Investigation of Physicochemical Properties of the Structurally Modified Nanosized Silicate-Substituted Hydroxyapatite Co-Doped with Eu3+ and Sr2+ Ions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of the Co-Doped Materials
2.2. Physical–Chemical Characterization
2.3. Spectroscopy Properties
3. Results and Discussion
3.1. X-ray Diffraction
3.2. Kröger–Vink Notation
3.3. Infrared Spectra
3.4. Spectroscopy Properties
3.5. Temperature-Dependent Emission
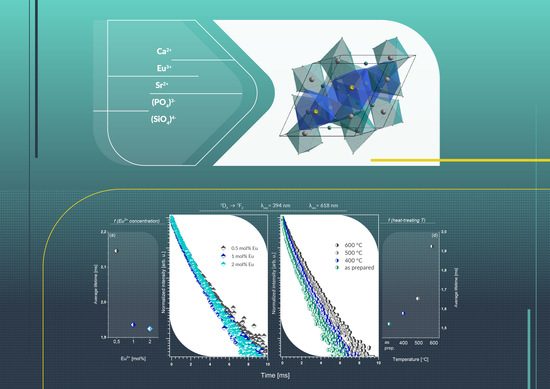
3.6. Decay Time
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zawisza, K.; Strzep, A.; Wiglusz, R.J. Influence of annealing temperature on the spectroscopic properties of hydroxyapatite analogues doped with Eu3+. New J. Chem. 2017, 41, 9990–9999. [Google Scholar] [CrossRef]
- Guo, Y.; Moon, B.K.; Choi, B.C.; Jeong, J.H.; Kim, J.H.; Choi, H. Fluorescence properties with red-shift of Eu2+ emission in novel phosphor-silicate apatite Sr3LaNa(PO4)2SiO4 phosphors. Ceram. Int. 2016, 42, 18324–18332. [Google Scholar] [CrossRef]
- Zawisza, K.; Wiglusz, R.J. Preferential site occupancy of Eu3+ ions in strontium hydroxyapatite nanocrystalline- Sr10(PO4)6(OH)2 -structural and spectroscopic characterisation. Dalton Trans. 2017, 46, 3265–3275. [Google Scholar] [CrossRef]
- Pogosova, M.A.; Eliseev, A.A.; Kazin, P.E.; Azarmi, F. Synthesis, structure, luminescence, and color features of the Eu- and Cu-doped calcium apatite. Dye Pigment. 2017, 141, 209–216. [Google Scholar] [CrossRef]
- Sokolnicki, J.; Zych, E. Synthesis and spectroscopic investigations of Sr2Y8(SiO4)6O2:Eu2+,Eu3+ phosphor for white LEDs. J. Lumin. 2015, 158, 65–69. [Google Scholar] [CrossRef]
- Xia, Z.; Molokeev, M.S.; Im, W.B.; Unithrattil, S.; Liu, Q. Crystal structure and photoluminescence evolution of La5(SxB1-x)(O13-xNx):Ce3+ solid solution phosphors. J. Phys. Chem. C 2015, 119, 9488–9495. [Google Scholar] [CrossRef]
- Sobierajska, P.; Wiglusz, R.J. Influence of the grain sizes on Stokes and anti-Stokes fluorescence in the Yb3+ and Tb3+ ions co-doped nanocrystalline fluorapatite. J. Alloy. Compd. 2019, 785, 808–818. [Google Scholar] [CrossRef]
- Liu, N.; Mei, L.; Liao, L.; Fu, J.; Yang, D. High thermal stability apatite phosphors Ca2La8(SiO4)6O2:Dy3+/Sm3+ for white light emission: Synthesis, structure, luminescence properties and energy transfer. Sci. Rep. 2019, 9. [Google Scholar] [CrossRef] [Green Version]
- Fleet, M.E.; Liu, X.; Pan, Y. Site preference of rare earth elements in hydroxyapatite [Ca10(PO4)6(OH)2]. J. Solid State Chem. 2000, 149, 391–398. [Google Scholar] [CrossRef]
- Chouard, N.; Caurant, D.; Majérus, O.; Dussossoy, J.L.; Loiseau, P.; Grygiel, C.; Peuget, S. External irradiation with heavy ions of neodymium silicate apatite ceramics and glass-ceramics. J. Nucl. Mater. 2019, 516, 11–29. [Google Scholar] [CrossRef]
- Zhu, G.; Shi, Y.; Mikami, M.; Shimomura, Y.; Wang, Y. Design, synthesis and characterization of a new apatite phosphor Sr4La2Ca4(PO4)6O2:Ce3+ with long wavelength Ce3+ emission. Opt. Mater. Express 2013, 3, 229. [Google Scholar] [CrossRef]
- Xia, Y.; Liu, Y.G.; Huang, Z.; Fang, M.; Molokeev, M.S.; Mei, L. Ca6La4(SiO4)2(PO4)4O2:Eu2+: A novel apatite green-emitting phosphor for near-ultraviolet excited w-LEDs. J. Mater. Chem. C 2016, 4, 4675–4683. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Yu, M.; Wu, Z.; Wang, Y.; Zhang, P. White emission enhancement of Ca5(PO4)3Cl:Dy3+ phosphor with Li+/Eu3+ co-doping for white light-emitting diodes. J. Mater. Sci. Mater. Electron. 2018, 29, 8224–8233. [Google Scholar] [CrossRef]
- Gu, Z.; Zhang, X.; Li, L.; Wang, Q.; Yu, X.; Feng, T. Acceleration of segmental bone regeneration in a rabbit model by strontium-doped calcium polyphosphate scaffold through stimulating VEGF and bFGF secretion from osteoblasts. Mater. Sci. Eng. C 2013, 33, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Wiens, M.; Wang, X.; Schröder, H.C.; Kolb, U.; Schloßmacher, U.; Ushijima, H.; Müller, W.E.G. The role of biosilica in the osteoprotegerin/RANKL ratio in human osteoblast-like cells. Biomaterials 2010, 31, 7716–7725. [Google Scholar] [CrossRef]
- Lin, K.; Xia, L.; Li, H.; Jiang, X.; Pan, H.; Xu, Y.; Lu, W.W.; Zhang, Z.; Chang, J. Enhanced osteoporotic bone regeneration by strontium-substituted calcium silicate bioactive ceramics. Biomaterials 2013, 34, 10028–10042. [Google Scholar] [CrossRef]
- Kikuchi, M.; Yamazaki, A.; Otsuka, R.; Akao, M.; Aoki, H. Crystal structure of Sr-substituted hydroxyapatite synthesized by hydrothermal method. J. Solid State Chem. 1994, 113, 373–378. [Google Scholar] [CrossRef]
- Rietveld, H.M. A profile refinement method for nuclear and magnetic structures. J. Appl. Crystallogr. 1969, 2, 65–71. [Google Scholar] [CrossRef]
- Lutterotti, L.; Matthies, S.; Wenk, H.-R. MAUD: A friendly Java program for material analysis using diffraction. IUCr Newsl. CPD 1999, 21, 14–15. [Google Scholar] [CrossRef] [Green Version]
- Pham, V.H.; Ngoc Trung, N. Luminescence of europium doped silicon-substituted hydroxyapatite nanobiophosphor via a coprecipitation method. Mater. Lett. 2014, 136, 359–361. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomie distances in halides and chaleogenides. Acta Cryst. 1976, A32, 751–767. [Google Scholar] [CrossRef]
- Balan, E.; Delattre, S.; Roche, D.; Segalen, L.; Morin, G.; Guillaumet, M.; Blanchard, M.; Lazzeri, M.; Brouder, C.; Salje, E.K.H. Line-broadening effects in the powder infrared spectrum of apatite. Phys. Chem. Miner. 2011, 38, 111–122. [Google Scholar] [CrossRef]
- Williams, R.L.; Hadley, M.J.; Jiang, P.J.; Rowson, N.A.; Mendes, P.M.; Rappoport, J.Z.; Grover, L.M. Thiol modification of silicon-substituted hydroxyapatite nanocrystals facilitates fluorescent labelling and visualisation of cellular internalisation. J. Mater. Chem. B 2013, 1, 4370–4378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Targonska, S.; Szyszka, K.; Rewak-Soroczynska, J.; Wiglusz, R.J. A new approach to spectroscopic and structural studies of the nano-sized silicate-substituted hydroxyapatite doped with Eu3+ ions. Dalton Trans. 2019, 48, 8303–8316. [Google Scholar] [CrossRef]
- Wiglusz, R.J.; Bednarkiewicz, A.; Strek, W. Synthesis and optical properties of Eu3+ ion doped nanocrystalline hydroxya patites embedded in PMMA matrix. J. Rare Earths 2011, 29, 1111–1116. [Google Scholar] [CrossRef]
- Marycz, K.; Sobierajska, P.; Smieszek, A.; Maredziak, M.; Wiglusz, K.; Wiglusz, R.J. Li+ activated nanohydroxyapatite doped with Eu3+ ions enhances proliferative activity and viability of human stem progenitor cells of adipose tissue and olfactory ensheathing cells. Further perspective of nHAP:Li+, Eu3+ application in theranostics. Mater. Sci. Eng. C 2017, 78, 151–162. [Google Scholar] [CrossRef]
- Lima, T.A.R.M.; Valerio, M.E.G. X-ray absorption fine structure spectroscopy and photoluminescence study of multifunctional europium (III)-doped hydroxyapatite in the presence of cationic surfactant medium. J. Lumin. 2018, 201, 70–76. [Google Scholar] [CrossRef]
- El Ouenzerfi, R.; Kbir-Ariguib, N.; Trabelsi-Ayedi, M.; Piriou, B. Spectroscopic study of Eu3+ in strontium hydroxyapatite Sr10(PO4)6(OH)2. J. Lumin. 1999, 85, 71–77. [Google Scholar] [CrossRef]
- Huang, Y.; Gan, J.; Seo, H.J. Luminescence investigation of Eu-activated Sr5(PO4)2SiO4 phosphor by combustion synthesis. J. Am. Ceram. Soc. 2011, 94, 1143–1148. [Google Scholar] [CrossRef]
- Yu, R.; Jeong, J.H.; Wang, Y.F. A novel Eu3+- and self-activated vanadate phosphor of Ca4La(VO4)3O with oxyvanadate apatite structure. J. Am. Ceram. Soc. 2017, 100, 5649–5658. [Google Scholar] [CrossRef]
- Doat, A.; Fanjul, M.; Pellé, F.; Hollande, E.; Lebugle, A. Europium-doped bioapatite: A new photostable biological probe, internalizable by human cells. Biomaterials 2003, 24, 3365–3371. [Google Scholar] [CrossRef]
- Martin, P.; Carlot, G.; Chevarier, A.; Den-Auwer, C.; Panczer, G. Mechanisms involved in thermal diffusion of rare earth elements in apatite. J. Nucl. Mater. 1999, 275, 268–276. [Google Scholar] [CrossRef] [Green Version]
- Judd, B.R. Optical absorption intensities of rare-earth ions. Phys. Rev. 1962, 127, 750–761. [Google Scholar] [CrossRef]
- Ofelt, G.S. Intensities of crystal spectra of rare-earth ions. J. Chem. Phys. 1962, 37, 511–520. [Google Scholar] [CrossRef]
- Kodaira, C.A.; Brito, H.F.; Malta, O.L.; Serra, O.A. Luminescence and energy transfer of the europium (III) tungstate obtained via the Pechini method. J. Lumin. 2003, 101, 11–21. [Google Scholar] [CrossRef]
- Hreniak, D.; Strek, W.; Amami, J.; Guyot, Y.; Boulon, G.; Goutaudier, C.; Pazik, R. The size-effect on luminescence properties of BaTiO3:Eu3+ nanocrystallites prepared by the sol-gel method. J. Alloy. Compd. 2004, 380, 348–351. [Google Scholar] [CrossRef]
- Sobierajska, P.; Wiglusz, R.J. Influence of Li+ ions on the physicochemical properties of nanocrystalline calcium–strontium hydroxyapatite doped with Eu3+ ions. New J. Chem. 2019, 43, 14908–14916. [Google Scholar] [CrossRef]
- Shionoya, S.; Yen, W.; Yamamoto, H. Phosphor Handbook; Phosphor Research Society; CRC Press: Cleveland, OH, USA, 2007. [Google Scholar]









| Sample | Ca9.8-xSr0.2Eux(PO4)2(SiO4)4(OH)2 600 °C | Ca9.6Sr0.2Eu0.2(PO4)2(SiO4)4(OH)2 | |||||
|---|---|---|---|---|---|---|---|
| x = 0.5 mol% Eu3+ | x = 1.0 mol% Eu3+ | x = 2.0 mol% Eu3+ | as prepared | 400 °C | 500 °C | 600 °C | |
| Ca(1)/Ca(2) | 0.42 | 2.08 | 3.06 | 2.46 | 2.64 | 2.78 | 3.06 |
| Arad (s−1) | 159.02 | 207.91 | 192.34 | 147.11 | 158.88 | 168.89 | 192.34 |
| Anrad (s−1) | 306.85 | 308.56 | 327.17 | 509.69 | 475.04 | 435.80 | 327.17 |
| Atot (s−1) | 465.87 | 516.48 | 519.51 | 656.80 | 633.92 | 604.69 | 519.51 |
| τ (ms) | 2.15 | 1.94 | 1.92 | 1.52 | 1.58 | 1.65 | 1.92 |
| Ω2 (10−20 cm2) | 4.084 | 5.898 | 5.377 | 3.678 | 4.166 | 4.506 | 5.377 |
| Ω4 (10−20 cm2) | 1.078 | 1.119 | 0.994 | 0.997 | 0.907 | 0.977 | 0.995 |
| h (%) | 34.13 | 40.26 | 37.02 | 22.40 | 25.06 | 27.93 | 37.02 |
| R | 2.85 | 4.11 | 3.75 | 2.57 | 2.91 | 3.14 | 3.75 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Targonska, S.; Wiglusz, R.J. Investigation of Physicochemical Properties of the Structurally Modified Nanosized Silicate-Substituted Hydroxyapatite Co-Doped with Eu3+ and Sr2+ Ions. Nanomaterials 2021, 11, 27. https://doi.org/10.3390/nano11010027
Targonska S, Wiglusz RJ. Investigation of Physicochemical Properties of the Structurally Modified Nanosized Silicate-Substituted Hydroxyapatite Co-Doped with Eu3+ and Sr2+ Ions. Nanomaterials. 2021; 11(1):27. https://doi.org/10.3390/nano11010027
Chicago/Turabian StyleTargonska, Sara, and Rafal J. Wiglusz. 2021. "Investigation of Physicochemical Properties of the Structurally Modified Nanosized Silicate-Substituted Hydroxyapatite Co-Doped with Eu3+ and Sr2+ Ions" Nanomaterials 11, no. 1: 27. https://doi.org/10.3390/nano11010027
APA StyleTargonska, S., & Wiglusz, R. J. (2021). Investigation of Physicochemical Properties of the Structurally Modified Nanosized Silicate-Substituted Hydroxyapatite Co-Doped with Eu3+ and Sr2+ Ions. Nanomaterials, 11(1), 27. https://doi.org/10.3390/nano11010027