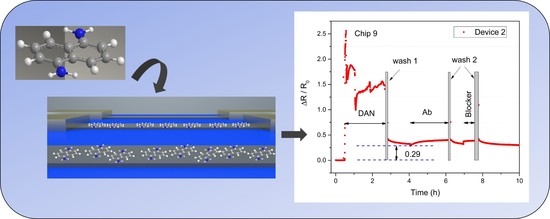
2.2.2. Functionalization of Graphene
2.2.3. Electrical Measurements
3. Results and Discussion
3.1. Cyclic Voltammetry
3.2. Surface Characterization-Raman Spectroscopy
3.3. Surface Characterization—X-ray Photoelectron Spectroscopy (XPS)
3.4. Electrical Measurements
3.5. Investigation of Wash Steps
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Neto, A.H.C.; Guinea, F.; Peres, N.M.R.; Novoselov, K.S.; Geim, A.K. The electronic properties of graphene. Rev. Mod. Phys. 2009, 81, 109–162. [Google Scholar] [CrossRef] [Green Version]
- Novoselov, K.S.; Fal’ko, V.I.; Colombo, L.; Gellert, P.R.; Schwab, M.G.; Kim, K. A roadmap for graphene. Nature 2012, 490, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Macucci, M.; Marconcini, P. Approximate calculation of the potential profile in a graphene-based device. IET Circuits Devices Syst. 2015, 9, 30–38. [Google Scholar]
- Liu, J.; Liu, Z.; Barrow, C.J.; Yang, W. Molecularly engineered graphene surfaces for sensing applications: A review. Anal. Chim. Acta 2015, 859, 1–19. [Google Scholar] [CrossRef]
- Walters, F.; Rozhko, S.; Buckley, D.; Ahmadi, E.D.A.; Ali, M.; Tehrani, Z.; Mitchell, J.; Burwell, G.; Liu, Y.; Kazakova, O.; et al. Real-time detection of hepatitis B surface antigen using a hybrid graphene-gold nanoparticle biosensor. 2D Mater. 2020, 7, 024009. [Google Scholar] [CrossRef]
- Zou, X.; Wei, S.; Jasensky, J.; Xiao, M.; Wang, M.; Wang, Q.; Brooks, C.L., III; Chen, Z. Molecular interactions between graphene and biological molecules. J. Am. Chem. Soc. 2017, 139, 1928–1936. [Google Scholar] [CrossRef]
- Russell, S.R.; Claridge, S.A. Peptide interfaces with graphene: An emerging intersection of analytical chemistry, theory, and materials young investigators in analytical and bioanalytical science. Anal. Bioanal. Chem. 2016, 408, 2649–2658. [Google Scholar] [CrossRef]
- Roach, P.; Farrar, D.; Perry, C.C. Interpretation of protein adsorption: Surface-induced conformational changes. J. Am. Chem. Soc. 2005, 127, 8168–8173. [Google Scholar] [CrossRef]
- Trzeciakiewicz, H.; Esteves-Villanueva, J.; Soudy, R.; Kaur, K.; Martic-Milne, S. Electrochemical characterization of protein adsorption onto YNGRT-Au and VLGXE-Au surfaces. Sensors (Switz.) 2015, 15, 19429–19442. [Google Scholar] [CrossRef] [Green Version]
- Alava, T.; Mann, J.A.; Theodore, C.; Beniyez, J.J.; Dichtel, W.R.; Parpia, J.M.; Craighead, H.G. Control of the graphene–protein interface is required to preserve adsorbed protein function. Anal. Chem. 2013, 85, 2754–2759. [Google Scholar] [CrossRef]
- Tehrani, Z.; Burwell, G.; Mohd Azmi, M.A.; Castaing, A.; Rickman, R.; Almarashi, J.Q.M.; Dunstan, P.; Beigi, A.M.; Doak, S.H.; Guy, O.J. Generic epitaxial graphene biosensors for ultrasensitive detection of cancer risk biomarker. 2D Mater. 2014, 1, 025004. [Google Scholar] [CrossRef]
- Yin, P.T.; Shah, S.; Chhowalla, M.; Lee, K.-B. Design, synthesis, and characterization of graphene–nanoparticle hybrid materials for bioapplications. Chem. Rev. 2015, 115, 2483–2531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, W.; Jiang, L.; van Geest, E.P.; Lima, L.M.C.C.; Schneider, G.F. Sensing at the surface of graphene field-effect transistors. Adv. Mater. 2017, 29, 1–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katoch, J.; Kim, S.N.; Kuang, Z.; Farmer, B.L.; Naik, R.R.; Tatulian, S.A.; Ishigami, M. Structure of a peptide adsorbed on graphene and graphite. Nano Lett. 2012, 12, 2342–2346. [Google Scholar] [CrossRef] [PubMed]
- Bosch-Navarro, C.; Laker, Z.P.L.; Marsden, A.J.; Wilson, N.R.; Rourke, J.P. Non-covalent functionalization of graphene with a hydrophilic self-limiting monolayer for macro-molecule immobilization. FlatChem 2017, 1, 52–56. [Google Scholar] [CrossRef] [Green Version]
- Hao, Z.; Zhu, Y.; Wang, X.; Rotti, P.G.; DiMarco, C.; Tyler, S.R.; Zhao, X.; Engelhardt, J.F.; Hone, J.; Lin, Q. Real-time monitoring of insulin using a graphene field-effect transistor aptameric nanosensor. ACS Appl. Mater. Interfaces 2017, 9, 27504–27511. [Google Scholar] [CrossRef]
- Haslam, C.; Damiati, S.; Whitley, T.; Davey, P.; Ifeachor, E.; Awan, S. Label-free sensors based on graphene field-effect transistors for the detection of human chorionic gonadotropin cancer risk biomarker. Diagnostics 2018, 8, 5. [Google Scholar] [CrossRef] [Green Version]
- Zhou, L.; Mao, H.; Wu, C.; Tang, L.; Wu, Z.; Sun, H.; Zhang, H.; Zhou, H.; Jia, C.; Jin, Q.; et al. Label-free graphene biosensor targeting cancer molecules based on non-covalent modification. Biosens. Bioelectron. 2017, 87, 701–707. [Google Scholar] [CrossRef] [Green Version]
- Vishnubhotla, R.; Ping, J.; Gao, Z.; Lee, A.; Saouaf, O.; Vrudhula, A.; Johnson, A.T. C Scalable graphene aptasensors for drug quantification. AIP Adv. 2017, 7, 1–7. [Google Scholar] [CrossRef]
- Cooper, V.R.; Lam, C.N.; Wang, Y.; Sumpter, B.G. Noncovalent interactions in nanotechnology. In Non-Covalent Interactions in Quantum Chemistry and Physics; Elsevier: Amsterdam, The Netherlands, 2017; pp. 417–451. [Google Scholar]
- Teixeira, S.; Conlan, R.S.; Guy, O.J.; Sales, M.G.F. Label-free human chorionic gonadotropin detection at picogram levels using oriented antibodies bound to graphene screen-printed electrodes. J. Mater. Chem. B 2014, 2, 1852–1865. [Google Scholar] [CrossRef] [Green Version]
- Yukird, J.; Wongtangprasert, T.; Rangkupan, R.; Chailapakul, O.; Pisitkun, T.; Rodthongkum, N. Label-free immunosensor based on graphene/polyaniline nanocomposite for neutrophil gelatinase-associated lipocalin detection. Biosens. Bioelectron. 2017, 87, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Chen, X.; Zhu, S.; Yao, Y.; Quan, W.; Liu, B. Enhanced sensitivity of ammonia sensor using graphene/polyaniline nanocomposite. Sens. Actuators B Chem. 2013, 178, 485–493. [Google Scholar] [CrossRef]
- Rosy, R.; Goyal, R.N.; Shim, Y.-B. Glutaraldehyde sandwiched amino functionalized polymer based aptasensor for the determination and quantification of chloramphenicol. RSC Adv. 2015, 5, 69356–69364. [Google Scholar] [CrossRef]
- Chasta, H.; Goyal, R.N. A Simple and sensitive poly-1,5-diaminonaphthalene modified sensor for the determination of sulfamethoxazole in biological samples. Electroanalysis 2015, 27, 1229–1237. [Google Scholar] [CrossRef]
- Tu, J.; Gan, Y.; Liang, T.; Hu, Q.; Wang, Q.; Ren, T.; Sun, Q.; Wan, H.; Wang, P. Graphene FET array biosensor based on ssDNA aptamer for ultrasensitive Hg2+ detection in environmental pollutants. Front. Chem. 2018, 6, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Shin, D.H.; Lee, J.S.; Jun, J.; An, J.H.; Kim, S.G.; Cho, K.H.; Jang, J. Flower-like Palladium Nanoclusters decorated graphene electrodes for ultrasensitive and flexible hydrogen gas sensing. Sci. Rep. 2015, 5, 12294. [Google Scholar] [CrossRef] [Green Version]
- Devadoss, A.; Forsyth, R.; Bigham, R.; Abbasi, H.; Ali, M.; Tehrani, Z.; Liu, Y.; Guy, O.J. Ultrathin functional polymer modified graphene for enhanced enzymatic electrochemical sensing. Biosensors 2019, 9, 16. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, B.H.; Nguyen, B.T.; Vu, H.V.; Nguyen, C.V.; Nguyen, D.T.; Nguyen, L.T.; Vu, T.T.; Tran, L.D. Development of label-free electrochemical lactose biosensor based on graphene/poly(1,5-diaminonaphthalene) film. Curr. Appl. Phys. 2016, 16, 135–140. [Google Scholar] [CrossRef]
- Elgrishi, N.; Rountree, K.J.; McCarthy, B.D.; Rountree, E.S.; Eisenhart, T.T.; Dempsey, J.L. A practical beginner’s guide to cyclic voltammetry. J. Chem. Educ. 2018, 95, 197–206. [Google Scholar] [CrossRef]
- Bocarsly, A.B. Cyclic voltammetry. In Characterization of Materials; John Wiley and Sons: Hoboken, NJ, USA, 2012; pp. 57–106. [Google Scholar]
- Gupta, P.; Yadav, S.K.; Agrawal, B.; Goyal, R.N. A novel graphene and conductive polymer modified pyrolytic graphite sensor for determination of propranolol in biological fluids. Sens. Actuators B Chem. 2014, 204, 791–798. [Google Scholar] [CrossRef]
- Jijie, R.; Kahlouche, K.; Barras, A.; Yamakawa, N.; Bouckaert, J.; Gharbi, T.; Szunerits, S.; Boukherroub, R. Reduced graphene oxide/polyethylenimine based immunosensor for the selective and sensitive electrochemical detection of uropathogenic Escherichia coli. Sens. Actuators B Chem. 2018, 260, 255–263. [Google Scholar] [CrossRef]
- Vu, H.D.; Nguyen, L.-H.; Nguyen, T.D.; Nguyen, H.B.; Nguyen, T.L.; Tran, D.L. Anodic stripping voltammetric determination of Cd2+ and Pb2+ using interpenetrated MWCNT/P1,5-DAN as an enhanced sensing interface. Ionics 2015, 21, 571–578. [Google Scholar] [CrossRef]
- Bard, L.; Faulkner, A.J. Electrochemical Methods: Fundamentals and Applications, 2nd ed.; Wiley: Chichester, UK, 2001. [Google Scholar]
- Ferrari, A.C.; Basko, D.M. Raman spectroscopy as a versatile tool for studying the properties of graphene. Nat. Nanotechnol. 2013, 8, 235–246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beams, R.; Gustavo, L.; Cançado, L.G.; Novotny, L. Raman characterization of defects and dopants in graphene. J. Phys. Condens. Matter. 2015, 27, 083002. [Google Scholar] [CrossRef] [PubMed]
- Vincent, T.; Panhal, V.; Booth, T.; Power, S.R.; Jauho, A.-P.; Antonov, V.; Kazakova, O. Probing the nanoscale origin of strain and doping in graphene-hBN heterostructures. 2D Mater. 2018, 6, 015022. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.E.; Ahn, G.; Shim, J.; Lee, Y.S.; Ryu, S. Optical separation of mechanical strain from charge doping in graphene. Nat. Commun. 2012, 3, 1024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banszerus, L.; Janssen, H.; Otto, M.; Epping, A.; Taniguchi, T.; Watanabe, K.; Beschoten, B.; Neumaier, D.; Stampfer, C. Identifying suitable substrates for high-quality graphene-based heterostructures. 2D Mater. 2017, 4, 025030. [Google Scholar] [CrossRef]
- Bartolomeo, A.D.; Giubileo, F.; Romeo, F.; Sabatino, P.; Carapella, G.; Iemmo, L.; Schroeder, T.; Lupina, G. Graphene field effect transistors with niobium contacts and asymmetric transfer characteristics. Nanotechnology 2015, 26, 475202. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.G.; Tang, M.; Lai, K.W.C. Doping effects of surface functionalization on graphene with aromatic molecule and organic solvents. Appl. Surf. Sci. 2017, 425, 713–721. [Google Scholar] [CrossRef]
- Habiba, K.; Makarov, V.I.; Avalos, J.; Guinel, M.J.; Weiner, B.R.; Morell, G. Surface structure of few layer graphene. Carbon N. Y. 2018, 136, 255–261. [Google Scholar]
- Alzate-Carvajal, N.; Acevedo-Guzmán, D.A.; Meza-Laguna, V.; Farías, M.H.; Pérez-Rey, L.A.; Abarca-Morales, E.; García-Ramírez, V.A.; Basiuk, V.A.; Basiuk, E.V. One-step nondestructive functionalization of graphene oxide paper with amines. RSC Adv. 2018, 8, 15253–15265. [Google Scholar] [CrossRef] [Green Version]
- Pinto, H.; Markevich, A. Electronic and electrochemical doping of graphene by surface adsorbates. Beilstein J. Nanotechnol. 2014, 5, 1842–1848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodríguez, S.J.; Makinistian, L.; Albanesi, E.A.; Albanesi, E.A. Electronic transport upon adsorption of biomolecules on graphene. In Handbook of Graphene; John Wiley and Sons: Hoboken, NJ, USA, 2019; pp. 767–792. [Google Scholar]
- Phillipson, R.; Lockhart de la Rosa, C.J.; Teyssandier, J.; Walke, P.; Waghray, D.; Fujita, Y.; Adisoejoso, J.; Mali, K.S.; Asselberghs, I.; Huyghebaert, C.; et al. Tunable doping of graphene by using physisorbed self-assembled networks. Nanoscale 2016, 8, 20017–20026. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klar, P.; Casiraghi, C. Raman spectroscopy of graphene in different dielectric solvents. Phys. Status Solidi 2010, 7, 2735–2738. [Google Scholar] [CrossRef]
- Yang, X.; Yan, M. Removing contaminants from transferred CVD graphene. Nano Res. 2020, 13, 599–610. [Google Scholar] [CrossRef]
- Lipatov, A.; Varezhnikov, A.; Augustin, M.; Bruns, M.; Sommer, M.; Sysoev, V.V.; Kolmakov, A.; Sinitskii, A. Intrinsic device-to-device variation in graphene field-effect transistors on a Si/SiO2 substrate as a platform for discriminative gas sensing. Appl. Phys. Lett. 2014, 104, 013114. [Google Scholar] [CrossRef] [Green Version]
- Ishigami, M.; Chen, J.H.; Cullen, W.G.; Fuhrer, M.S.; Williams, E.D. Atomic structure of graphene on SiO2. Nano Lett. 2007, 7, 1643–1648. [Google Scholar] [CrossRef] [Green Version]
- Khamis, S.M.; Jones, R.A.; Johnson, A.T.C. Optimized photolithographic fabrication process for carbon nanotube devices. AIP Adv. 2011, 1, 022106. [Google Scholar] [CrossRef]
- Zhang, Z.; Huang, H.; Yang, X.; Zang, L. Tailoring electronic properties of graphene by π-π Stacking with aromatic molecules. J. Phys. Chem. Lett. 2011, 2, 2897–2905. [Google Scholar] [CrossRef]
- Cunge, G.; Petit-Etienne, C.; Davydova, A.; Ferrah, D.; Renault, O.; Okuno, H.; Kalita, D.; Bouchiat, V. Dry efficient cleaning of poly-methyl-methacrylate residues from graphene with high-density H2 and H2-N2 plasmas. J. Appl. Phys. 2015, 118, 123302. [Google Scholar] [CrossRef]
- Yulaev, A.; Cheng, G.; Walker, A.R.H.; Vlassiouk, I.V.; Myers, A.; Leite, M.S.; Kolmakov, A. Toward clean suspended CVD graphene. RSC Adv. 2016, 6, 83954–83962. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zorn, G.; Liu, L.-H.; Árnadóttir, L.; Wang, H.; Gamble, L.J.; Castner, D.G.; Yan, M. X-ray photoelectron spectroscopy investigation of the nitrogen species in photoactive perfluorophenylazide-modified surfaces. J. Phys. Chem. C 2014, 118, 376–383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pham, M.C.; Oulahyane, M.; Mostefai, M.; Chehimi, M.M. Multiple internal reflection FT-IR spectroscopy (MIRFTIRS) study of the electrochemical synthesis and the redox process of poly(1,5-diaminonaphthalene). Synth. Met. 1998, 93, 89–96. [Google Scholar] [CrossRef]
- Melios, C.; Giusca, C.E.; Panchal, V.; Kazakova, O. Water on graphene: Review of recent progress. 2D Mater. 2018, 5, 022001. [Google Scholar] [CrossRef] [Green Version]





| Sample | O 1s | N 1s | C 1s | Si 2p |
|---|---|---|---|---|
| Blank Graphene | 36.0 ± 0.4% | – | 45.6 ± 1.1% | 18.4 ± 1.5% |
| DAN | 30.9 ± 1.9% | 2.2 ± 0.1% | 52.3 ± 3.7% | 14.5 ± 1.8% |
| DAN + Ab | 20.4 ± 0.1% | 10.9 ± 0.1% | 62.8 ± 0.1% | 5.9 ± 0.1% |
| Sample | Element | Binding Energy Position (eV) | Atomic Concentration (%) |
|---|---|---|---|
| Blank Graphene (Gr) | O 1s | 532.22 | 61.83 ± 0.32 |
| N 1s | N/A | N/A | |
| C 1s | 284.02 | 38.17 ± 0.32 | |
| Gr + EtOH Wash Only | O 1s | 532.10 | 50.94 ± 0.35 |
| N 1s | 399.30 | 2.56 ± 0.18 | |
| C 1s | 284.03 | 46.50 ± 0.36 | |
| Gr + EtOH Wash + 1 DI Water Wash | O 1s | 531.90 | 48.60 ± 0.33 |
| N 1s | 398.70 | 2.59 ± 0.15 | |
| C 1s | 284.00 | 48.81 ± 0.34 | |
| Gr + EtOH Wash + 2 DI Water Wash | O 1s | 532.00 | 46.04 ± 0.34 |
| N 1s | 399.30 | 2.69 ± 0.14 | |
| C 1s | 283.98 | 51.27 ± 0.35 | |
| Gr + EtOH Wash + 3 DI Water Wash | O 1s | 531.90 | 46.52 ± 0.34 |
| N 1s | 399.20 | 2.60 ± 0.17 | |
| C 1s | 283.99 | 50.88 ± 0.35 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Walters, F.; Ali, M.M.; Burwell, G.; Rozhko, S.; Tehrani, Z.; Daghigh Ahmadi, E.; Evans, J.E.; Abbasi, H.Y.; Bigham, R.; Mitchell, J.J.; et al. A Facile Method for the Non-Covalent Amine Functionalization of Carbon-Based Surfaces for Use in Biosensor Development. Nanomaterials 2020, 10, 1808. https://doi.org/10.3390/nano10091808
Walters F, Ali MM, Burwell G, Rozhko S, Tehrani Z, Daghigh Ahmadi E, Evans JE, Abbasi HY, Bigham R, Mitchell JJ, et al. A Facile Method for the Non-Covalent Amine Functionalization of Carbon-Based Surfaces for Use in Biosensor Development. Nanomaterials. 2020; 10(9):1808. https://doi.org/10.3390/nano10091808
Chicago/Turabian StyleWalters, Ffion, Muhammad Munem Ali, Gregory Burwell, Sergiy Rozhko, Zari Tehrani, Ehsaneh Daghigh Ahmadi, Jon E. Evans, Hina Y. Abbasi, Ryan Bigham, Jacob John Mitchell, and et al. 2020. "A Facile Method for the Non-Covalent Amine Functionalization of Carbon-Based Surfaces for Use in Biosensor Development" Nanomaterials 10, no. 9: 1808. https://doi.org/10.3390/nano10091808
APA StyleWalters, F., Ali, M. M., Burwell, G., Rozhko, S., Tehrani, Z., Daghigh Ahmadi, E., Evans, J. E., Abbasi, H. Y., Bigham, R., Mitchell, J. J., Kazakova, O., Devadoss, A., & Guy, O. J. (2020). A Facile Method for the Non-Covalent Amine Functionalization of Carbon-Based Surfaces for Use in Biosensor Development. Nanomaterials, 10(9), 1808. https://doi.org/10.3390/nano10091808