Advances in Nanomaterials Used in Co-Delivery of siRNA and Small Molecule Drugs for Cancer Treatment
Abstract
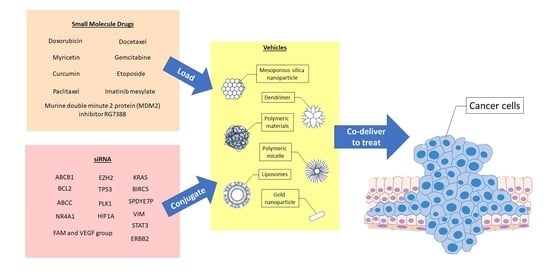
:1. Introduction
2. Desirable Properties of Co-Delivery Systems
3. Classes of Co-Delivery Systems
3.1. Mesoporous Silica Nanoparticles
3.1.1. General Properties
3.1.2. Applications in Cancer Treatment
3.2. Polymeric Materials
3.2.1. General Properties
3.2.2. Applications in Cancer Treatment
3.3. Liposomes
3.3.1. General Properties
3.3.2. Applications in Cancer Treatment
3.4. Dendrimers
3.4.1. General Properties
3.4.2. Applications in Cancer Treatment
3.5. Gold Nanoparticles
3.5.1. General Properties
3.5.2. Applications in Cancer Treatment
4. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization. WHO Report on Cancer: Setting Priorities, Investing Wisely and Providing Care for All; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Alberts, B.; Johnson, A.; L., J. The Molecular Basis of Cancer-Cell Behavior, 4th ed.; Garland Science: New York, NY, USA, 2002. [Google Scholar]
- Institute, N.C. Types of Cancer Treatment. Available online: https://www.cancer.gov/about-cancer/treatment/types (accessed on 28 August 2021).
- Jin, J.O.; Kim, G.; Hwang, J.; Han, K.H.; Kwak, M.; Lee, P.C.W. Nucleic acid nanotechnology for cancer treatment. Biochim. Biophys. Acta-Rev. Cancer 2020, 1874, 188377. [Google Scholar] [CrossRef]
- Lee, S.J.; Kim, M.J.; Kwon, I.C.; Roberts, T.M. Delivery strategies and potential targets for siRNA in major cancer types. Adv. Drug Deliv. Rev. 2016, 104, 2–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Wang, Y.; Zhu, Y.; Oupický, D. Recent advances in delivery of drug-nucleic acid combinations for cancer treatment. J. Control. Release 2013, 172, 589–600. [Google Scholar] [CrossRef] [Green Version]
- Dong, Y.; Siegwart, D.J.; Anderson, D.G. Strategies, design, and chemistry in siRNA delivery systems. Adv. Drug Deliv. Rev. 2019, 144, 133–147. [Google Scholar] [CrossRef] [PubMed]
- Oh, Y.K.; Park, T.G. siRNA delivery systems for cancer treatment. Adv. Drug Deliv. Rev. 2009, 61, 850–862. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Xu, X.; Zhang, K.; Sun, B.; Wang, L.; Meng, L.; Liu, Q.; Zheng, C.; Yang, B.; Sun, H. Codelivery of doxorubicin and MDR1-siRNA by mesoporous silica nanoparticles-polymerpolyethylenimine to improve oral squamous carcinoma treatment. Int. J. Nanomed. 2018, 13, 187–198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, M.; Wang, J.; Li, B.; Meng, L.; Tian, Z. Recent advances in mechanism-based chemotherapy drug-siRNA pairs in co-delivery systems for cancer: A review. Colloids Surf. B Biointerfaces 2017, 157, 297–308. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, B.G.; Vit, F.F.; Carvalho, H.F.; Han, S.W.; de la Torre, L.G. Recent advances in co-delivery nanosystems for synergistic action in cancer treatment. J. Mater. Chem. B 2021, 9, 1208–1237. [Google Scholar] [CrossRef]
- Li, X.; Sun, A.N.; Liu, Y.J.; Zhang, W.J.; Pang, N.; Cheng, S.X.; Qi, X.R. Amphiphilic dendrimer engineered nanocarrier systems for co-delivery of siRNA and paclitaxel to matrix metalloproteinase-rich tumors for synergistic therapy. NPG Asia Mater. 2018, 10, 238–254. [Google Scholar] [CrossRef] [Green Version]
- Malhotra, M.; Tomaro-Duchesneau, C.; Saha, S.; Prakash, S. Intranasal Delivery of Chitosan—siRNA Nanoparticle Formulation to the Brain. In Drug Delivery System; Jain, K.K., Ed.; Springer: New York, NY, USA, 2014; pp. 233–247. ISBN 978-1-4939-0363-4. [Google Scholar]
- Ibraheem, D.; Elaissari, A.; Fessi, H. Gene therapy and DNA delivery systems. Int. J. Pharm. 2014, 459, 70–83. [Google Scholar] [CrossRef]
- Scholz, C.; Wagner, E. Therapeutic plasmid DNA versus siRNA delivery: Common and different tasks for synthetic carriers. J. Control. Release 2012, 161, 554–565. [Google Scholar] [CrossRef]
- Sajid, M.I.; Moazzam, M.; Tiwari, R.K.; Kato, S.; Cho, K.Y. Overcoming barriers for siRNA therapeutics: From bench to bedside. Pharmaceuticals 2020, 13, 294. [Google Scholar] [CrossRef]
- Hsiao, I.L.; Fritsch-Decker, S.; Leidner, A.; Al-Rawi, M.; Hug, V.; Diabaté, S.; Grage, S.L.; Meffert, M.; Stoeger, T.; Gerthsen, D.; et al. Biocompatibility of Amine-Functionalized Silica Nanoparticles: The Role of Surface Coverage. Small 2019, 15, 1805400. [Google Scholar] [CrossRef] [PubMed]
- Moreira, A.F.; Dias, D.R.; Correia, I.J. Stimuli-responsive mesoporous silica nanoparticles for cancer therapy: A review. Microporous Mesoporous Mater. 2016, 236, 141–157. [Google Scholar] [CrossRef]
- Holban, A.M.; Grumezescu, A.M.; Andronescu, E. Inorganic Nanoarchitectonics Designed for Drug Delivery and Anti-Infective Surfaces; Elsevier Inc.: Amsterdam, The Netherlands, 2016; ISBN 9780323428613. [Google Scholar]
- Holban, A.M.; Grumezescu, A. Nanocomposite Drug Carriers. In Nanoarchitectonics for Smart Delivery and Drug Targeting; William Andrew: Norwich, NY, USA, 2016; pp. 261–284. ISBN 9780323477222. [Google Scholar]
- Mamaeva, V.; Sahlgren, C.; Lindén, M. Mesoporous silica nanoparticles in medicine-Recent advances. Adv. Drug Deliv. Rev. 2013, 65, 689–702. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Liang, S.; Long, M.; Xu, H. Mesoporous silica nanoparticles as potential carriers for enhanced drug solubility of paclitaxel. Mater. Sci. Eng. C 2017, 78, 12–17. [Google Scholar] [CrossRef]
- Santoso, A.V.; Susanto, A.; Irawaty, W.; Hadisoewignyo, L.; Hartono, S.B. Chitosan modified mesoporous silica nanoparticles as a versatile drug carrier with pH dependent properties. AIP Conf. Proc. 2019, 2114, 020011. [Google Scholar] [CrossRef]
- Kumar, P.; Tambe, P.; Paknikar, K.M.; Gajbhiye, V. Mesoporous silica nanoparticles as cutting-edge theranostics: Advancement from merely a carrier to tailor-made smart delivery platform. J. Control. Release 2018, 287, 35–57. [Google Scholar] [CrossRef]
- Elbialy, N.S.; Aboushoushah, S.F.; Sofi, B.F.; Noorwali, A. Multifunctional curcumin-loaded mesoporous silica nanoparticles for cancer chemoprevention and therapy. Microporous Mesoporous Mater. 2020, 291, 109540. [Google Scholar] [CrossRef]
- Qi, S.S.; Sun, J.H.; Yu, H.H.; Yu, S.Q. Co-delivery nanoparticles of anti-cancer drugs for improving chemotherapy efficacy. Drug Deliv. 2017, 24, 1909–1926. [Google Scholar] [CrossRef] [Green Version]
- Sun, B.; Wang, X.; Liao, Y.P.; Ji, Z.; Chang, C.H.; Pokhrel, S.; Ku, J.; Liu, X.; Wang, M.; Dunphy, D.R.; et al. Repetitive Dosing of Fumed Silica Leads to Profibrogenic Effects through Unique Structure-Activity Relationships and Biopersistence in the Lung. ACS Nano 2016, 10, 8054–8066. [Google Scholar] [CrossRef] [Green Version]
- Hegde, S.; Krisnawan, V.E.; Herzog, B.H.; Zuo, C.; Breden, M.A.; Knolhoff, B.L.; Hogg, G.D.; Tang, J.P.; Baer, J.M.; Mpoy, C.; et al. Dendritic Cell Paucity Leads to Dysfunctional Immune Surveillance in Pancreatic Cancer. Cancer Cell 2020, 37, 289–307.e9. [Google Scholar] [CrossRef] [PubMed]
- Seelige, R.; Searles, S.; Bui, J.D. Mechanisms regulating immune surveillance of cellular stress in cancer. Cell. Mol. Life Sci. 2018, 75, 225–240. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Zhou, B.; Du, X.; Wang, Y.; Zhang, J.; Ai, Y.; Xia, Z.; Zhao, G. Folic acid (FA)-conjugated mesoporous silica nanoparticles combined with MRP-1 siRNA improves the suppressive effects of myricetin on non-small cell lung cancer (NSCLC). Biomed. Pharmacother. 2020, 125, 109561. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Xu, M.; Cao, C.; Yu, Q.; Zhou, Y.; Liu, J. A redox-responsive strategy using mesoporous silica nanoparticles for co-delivery of siRNA and doxorubicin. J. Mater. Chem. B 2017, 5, 6908–6919. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Chen, Y.; Zhang, Y.; He, Z.; Wu, X.; Jiang, L.P. PH-sensitive CAP/SiO2 composite for efficient co-delivery of doxorubicin and siRNA to overcome multiple drug resistance. RSC Adv. 2020, 10, 4251–4257. [Google Scholar] [CrossRef]
- Yu, X.; Zhu, Y. Preparation of magnetic mesoporous silica nanoparticles as a multifunctional platform for potential drug delivery and hyperthermia. Sci. Technol. Adv. Mater. 2016, 17, 229–238. [Google Scholar] [CrossRef] [Green Version]
- Maeda, H. Polymer therapeutics and the EPR effect. J. Drug Target. 2017, 25, 781–785. [Google Scholar] [CrossRef]
- Park, J.; Choi, Y.; Chang, H.; Um, W.; Ryu, J.H.; Kwon, I.C. Alliance with EPR effect: Combined strategies to improve the EPR effect in the tumor microenvironment. Theranostics 2019, 9, 8073–8090. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Chen, L.; Nie, W.; Wang, W.; Qin, M.; Mo, X.; Wang, H.; He, C. Dual-responsive mesoporous silica nanoparticles mediated codelivery of doxorubicin and Bcl-2 SiRNA for targeted treatment of breast cancer. J. Phys. Chem. C 2016, 120, 22375–22387. [Google Scholar] [CrossRef]
- Pandey, S.P.; Shukla, T.; Dhote, V.K.; Mishra, D.K.; Maheshwari, R.; Tekade, R.K. Use of Polymers in Controlled Release of Active Agents. In Basic Fundamentals of Drug Delivery; Academic Press: Cambridge, MA, USA, 2019; pp. 113–172. [Google Scholar] [CrossRef]
- Deb, P.K.; Kokaz, S.F.; Abed, S.N.; Paradkar, A.; Tekade, R.K. Pharmaceutical and Biomedical Applications of Polymers. In Basic Fundamentals of Drug Delivery; Academic Press: Cambridge, MA, USA, 2019; pp. 203–267. [Google Scholar] [CrossRef]
- Wechsler, M.E.; Clegg, J.R.; Peppas, N.A. The Interface of Drug Delivery and Regenerative Medicine; Elsevier Inc.: Amsterdam, The Netherlands, 2019; Volume 1–3, ISBN 9780128136997. [Google Scholar]
- dos Santos Rodrigues, B.; Lakkadwala, S.; Sharma, D.; Singh, J. Chitosan for Gene, DNA Vaccines, and Drug Delivery; Elsevier Inc.: Amsterdam, The Netherlands, 2019; ISBN 9780128184332. [Google Scholar]
- Sung, Y.K.; Kim, S.W. Recent advances in the development of gene delivery systems. Biomater. Res. 2019, 23, 8. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Wang, L.; Wei, X.; Zhou, S. Polymer-based drug delivery systems for cancer treatment. J. Polym. Sci. Part A Polym. Chem. 2016, 54, 3525–3550. [Google Scholar] [CrossRef]
- Yan, T.; Zhu, S.; Hui, W.; He, J.; Liu, Z.; Cheng, J. Chitosan based pH-responsive polymeric prodrug vector for enhanced tumor targeted co-delivery of doxorubicin and siRNA. Carbohydr. Polym. 2020, 250, 116781. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Chen, Z.; Wu, S.; Han, Y.; Wang, H.; Sun, H.; Kong, D.; Leng, X.; Wang, C.; Zhang, L.; et al. Micelle or polymersome formation by PCL-PEG-PCL copolymers as drug delivery systems. Chin. Chem. Lett. 2017, 28, 1905–1909. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Feng, H.; Jin, L.; Zhang, Y.; Tian, X.; Li, J. Polymeric micelle with pH-induced variable size and doxorubicin and siRNA co-delivery for synergistic cancer therapy. Appl. Nanosci. 2020, 10, 1903–1913. [Google Scholar] [CrossRef]
- Arote, R.B.; Jere, D.; Jiang, H.-L.; Kim, Y.-K.; Choi, Y.-J.; Cho, M.-H.; Cho, C.-S. Injectable polymeric carriers for gene delivery systems. In Injectable Biomaterials; Woodhead Publishing: Cambridge, UK, 2011; pp. 235–259. [Google Scholar] [CrossRef]
- Gardini, D.; Lüscher, C.J.; Struve, C.; Krogfelt, K.A. Tailored Nanomaterials for Antimicrobial Applications; Elsevier Inc.: Amsterdam, The Netherlands, 2018; ISBN 9780323512558. [Google Scholar]
- Zhao, X.; Li, F.; Li, Y.; Wang, H.; Ren, H.; Chen, J.; Nie, G.; Hao, J. Co-delivery of HIF1α siRNA and gemcitabine via biocompatible lipid-polymer hybrid nanoparticles for effective treatment of pancreatic cancer. Biomaterials 2015, 46, 13–25. [Google Scholar] [CrossRef]
- Lv, H.; Zhang, S.; Wang, B.; Cui, S.; Yan, J. Toxicity of cationic lipids and cationic polymers in gene delivery. J. Control. Release 2006, 114, 100–109. [Google Scholar] [CrossRef]
- Fischer, D.; Bieber, T.; Li, Y.; Elsässer, H.P.; Kissel, T. A novel non-viral vector for DNA delivery based on low molecular weight, branched polyethylenimine: Effect of molecular weight on transfection efficiency and cytotoxicity. Pharm. Res. 1999, 16, 1273–1279. [Google Scholar] [CrossRef]
- Alinejad-Mofrad, E.; Malaekeh-Nikouei, B.; Gholami, L.; Mousavi, S.H.; Sadeghnia, H.R.; Mohajeri, M.; Darroudi, M.; Oskuee, R.K. Evaluation and comparison of cytotoxicity, genotoxicity, and apoptotic effects of poly-l-lysine/plasmid DNA micro- and nanoparticles. Hum. Exp. Toxicol. 2019, 38, 983–991. [Google Scholar] [CrossRef]
- Rimann, M.; Lühmann, T.; Textor, M.; Guerino, B.; Ogier, J.; Hall, H. Characterization of PLL-g-PEG-DNA nanoparticles for the delivery of therapeutic DNA. Bioconjug. Chem. 2008, 19, 548–557. [Google Scholar] [CrossRef]
- Hershberger, K.K.; Gauger, A.J.; Bronstein, L.M. Utilizing Stimuli Responsive Linkages to Engineer and Enhance Polymer Nanoparticle-Based Drug Delivery Platforms. ACS Appl. Bio Mater. 2021, 4, 4720–4736. [Google Scholar] [CrossRef]
- Hopkins, E.; Sanvictores, T.; Sharma, S. Physiology, Acid Base Balance. In Urolithiasis; StatPearls Publishing: Treasure Island, FL, USA, 2020; pp. 19–22. [Google Scholar]
- Suo, A.; Qian, J.; Xu, M.; Xu, W.; Zhang, Y.; Yao, Y. Folate-decorated PEGylated triblock copolymer as a pH/reduction dual-responsive nanovehicle for targeted intracellular co-delivery of doxorubicin and Bcl-2 siRNA. Mater. Sci. Eng. C 2017, 76, 659–672. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, Y.; Zhang, W.; Sun, C.; Wu, J.; Tang, J. Reversing of multidrug resistance breast cancer by co-delivery of P-gp siRNA and doxorubicin via folic acid-modified core-shell nanomicelles. Colloids Surf. B Biointerfaces 2016, 138, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Norouzi, P.; Amini, M.; Dinarvand, R.; Arefian, E.; Seyedjafari, E.; Atyabi, F. Co-delivery of gemcitabine prodrug along with anti NF-κB siRNA by tri-layer micelles can increase cytotoxicity, uptake and accumulation of the system in the cancers. Mater. Sci. Eng. C 2020, 116, 111161. [Google Scholar] [CrossRef]
- Lin, C.; Hu, Z.; Yuan, G.; Su, H.; Zeng, Y.; Guo, Z.; Zhong, F.; Jiang, K.; He, S. HIF1α-siRNA and gemcitabine combination-based GE-11 peptide antibody-targeted nanomedicine for enhanced therapeutic efficacy in pancreatic cancers. J. Drug Target. 2019, 27, 797–805. [Google Scholar] [CrossRef]
- Yuan, Z.Q.; Chen, W.L.; You, B.G.; Liu, Y.; Li, J.Z.; Zhu, W.J.; Zhou, X.F.; Liu, C.; Zhang, X.N. Multifunctional nanoparticles co-delivering EZH2 siRNA and etoposide for synergistic therapy of orthotopic non-small-cell lung tumor. J. Control. Release 2017, 268, 198–211. [Google Scholar] [CrossRef]
- Wen, Z.M.; Jie, J.; Zhang, Y.; Liu, H.; Peng, L.P. A self-assembled polyjuglanin nanoparticle loaded with doxorubicin and anti-Kras siRNA for attenuating multidrug resistance in human lung cancer. Biochem. Biophys. Res. Commun. 2017, 493, 1430–1437. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.; Jin, G.; Kang, L.; Chen, L.; Gao, Z.; Huang, W. Smart polymeric nanoparticles with pH-responsive and PEG-detachable properties for co-delivering paclitaxel and survivin siRNA to enhance antitumor outcomes. Int. J. Nanomed. 2018, 13, 2405–2426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, C.; Liu, W.; Hu, Y.; Li, W.; Di, W. Bioinspired tumor-homing nanoplatform for co-delivery of paclitaxel and siRNA-E7 to HPV-related cervical malignancies for synergistic therapy. Theranostics 2020, 10, 3325–3339. [Google Scholar] [CrossRef] [PubMed]
- Nasab, S.H.; Amani, A.; Ebrahimi, H.A.; Hamidi, A.A. Design and preparation of a new multi-targeted drug delivery system using multifunctional nanoparticles for co-delivery of siRNA and paclitaxel. J. Pharm. Anal. 2021, 11, 163–173. [Google Scholar] [CrossRef]
- Joshi, U.; Filipczak, N.; Khan, M.M.; Attia, S.A.; Torchilin, V. Hypoxia-sensitive micellar nanoparticles for co-delivery of siRNA and chemotherapeutics to overcome multi-drug resistance in tumor cells. Int. J. Pharm. 2020, 590, 119915. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, B.; Chen, X.; Wu, M.; Ji, Y.; Tang, G.; Ping, Y. A supramolecular co-delivery strategy for combined breast cancer treatment and metastasis prevention. Chin. Chem. Lett. 2020, 31, 1153–1158. [Google Scholar] [CrossRef]
- Yang, Y.; Meng, Y.; Ye, J.; Xia, X.; Wang, H.; Li, L.; Dong, W.; Jin, D.; Liu, Y. Sequential delivery of VEGF siRNA and paclitaxel for PVN destruction, anti-angiogenesis, and tumor cell apoptosis procedurally via a multi-functional polymer micelle. J. Control. Release 2018, 287, 103–120. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, K.S.; Hussein, S.A.; Ali, A.H.; Korma, S.A.; Lipeng, Q.; Jinghua, C. Liposome: Composition, characterisation, preparation, and recent innovation in clinical applications. J. Drug Target 2019, 27, 742–761. [Google Scholar] [CrossRef]
- Sercombe, L.; Veerati, T.; Moheimani, F.; Wu, S.Y.; Sood, A.K.; Hua, S. Advances and challenges of liposome assisted drug delivery. Front. Pharmacol. 2015, 6, 286. [Google Scholar] [CrossRef] [Green Version]
- Akbarzadeh, A.; Rezaei-sadabady, R.; Davaran, S.; Joo, S.W.; Zarghami, N. Liposome: Classification, prepNew aspects of liposomesaration, and applications. Nanoscale Res. Lett. 2013, 8, 102. [Google Scholar] [CrossRef] [Green Version]
- Kiaie, S.H.; Mojarad-Jabali, S.; Khaleseh, F.; Allahyari, S.; Taheri, E.; Zakeri-Milani, P.; Valizadeh, H. Axial pharmaceutical properties of liposome in cancer therapy: Recent advances and perspectives. Int. J. Pharm. 2020, 581, 119269. [Google Scholar] [CrossRef]
- Filipczak, N.; Pan, J.; Yalamarty, S.S.K.; Torchilin, V.P. Recent advancements in liposome technology. Adv. Drug Deliv. Rev. 2020, 156, 4–22. [Google Scholar] [CrossRef]
- Jose, A.; Labala, S.; Ninave, K.M.; Gade, S.K.; Venuganti, V.V.K. Effective Skin Cancer Treatment by Topical Co-delivery of Curcumin and STAT3 siRNA Using Cationic Liposomes. AAPS PharmSciTech 2018, 19, 166–175. [Google Scholar] [CrossRef]
- Qu, M.H.; Zeng, R.F.; Fang, S.; Dai, Q.S.; Li, H.P.; Long, J.T. Liposome-based co-delivery of siRNA and docetaxel for the synergistic treatment of lung cancer. Int. J. Pharm. 2014, 474, 112–122. [Google Scholar] [CrossRef]
- Oh, H.R.; Jo, H.Y.; Park, J.S.; Kim, D.E.; Cho, J.Y.; Kim, P.H.; Kim, K.S. Galactosylated liposomes for targeted co-delivery of doxorubicin/vimentin sirna to hepatocellular carcinoma. Nanomaterials 2016, 6, 141. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Zhang, S.; Zhi, D.; Zhao, Y.; Cui, S.; Cui, J. Co-delivery of paclitaxel and survivin siRNA with cationic liposome for lung cancer therapy. Colloids Surf. A Physicochem. Eng. Asp. 2020, 585, 124054. [Google Scholar] [CrossRef]
- Yao, Y.; Su, Z.; Liang, Y.; Zhang, N. pH-Sensitive carboxymethyl chitosan-modified cationic liposomes for sorafenib and siRNa co-delivery. Int. J. Nanomed. 2015, 10, 6185–6198. [Google Scholar] [CrossRef] [Green Version]
- Lymberopoulos, A.; Demopoulou, C.; Kyriazi, M.; Katsarou, M.; Demertzis, N.; Hatziandoniou, S.; Maswadeh, H.; Papaioanou, G.; Demetzos, C.; Maibach, H.; et al. Liposome percutaneous penetration in vivo. Toxicol. Res. Appl. 2017, 1, 239784731772319. [Google Scholar] [CrossRef] [Green Version]
- Subongkot, T.; Ngawhirunpat, T.; Opanasopit, P. Development of ultradeformable liposomes with fatty acids for enhanced dermal rosmarinic acid delivery. Pharmaceutics 2021, 13, 404. [Google Scholar] [CrossRef]
- Jiang, T.; Jin, K.; Liu, X.; Pang, Z. Nanoparticles for Tumor Targeting; Elsevier Ltd.: Amsterdam, The Netherlands, 2017; ISBN 9780081019153. [Google Scholar]
- Abedi-Gaballu, F.; Dehghan, G.; Ghaffari, M.; Yekta, R.; Abbaspour-Ravasjani, S.; Baradaran, B.; Ezzati Nazhad Dolatabadi, J.; Hamblin, M.R. PAMAM dendrimers as efficient drug and gene delivery nanosystems for cancer therapy. Appl. Mater. Today 2018, 12, 177–190. [Google Scholar] [CrossRef]
- Thakore, S.I.; Solanki, A.; Das, M. Exploring Potential of Polymers in Cancer Management; Elsevier Inc.: Amsterdam, The Netherlands, 2019; ISBN 9780128184332. [Google Scholar]
- Li, Y.; Wang, H.; Wang, K.; Hu, Q.; Yao, Q.; Shen, Y.; Yu, G.; Tang, G. Targeted Co-delivery of PTX and TR3 siRNA by PTP Peptide Modified Dendrimer for the Treatment of Pancreatic Cancer. Small 2017, 13, 1602697. [Google Scholar] [CrossRef] [PubMed]
- Jain, K. Dendrimers: Smart Nanoengineered Polymers for Bioinspired Applications in Drug Delivery; Elsevier Ltd.: Amsterdam, The Netherlands, 2017; ISBN 9780081019153. [Google Scholar]
- Li, J.; Liang, H.; Liu, J.; Wang, Z. Poly (amidoamine) (PAMAM) dendrimer mediated delivery of drug and pDNA/siRNA for cancer therapy. Int. J. Pharm. 2018, 546, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Pan, J.; Yao, M.; Palmerston Mendes, L.; Sarisozen, C.; Mao, S.; Torchilin, V.P. Charge reversible hyaluronic acid-modified dendrimer-based nanoparticles for siMDR-1 and doxorubicin co-delivery. Eur. J. Pharm. Biopharm. 2020, 154, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Xin, X.; Qiu, L.; Li, W.; Guan, G.; Li, G.; Qiao, M.; Zhao, X.; Hu, H.; Chen, D. Co-delivery of p53 and MDM2 inhibitor RG7388 using a hydroxyl terminal PAMAM dendrimer derivative for synergistic cancer therapy. Acta Biomater. 2019, 100, 118–131. [Google Scholar] [CrossRef] [PubMed]
- Du Rietz, H.; Hedlund, H.; Wilhelmson, S.; Nordenfelt, P.; Wittrup, A. Imaging small molecule-induced endosomal escape of siRNA. Nat. Commun. 2020, 11, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Bus, T.; Traeger, A.; Schubert, U.S. The great escape: How cationic polyplexes overcome the endosomal barrier. J. Mater. Chem. B 2018, 6, 6904–6918. [Google Scholar] [CrossRef]
- Vermeulen, L.M.P.; Brans, T.; Samal, S.K.; Dubruel, P.; Demeester, J.; De Smedt, S.C.; Remaut, K.; Braeckmans, K. Endosomal Size and Membrane Leakiness Influence Proton Sponge-Based Rupture of Endosomal Vesicles. ACS Nano 2018, 12, 2332–2345. [Google Scholar] [CrossRef] [Green Version]
- Ghaffari, M.; Dehghan, G.; Baradaran, B.; Zarebkohan, A.; Mansoori, B.; Soleymani, J.; Ezzati Nazhad Dolatabadi, J.; Hamblin, M.R. Co-delivery of curcumin and Bcl-2 siRNA by PAMAM dendrimers for enhancement of the therapeutic efficacy in HeLa cancer cells. Colloids Surf. B Biointerfaces 2020, 188, 110762. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, A.F.A.; Higashi, T.; Motoyama, K.; Ohyama, A.; Onodera, R.; Khaled, K.A.; Sarhan, H.A.; Hussein, A.K.; Arima, H. In Vitro and In Vivo Co-delivery of siRNA and Doxorubicin by Folate-PEG-Appended Dendrimer/Glucuronylglucosyl-β-Cyclodextrin Conjugate. AAPS J. 2019, 21, 54. [Google Scholar] [CrossRef] [PubMed]
- Yaqoob, S.B.; Adnan, R.; Rameez Khan, R.M.; Rashid, M. Gold, Silver, and Palladium Nanoparticles: A Chemical Tool for Biomedical Applications. Front. Chem. 2020, 8, 376. [Google Scholar] [CrossRef]
- Sztandera, K.; Gorzkiewicz, M.; Klajnert-Maculewicz, B. Gold Nanoparticles in Cancer Treatment. Mol. Pharm. 2019, 16, 1–23. [Google Scholar] [CrossRef]
- Yin, F.; Yang, C.; Wang, Q.; Zeng, S.; Hu, R.; Lin, G.; Tian, J.; Hu, S.; Lan, R.F.; Yoon, H.S.; et al. A light-driven therapy of pancreatic adenocarcinoma using gold nanorods-based nanocarriers for co-delivery of doxorubicin and siRNA. Theranostics 2015, 5, 818–833. [Google Scholar] [CrossRef] [Green Version]
- Beik, J.; Khateri, M.; Khosravi, Z.; Kamrava, S.K.; Kooranifar, S.; Ghaznavi, H.; Shakeri-Zadeh, A. Gold nanoparticles in combinatorial cancer therapy strategies. Coord. Chem. Rev. 2019, 387, 299–324. [Google Scholar] [CrossRef]
- Dykman, L.A.; Khlebtsov, N.G. Immunological properties of gold nanoparticles. Chem. Sci. 2017, 8, 1719–1735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yafout, M.; Ousaid, A.; Khayati, Y.; El Otmani, I.S. Gold nanoparticles as a drug delivery system for standard chemotherapeutics: A new lead for targeted pharmacological cancer treatments. Sci. Afr. 2021, 11, e00685. [Google Scholar] [CrossRef]
- Singh, S.; Maurya, P.K. Nanotechnology in Modern Animal Biotechnology: Recent Trends and Future Perspectives; Springer: New York, NY, USA, 2019; pp. 29–65. ISBN 9789811360046. [Google Scholar]
- Kotcherlakota, R.; Srinivasan, D.J.; Mukherjee, S.; Haroon, M.M.; Dar, G.H.; Venkatraman, U.; Patra, C.R.; Gopal, V. Engineered fusion protein-loaded gold nanocarriers for targeted co-delivery of doxorubicin and erbB2-siRNA in human epidermal growth factor receptor-2+ ovarian cancer. J. Mater. Chem. B 2017, 5, 7082–7098. [Google Scholar] [CrossRef]
- Labala, S.; Jose, A.; Chawla, S.R.; Khan, M.S.; Bhatnagar, S.; Kulkarni, O.P.; Venuganti, V.V.K. Effective melanoma cancer suppression by iontophoretic co-delivery of STAT3 siRNA and imatinib using gold nanoparticles. Int. J. Pharm. 2017, 525, 407–417. [Google Scholar] [CrossRef] [PubMed]




| Delivery System | Advantages | Disadvantages |
|---|---|---|
| Mesoporous Silica Nanoparticles |
|
|
| Polymeric Materials |
|
|
| Liposomes |
|
|
| Dendrimers |
|
|
| Gold nanoparticles |
|
|
| Delivery System | Small Molecule Drug | siRNA Target | Type of Cancer | Cell Line | Testing Stage | Ref. |
|---|---|---|---|---|---|---|
| Mesoporous silica nanoparticles modified with polyethylenimine | Doxorubicin | ABCB1 (or MDR1) | Oral squamous carcinoma | KBV | In vitro In vivo | [10] |
| Folic acid (FA)-conjugated mesoporous silica nanoparticles | Myricetin | ABCC1 (or MRP1) | Non-small cell lung cancer | A594 NCI-H1299 | In vitro In vivo | [31] |
| Mesoporous silica nanoparticles (MSNPs) | Doxorubicin | BCL2 | Breast Cancer | MCF-7 HEK 293 | In vitro In vivo | [32] |
| Acid-sensitive calcium phosphate/silica dioxide (CAP/SiO2) composite | Doxorubicin | ABCB1 (or MDR1) | Leukaemia | K562/ADR | In vitro | [33] |
| Mesoporous silica nanoparticles modified with polyethylenimine− polylysine and folate-linked poly(ethylene glycol) | Doxorubicin | BCL2 | Breast Cancer | MDA-MB-231 RAW 264.7 | In vitro | [37] |
| Delivery System | Small Molecule Drug | siRNA Target | Type of Cancer | Cell Line | Testing Stage | Ref. |
|---|---|---|---|---|---|---|
| Chitosan based pH-responsive polymeric prodrug vector (GA-CS-PEI-HBA-DOX) where GA-CS-PEI-HBA-DOX is prodrug chitosan-polyethylenimine-4-hydrazino-benzoic acid doxorubicin | Doxorubicin | BCL2 | Liver cancer | HUVEC, HepG2 | In vitro In vivo | [44] |
| Amphiphilic block copolymer of monomethoxylpoly(ethylene glycol), poly(l-lysine), and poly(aspartyl(Benzylamine-co-(Diisopropylamino)ethylamine)) mPEG-PLLys-PLAsp(BzA-co-DIP), abbreviated as PELABD micelles | Doxorubicin | siRNA | Ovarian cancer | SKOV3 | In vitro | [46] |
| Triblock copolymer nanocarrier of PAH-b-PDMAPMA-b-PAH where PAH-b-PDMAPMA-b-PAH is poly(acrylhydrazine)-block-poly(3-dimethylaminopropyl methacrylamide)-block-poly(acrylhydrazine) (PAH-b-PDMAPMA-b-PAH) | Doxorubicin | BCL2 | Breast cancer | MCF-7 | In vitro | [56] |
| PEG-PCL-PEI triblock copolymer nanomicelle functionalized with folic acid | Doxorubicin | (P-gp) siRNA | Breast cancer | MCF-7/ADR | In vitro | [57] |
| Poly(ε-caprolactone), polyethylenimine and polyethylene glycol (PCL-PEI-PEG) copolymers | 4-(N)-stearoyl gemcitabine | RELA | Pancreatic cancer and breast cancer | AsPC-1, MCF-7 | In vitro | [58] |
| Lipid-polymer hybrid nanoparticles where cationic ε-polylysine co-polymer nanoparticles (ENPs) are coated with PEGylated lipid bilayer resulted formation of LENPs, with reversed surface charge | Gemcitabine | HIF1A | Pancreatic cancer | Panc-1 | In vitro In vivo | [59] |
| pH/redox dual-sensitive polymeric materials (cPCPL) where cPCPL is poly(ethylene glycol))x-(chitosan-polymine)y-(lipoic acid)z grafted with cRGDyC-PEG-NHS, cRGDyC is a kind of peptide, PEG is poly(ethylene glycol) and NHS is hydroxysuccinimide. | Etoposide | EZH2 | Orthotopic non-small-cell lung tumour | luc-A549 | In vitro In vivo | [60] |
| Self-assembled polyjuglanin nanoparticles (PJAD-PEG) where PJAD-PEG is poly(juglanin (Jug) dithiodipropionic acid (DA))-b-poly(ethylene glycol) (PEG) | Doxorubicin | KRAS | Lung cancer | A549, H69 | In vitro In vivo | [61] |
| Cationic polyethylenimine-block-polylactic acid (PEI-PLA) | Paclitaxel | BIRC5 | Lung Adenocarcinoma | 4T1, A549 | In vitro In vivo | [62] |
| Lactic-co-glycolic acid (PLGA) nanoparticles | Paclitaxel | SPDYE7P | Cervical cancer | HeLa | In vitro In vivo | [63] |
| FeCo-polyethylenimine (FeCo-PEI) nanoparticles and polylactic acid-polyethylene glycol (PLA-PEG) | Paclitaxel | FAM group | Breast cancer | MCF-7, BT-474 | In vitro | [64] |
| Hypoxia-sensitive PEG-azobenzene-PEI-DOPE (PAPD) nanoparticles | Doxorubicin | ABCB1 | Ovarian cancer and breast cancer | A2780 ADR, MCF7 ADR | In vitro | [65] |
| Chondroitin sulfate (CS)-coated β-cyclodextrin polyethylenemine polymer | Paclitaxel | MCAM | Breast Cancer | MDA-MB-231, MCF-7 | In vitro | [66] |
| Targeted multifunctional polymeric micelle (TMPM) where TMPM is made up of triblock copolymer poly(ε-caprolactone)-polyethyleneglycol-poly(L-histidine) (PCL-PEGPHIS) | Paclitaxel | VEGF group | Breast Cancer | HUVECs, MCF-7 | In vitro | [67] |
| Delivery System | Small Molecule Drug | siRNA Target | Types of Cancer | Cell Line | Testing Stage | Ref. |
|---|---|---|---|---|---|---|
| GE-11 peptide conjugated liposome | Gemcitabine | HIF1A | Pancreatic cancer | Panc-1 | In vitro In vivo | [59] |
| 1,2-Dioleoyl-3-trimethylammonium propane (DOTAP) -based cationic liposomes | Curcumin | STAT3 | Skin cancer | B16F10 | In vitro In vivo | [73] |
| PEGylated liposomes | Docetaxel | BCL2 | Lung cancer | A549, H226 | In vitro In vivo | [74] |
| Galactosylated Liposomes | Doxorubicin | VIM | Hepatocellular Carcinoma | Huh7, A549 | In vitro In vivo | [75] |
| Carbamate-linked cationic lipid (Cationic Liposome) | Paclitaxel | BIRC5 | Lung Cancer | NCI-H460 | In vitro | [76] |
| Delivery System | Small Molecule Drug | siRNA Target | Type of Cancer | Cell Line | Testing Stage | Ref. |
|---|---|---|---|---|---|---|
| Amphiphilic dendrimer engineered nanocarrier system (ADENS) modified by tumour microenvironment-sensitive polypeptides (TMSP) (TMSP-ADENS) | Paclitaxel | FAM and VEGF group | Melanoma, prostate cancer | A375, PC-3, HT-1080 | In vitro In vivo | [13] |
| PTP (plectin-1 targeted peptide, NH2-KTLLPTP-COOH), biomarker for pancreatic cancer, integrated with the PSPG vector to form peptide-conjugated PSPG (PSPGP) where PSPG is branched poly(ethylene glycol) with G2 dendrimers through disulfide linkages | Paclitaxel | NR4A1 (or TR3) | Pancreatic Cancer | Panc-1 | In vitro In vivo | [83] |
| Hyaluronic acid (HA) modified MDMs where MDMs is the PAMAM-PEG2k-DOPE co-polymer, together with mPEG2k-DOPE, was formulated into mixed dendrimer micelles, and PAMAM is the generation 4 polyamidoamine | Doxorubicin | ABCB1 (or MDR1) | Ovarian cancer, colorectal carcinoma and breast cancer | A2780 ADR, HCT 116, MDA-MB-231 | In vitro | [86] |
| PAMAM-OH derivative (PAMSPF) | Murine double minute 2 protein (MDM2) inhibitor RG7388 | TP53 | Breast cancer | P53-wild type MCF-7 cells (MCF-7/WT), MDA-MB-435 | In vitro In vivo | [87] |
| Polyamidoamine (PAMAM) dendrimer | Curcumin | BCL2 | Cervical cancer | HeLa | In vitro | [91] |
| Folate-polyethylene glycol appended dendrimer conjugate with glucuronylglucosyl-β cyclodextrin (Fol-PEG-GUG-β-CDE) (generation 3) | Doxorubicin | PLK1 | Cervical cancer | KB | In vitro In vivo | [92] |
| Delivery System | Small Molecule Drug | siRNA Target | Type of Cancer | Cell Line | Testing Stage | Ref. |
|---|---|---|---|---|---|---|
| Polyelectrolyte polymers coated gold nanorods (AuNRs) | Doxorubicin | KRAS | Pancreatic Cancer | Panc-1 | In vitro In vivo | [95] |
| Gold nanoparticles (AuNPs) combined with an engineered bi-functional recombinant fusion protein TRAF(C) (TR) | Doxorubicin | ERBB2 | Ovarian cancer | SK-OV-3, MDA-MB-231, A549, PANC-1, B16F10 | In vitro In vivo | [100] |
| Layer-by-layer assembled gold nanoparticles (LbL-AuNP) | Imatinib mesylate | STAT3 | Melanoma cancer | B16F10 | In vivo | [101] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chung, S.L.; Yee, M.S.-L.; Hii, L.-W.; Lim, W.-M.; Ho, M.Y.; Khiew, P.S.; Leong, C.-O. Advances in Nanomaterials Used in Co-Delivery of siRNA and Small Molecule Drugs for Cancer Treatment. Nanomaterials 2021, 11, 2467. https://doi.org/10.3390/nano11102467
Chung SL, Yee MS-L, Hii L-W, Lim W-M, Ho MY, Khiew PS, Leong C-O. Advances in Nanomaterials Used in Co-Delivery of siRNA and Small Molecule Drugs for Cancer Treatment. Nanomaterials. 2021; 11(10):2467. https://doi.org/10.3390/nano11102467
Chicago/Turabian StyleChung, Shei Li, Maxine Swee-Li Yee, Ling-Wei Hii, Wei-Meng Lim, Mui Yen Ho, Poi Sim Khiew, and Chee-Onn Leong. 2021. "Advances in Nanomaterials Used in Co-Delivery of siRNA and Small Molecule Drugs for Cancer Treatment" Nanomaterials 11, no. 10: 2467. https://doi.org/10.3390/nano11102467
APA StyleChung, S. L., Yee, M. S. -L., Hii, L. -W., Lim, W. -M., Ho, M. Y., Khiew, P. S., & Leong, C. -O. (2021). Advances in Nanomaterials Used in Co-Delivery of siRNA and Small Molecule Drugs for Cancer Treatment. Nanomaterials, 11(10), 2467. https://doi.org/10.3390/nano11102467