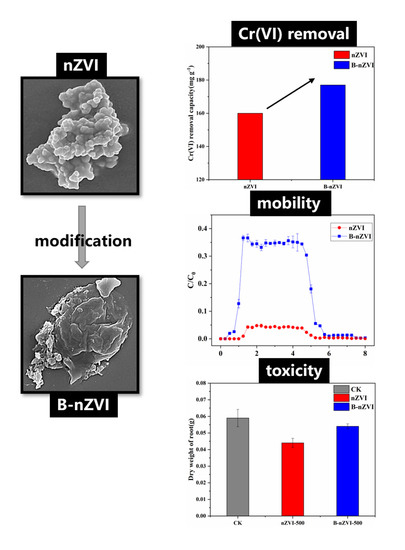
Nanoscale Zero-Valent Iron Modified by Bentonite with Enhanced Cr(VI) Removal Efficiency, Improved Mobility, and Reduced Toxicity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Preparation of nZVI and Bentonite-Supported nZVI
2.3. Characterization of nZVI and B-nZVI
2.4. Batch Experiments
2.5. Column Transport Experiments
2.6. Luminous Bacteria Toxicity Test
2.7. Ryegrass Hydroponic Experiment
3. Results and Discussion
3.1. Characterization of nZVI and B-nZVI
3.2. Cr(VI) Removal by nZVI and B-nZVI
3.3. Transport of nZVI and B-nZVI in Quartz Sand Columns
3.4. Transport of nZVI and B-nZVI in Soil Columns
3.5. Luminous Bacteria Toxicity Test
3.6. Ryegrass Hydroponic Experiment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jiang, D.; Zeng, G.; Huang, D.; Chen, M.; Zhang, C.; Huang, C.; Wan, J. Remediation of contaminated soils by enhanced nanoscale zero valent iron. Environ. Res. 2018, 163, 217–227. [Google Scholar] [CrossRef]
- Lefevre, E.; Bossa, N.; Wiesner, M.R.; Gunsch, C.K. A review of the environmental implications of in situ remediation by nanoscale zero valent iron (nZVI): Behavior, transport and impacts on microbial communities. Sci. Total Environ. 2016, 565, 889–901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, F.; Dionysiou, D.D.; Liu, H. The use of zero-valent iron for groundwater remediation and wastewater treatment: A review. J. Hazard. Mater. 2014, 267, 194–205. [Google Scholar] [CrossRef]
- Chen, H.; Cao, Y.; Wei, E.; Gong, T.; Xian, Q. Facile synthesis of graphene nano zero-valent iron composites and their efficient removal of trichloronitromethane from drinking water. Chemosphere 2016, 146, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, X.; Xu, D.; Yang, W.; Bai, S. Application of nanoscale zero-valent iron in hexavalent chromium-contaminated soil: A review. Nanotechnol. Rev. 2020, 9, 736–750. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, H.-P.; Zhu, L. Remediation of soil contaminated with organic compounds by nanoscale zero-valent iron: A review. Sci. Total Environ. 2021, 760, 143413. [Google Scholar] [CrossRef] [PubMed]
- Tosco, T.; Papini, M.P.; Viggi, C.C.; Sethi, R. Nanoscale zerovalent iron particles for groundwater remediation: A review. J. Clean. Prod. 2014, 77, 10–21. [Google Scholar] [CrossRef]
- Wang, B.; Deng, C.; Ma, W.; Sun, Y. Modified nanoscale zero-valent iron in persulfate activation for organic pollution remediation: A review. Environ. Sci. Pollut. Res. 2021, 28, 34229–34247. [Google Scholar] [CrossRef]
- Wilkin, R.T.; Su, C.; Ford, R.G.; Paul, C.J. Chromium-Removal Processes during Groundwater Remediation by a Zerovalent Iron Permeable Reactive Barrier. Environ. Sci. Technol. 2005, 39, 4599–4605. [Google Scholar] [CrossRef]
- Karn, B.; Kuiken, T.; Otto, M. Nanotechnology and in Situ Remediation: A Review of the Benefits and Potential Risks. Environ. Health Perspect. 2009, 117, 1813–1831. [Google Scholar] [CrossRef] [Green Version]
- Shi, L.-N.; Zhang, X.; Chen, Z.-L. Removal of Chromium (VI) from wastewater using bentonite-supported nanoscale zero-valent iron. Water Res. 2011, 45, 886–892. [Google Scholar] [CrossRef]
- Ma, L.; Du, Y.; Chen, S.; Zhang, F.; Zhan, W.; Du, D.; Zhang, T.C. Nanoscale zero-valent iron coupling with Shewanella oneidensis MR-1 for enhanced reduction/removal of aque-ous Cr(VI). Sep. Purif. Technol. 2021, 277, 119488. [Google Scholar] [CrossRef]
- Geng, B.; Jin, Z.; Li, T.; Qi, X. Preparation of chitosan-stabilized Fe0 nanoparticles for removal of hexavalent chromium in water. Sci. Total Environ. 2009, 407, 4994–5000. [Google Scholar] [CrossRef] [PubMed]
- Geng, B.; Jin, Z.; Li, T.; Qi, X. Kinetics of hexavalent chromium removal from water by chitosan-Fe0 nanoparticles. Chemosphere 2009, 75, 825–830. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, H.M.; Awad, M.; Al-Farraj, A.S.; Al-Turki, A.M. Stability and Dynamic Aggregation of Bare and Stabilized Zero-Valent Iron Nanoparticles under Variable Solution Chemistry. Nanomaterials 2020, 10, 192. [Google Scholar] [CrossRef] [Green Version]
- Dong, H.; Lo, I.M. Influence of calcium ions on the colloidal stability of surface-modified nano zero-valent iron in the absence or presence of humic acid. Water Res. 2013, 47, 2489–2496. [Google Scholar] [CrossRef] [PubMed]
- Jiao, C.; Tan, X.; Lin, A.; Yang, W. Preparation of Activated Carbon Supported Bead String Structure Nano Zero Valent Iron in a Polyethylene Glycol-Aqueous Solution and Its Efficient Treatment of Cr(VI) Wastewater. Molecules 2019, 25, 47. [Google Scholar] [CrossRef] [Green Version]
- Su, H.; Fang, Z.; Tsang, P.E.; Zheng, L.; Cheng, W.; Fang, J.; Zhao, D. Remediation of hexavalent chromium contaminated soil by biochar-supported zero-valent iron nanoparticles. J. Hazard. Mater. 2016, 318, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Mortazavian, S.; An, H.; Chun, D.; Moon, J. Activated carbon impregnated by zero-valent iron nanoparticles (AC/nZVI) optimized for simultaneous adsorption and reduction of aqueous hexavalent chromium: Material characterizations and kinetic studies. Chem. Eng. J. 2018, 353, 781–795. [Google Scholar] [CrossRef]
- Chen, S.; Bedia, J.; Li, H.; Ren, L.Y.; Naluswata, F.; Belver, C. Nanoscale zero-valent iron@mesoporous hydrated silica core-shell particles with enhanced dispersibility, transportability and degradation of chlorinated aliphatic hydrocarbons. Chem. Eng. J. 2018, 343, 619–628. [Google Scholar] [CrossRef]
- Li, Z.; Wang, L.; Wu, J.; Xu, Y.; Wang, F.; Tang, X.; Xu, J.; Ok, Y.S.; Meng, J.; Liu, X. Zeolite-supported nanoscale zero-valent iron for immobilization of cadmium, lead, and arsenic in farmland soils: Encapsulation mechanisms and indigenous microbial responses. Environ. Pollut. 2020, 260, 114098. [Google Scholar] [CrossRef]
- Zhang, W.; Qian, L.; Chen, Y.; Ouyang, D.; Han, L.; Shang, X.; Li, J.; Gu, M.; Chen, M. Nanoscale zero-valent iron supported by attapulgite produced at different acid modification: Synthesis mechanism and the role of silicon on Cr(VI) removal. Chemosphere 2021, 267, 129183. [Google Scholar] [CrossRef]
- Sun, Z.; Zheng, S.; Ayoko, G.; Frost, R.L.; Xi, Y. Degradation of simazine from aqueous solutions by diatomite-supported nanosized zero-valent iron composite materials. J. Hazard. Mater. 2013, 263, 768–777. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gholami, P.; Khataee, A.; Bhatnagar, A.; Vahid, B. Synthesis of N-Doped Magnetic WO3–x@Mesoporous Carbon Using a Diatom Template and Plasma Modification: Visible-Light-Driven Photocatalytic Activities. ACS Appl. Mater. Interfaces 2021, 13, 13072–13086. [Google Scholar] [CrossRef] [PubMed]
- Gholami, P.; Khataee, A.; Bhatnagar, A. Environmentally superior cleaning of diatom frustules using sono-Fenton process: Facile fabrication of nanoporous silica with homogeneous morphology and controlled size. Ultrason. Sonochem. 2020, 64, 105044. [Google Scholar] [CrossRef]
- Song, H.; Liu, W.; Meng, F.; Yang, Q.; Guo, N. Efficient Sequestration of Hexavalent Chromium by Graphene-Based Nanoscale Zero-Valent Iron Composite Coupled with Ultrasonic Pretreatment. Int. J. Environ. Res. Public Health 2021, 18, 5921. [Google Scholar] [CrossRef] [PubMed]
- Luckham, P.F.; Rossi, S. The colloidal and rheological properties of bentonite suspensions. Adv. Colloid Interface Sci. 1999, 82, 43–92. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Yi, K.; Zhang, X.; Han, P.; Liu, W.; Tong, M. Modification of zero valent iron nanoparticles by sodium alginate and bentonite: Enhanced transport, effective hexavalent chromium removal and reduced bacterial toxicity. J. Hazard. Mater. 2020, 388, 121822. [Google Scholar] [CrossRef] [PubMed]
- Wiesner, M.R.; Lowry, G.V.; Alvarez, P.; Dionysiou, D.; Biswas, P. Assessing the Risks of Manufactured Nanomaterials. Environ. Sci. Technol. 2006, 40, 4336–4345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patil, S.S.; Shedbalkar, U.U.; Truskewycz, A.; Chopade, B.A.; Ball, A.S. Nanoparticles for environmental clean-up: A review of potential risks and emerging solutions. Environ. Technol. Innov. 2016, 5, 10–21. [Google Scholar] [CrossRef]
- Vítková, M.; Puschenreiter, M.; Komárek, M. Effect of nano zero-valent iron application on As, Cd, Pb, and Zn availability in the rhizosphere of metal(loid) contaminated soils. Chemosphere 2018, 200, 217–226. [Google Scholar] [CrossRef]
- Wang, J.; Fang, Z.; Cheng, W.; Yan, X.; Tsang, P.E.; Zhao, D. Higher concentrations of nanoscale zero-valent iron (nZVI) in soil induced rice chlorosis due to inhibited active iron transportation. Environ. Pollut. 2016, 210, 338–345. [Google Scholar] [CrossRef]
- Saccà, M.L.; Fajardo, C.; Martinez-Gomariz, M.; Costa, G.; Nande, M.; Martin, M. Molecular Stress Responses to Nano-Sized Zero-Valent Iron (nZVI) Particles in the Soil Bacterium Pseudomonas stutzeri. PLoS ONE 2014, 9, e89677. [Google Scholar] [CrossRef] [Green Version]
- Saccà, M.L.; Fajardo, C.; Costa, G.; Lobo, C.; Nande, M.; Martin, M. Integrating classical and molecular approaches to evaluate the impact of nanosized zero-valent iron (nZVI) on soil organisms. Chemosphere 2014, 104, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Yang, Y.; Deng, R.; Gong, X.; Zhou, W.; Chen, S.; Li, B.; Wang, G. Remediation of Cd-Contaminated Soil by Modified Nanoscale Zero-Valent Iron: Role of Plant Root Exudates and Inner Mechanisms. Int. J. Environ. Res. Public Health 2021, 18, 5887. [Google Scholar] [CrossRef]
- Crane, R.; Scott, T. Nanoscale zero-valent iron: Future prospects for an emerging water treatment technology. J. Hazard. Mater. 2012, 211, 112–125. [Google Scholar] [CrossRef] [PubMed]
- Connolly, E.L.; Guerinot, M. Iron stress in plants. Genome Biol. 2002, 3, reviews1024.1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, L.; Zhuang, W.; Wang, X.; Yu, K.; Yang, S.; Xia, S. Potential effects of loading nano zero valent iron discharged on membrane fouling in an anoxic/oxic membrane bioreactor. Water Res. 2017, 111, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-B.; Zhang, W.-X. Synthesizing Nanoscale Iron Particles for Rapid and Complete Dechlorination of TCE and PCBs. Environ. Sci. Technol. 1997, 31, 2154–2156. [Google Scholar] [CrossRef]
- Wu, B.; Peng, D.; Hou, S.; Tang, B.; Wang, C.; Xu, H. Dynamic study of Cr (VI) removal performance and mechanism from water using multilayer material coated nanoscale zerovalent iron. Environ. Pollut. 2018, 240, 717–724. [Google Scholar] [CrossRef]
- Zheng, H.; Liu, L.; Lu, Y.; Long, Y.; Wang, L.; Ho, K.-P.; Wong, K.-Y. Rapid Determination of Nanotoxicity Using Luminous Bacteria. Anal. Sci. 2010, 26, 125–128. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.-P.; Li, X.-Q.; Cao, J.; Zhang, W.-X.; Wang, H.P. Characterization of zero-valent iron nanoparticles. Adv. Colloid Interface Sci. 2006, 120, 47–56. [Google Scholar] [CrossRef]
- Zhou, S.; Li, Y.; Chen, J.; Liu, Z.; Wang, Z.; Na, P. Enhanced Cr(VI) removal from aqueous solutions using Ni/Fe bimetallic nanoparticles: Characterization, kinetics and mechanism. RSC Adv. 2014, 4, 50699–50707. [Google Scholar] [CrossRef]
- Zhirong, L.; Uddin, A.; Zhanxue, S. FT-IR and XRD analysis of natural Na-bentonite and Cu(II)-loaded Na-bentonite. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2011, 79, 1013–1016. [Google Scholar] [CrossRef] [PubMed]
- Shih, Y.-H.; Hsu, C.-Y.; Su, Y.-F. Reduction of hexachlorobenzene by nanoscale zero-valent iron: Kinetics, pH effect, and degradation mechanism. Sep. Purif. Technol. 2011, 76, 268–274. [Google Scholar] [CrossRef]
- Lv, D.; Zhou, J.; Cao, Z.; Xu, J.; Liu, Y.; Li, Y.; Yang, K.; Lou, Z.; Lou, L.; Xu, X. Mechanism and influence factors of chromium(VI) removal by sulfide-modified nanoscale zerovalent iron. Chemosphere 2019, 224, 306–315. [Google Scholar] [CrossRef] [PubMed]
- Shih, Y.-H.; Tai, Y.-T. Reaction of decabrominated diphenyl ether by zerovalent iron nanoparticles. Chemosphere 2010, 78, 1200–1206. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Wen, J.; Zhang, H.; Wang, Q.; Hu, X. Enhancing Cr(VI) reduction and immobilization by magnetic core-shell structured NZVI@MOF derivative hybrids. Environ. Pollut. 2020, 260, 114021. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, L.; Wang, R.; Wang, Y.; Zhang, X. A novel cellulose hydrogel coating with nanoscale Fe0 for Cr(VI) adsorption and reduction. Sci. Total Environ. 2020, 726, 138625. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, S.; Oku, M.; Waseda, Y. Changes in the chemical state and composition of the clean surface of K2CrO4 and K2Cr2O7 due to air exposure and argon ion bombardment. Surf. Interface Anal. 1997, 25, 161–166. [Google Scholar] [CrossRef]
- Li, S.; You, T.; Guo, Y.; Yao, S.; Zang, S.; Xiao, M.; Zhang, Z.; Shen, Y. High dispersions of nano zero valent iron supported on biochar by one-step carbothermal synthesis and its application in chromate removal. RSC Adv. 2019, 9, 12428–12435. [Google Scholar] [CrossRef] [Green Version]
- Li, X.-Q.; Cao, J.; Zhang, W.-X. Stoichiometry of Cr(VI) Immobilization Using Nanoscale Zerovalent Iron (nZVI): A Study with High-Resolution X-Ray Photoelectron Spectroscopy (HR-XPS). Ind. Eng. Chem. Res. 2008, 47, 2131–2139. [Google Scholar] [CrossRef]
- Li, Z.; Xu, S.; Xiao, G.; Qian, L.; Song, Y. Removal of hexavalent chromium from groundwater using sodium alginate dispersed nano zero-valent iron. J. Environ. Manag. 2019, 244, 33–39. [Google Scholar] [CrossRef]
- Braun, A.; Klumpp, E.; Azzam, R.; Neukum, C. Transport and deposition of stabilized engineered silver nanoparticles in water saturated loamy sand and silty loam. Sci. Total Environ. 2015, 535, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Ben-Moshe, T.; Dror, I.; Berkowitz, B. Transport of metal oxide nanoparticles in saturated porous media. Chemosphere 2010, 81, 387–393. [Google Scholar] [CrossRef]
- Reginatto, C.; Cecchin, I.; Heineck, K.S.; Thomé, A.; Reddy, K.R. Use of Nanoscale Zero-Valent Iron for Remediation of Clayey Soil Contaminated with Hexavalent Chromium: Batch and Column Tests. Int. J. Environ. Res. Public Health 2020, 17, 1001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ibrahim, H.M.; Awad, M.; Al-Farraj, A.S.; Al-Turki, A.M. Effect of Flow Rate and Particle Concentration on the Transport and Deposition of Bare and Stabilized Zero-Valent Iron Nanoparticles in Sandy Soil. Sustainability 2019, 11, 6608. [Google Scholar] [CrossRef] [Green Version]
- Dong, H.; Lo, I.M.C. Transport of Surface-Modified Nano Zero-Valent Iron (SM-NZVI) in Saturated Porous Media: Effects of Surface Stabilizer Type, Subsurface Geochemistry, and Contaminant Loading. Water Air Soil Pollut. 2014, 225, 1–12. [Google Scholar] [CrossRef]
- Yakub, E.; Agarry, S.E.; Omoruwou, F.; Owabor, C.N. Comparative study of the batch adsorption kinetics and mass transfer in phenol-sand and phenol-clay adsorption systems. Part. Sci. Technol. 2019, 38, 801–811. [Google Scholar] [CrossRef]
- Alonso, M.M.; Martínez-Gaitero, R.; Gismera-Diez, S.; Puertas, F. PCE and BNS admixture adsorption in sands with different composition and particle size distribution. Mater. Constr. 2017, 67, e121. [Google Scholar] [CrossRef]
- Fajardo, C.; Ortiz, L.; Rodríguez-Membibre, M.; Nande, M.; Lobo, M.; Martin, M. Assessing the impact of zero-valent iron (ZVI) nanotechnology on soil microbial structure and functionality: A molecular approach. Chemosphere 2012, 86, 802–808. [Google Scholar] [CrossRef]
- Auffan, M.; Achouak, W.; Rose, J.; Roncato, M.-A.; Chanéac, C.; Waite, D.T.; Masion, A.; Woicik, J.C.; Wiesner, M.R.; Bottero, J.-Y. Relation between the Redox State of Iron-Based Nanoparticles and Their Cytotoxicity toward Escherichia coli. Environ. Sci. Technol. 2008, 42, 6730–6735. [Google Scholar] [CrossRef]
- Chen, J.; Xiu, Z.; Lowry, G.V.; Alvarez, P.J. Effect of natural organic matter on toxicity and reactivity of nano-scale zero-valent iron. Water Res. 2011, 45, 1995–2001. [Google Scholar] [CrossRef]
- Li, Z.; Greden, K.; Alvarez, P.J.J.; Gregory, K.B.; Lowry, G.V. Adsorbed Polymer and NOM Limits Adhesion and Toxicity of Nano Scale Zerovalent Iron to E. coli. Environ. Sci. Technol. 2010, 44, 3462–3467. [Google Scholar] [CrossRef]
- Qiu, X.; Fang, Z.; Yan, X.; Cheng, W.; Lin, K. Chemical stability and toxicity of nanoscale zero-valent iron in the remediation of chromium-contaminated watershed. Chem. Eng. J. 2013, 220, 61–66. [Google Scholar] [CrossRef]
- Xu, C.; Peng, C.; Sun, L.; Zhang, S.; Huang, H.; Chen, Y.; Shi, J. Distinctive effects of TiO2 and CuO nanoparticles on soil microbes and their community structures in flooded paddy soil. Soil Biol. Biochem. 2015, 86, 24–33. [Google Scholar] [CrossRef]
- Lee, C.; Kim, J.Y.; Lee, W.I.; Nelson, K.L.; Yoon, J.; Sedlak, D.L. Bactericidal Effect of Zero-Valent Iron Nanoparticles on Escherichia coli. Environ. Sci. Technol. 2008, 42, 4927–4933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gillissen, A.; Schärling, B.; Jaworska, M.; Bartling, A.; Rasche, K.; Schultze-Werninghaus, G. Oxidant scavenger function of ambroxol in vitro: A comparison with N-acetylcysteine. Res. Exp. Med. 1997, 196, 389–398. [Google Scholar] [CrossRef]
- Dwivedi, A.D.; Yoon, H.; Singh, J.P.; Chae, K.H.; Rho, S.-C.; Hwang, D.S.; Chang, Y.-S. Uptake, Distribution, and Transformation of Zerovalent Iron Nanoparticles in the Edible Plant Cucumis sativus. Environ. Sci. Technol. 2018, 52, 10057–10066. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Xing, B. Root Uptake and Phytotoxicity of ZnO Nanoparticles. Environ. Sci. Technol. 2008, 42, 5580–5585. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, I.; Mukherjee, A.; Mukherjee, A. In planta genotoxicity of nZVI: Influence of colloidal stability on uptake, DNA damage, oxidative stress and cell death. Mutagenesis 2017, 32, 371–387. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.; Huang, D.; Liu, Y.; Zeng, G.; Wang, R.; Wan, J.; Zhang, C.; Chen, Z.; Qin, X.; Xue, W. Stabilized Nanoscale Zerovalent Iron Mediated Cadmium Accumulation and Oxidative Damage of Boehmeria nivea (L.) Gaudich Cultivated in Cadmium Contaminated Sediments. Environ. Sci. Technol. 2017, 51, 11308–11316. [Google Scholar] [CrossRef]
- Guha, T.; Gopal, G.; Chatterjee, R.; Mukherjee, A.; Kundu, R. Differential growth and metabolic responses induced by nano-scale zero valent iron in germinating seeds and seedlings of Oryza sativa L. cv. Swarna. Ecotoxicol. Environ. Saf. 2020, 204, 111104. [Google Scholar] [CrossRef]
- Wang, J.; Fang, Z.; Cheng, W.; Tsang, P.E.; Zhao, D. Ageing decreases the phytotoxicity of zero-valent iron nanoparticles in soil cultivated with Oryza sativa. Ecotoxicology 2016, 25, 1202–1210. [Google Scholar] [CrossRef] [PubMed]
- Diao, M.; Yao, M. Use of zero-valent iron nanoparticles in inactivating microbes. Water Res. 2009, 43, 5243–5251. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Kou, X.; Pei, Z.; Xiao, J.Q.; Shan, X.; Xing, B. Physiological effects of magnetite (Fe3O4) nanoparticles on perennial ryegrass (Lolium perenne L.) and pumpkin (Cucurbita mixta) plants. Nanotoxicology 2010, 5, 30–42. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhang, P.; Adeel, M.; Guo, Z.; Chetwynd, A.J.; Ma, C.; Bai, T.; Hao, Y.; Rui, Y. Physiological impacts of zero valent iron, Fe3O4 and Fe2O3 nanoparticles in rice plants and their potential as Fe fertilizers. Environ. Pollut. 2021, 269, 116134. [Google Scholar] [CrossRef] [PubMed]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, J.; Luo, Y.; Sun, J.; Shi, J. Nanoscale Zero-Valent Iron Modified by Bentonite with Enhanced Cr(VI) Removal Efficiency, Improved Mobility, and Reduced Toxicity. Nanomaterials 2021, 11, 2580. https://doi.org/10.3390/nano11102580
Ye J, Luo Y, Sun J, Shi J. Nanoscale Zero-Valent Iron Modified by Bentonite with Enhanced Cr(VI) Removal Efficiency, Improved Mobility, and Reduced Toxicity. Nanomaterials. 2021; 11(10):2580. https://doi.org/10.3390/nano11102580
Chicago/Turabian StyleYe, Jien, Yating Luo, Jiacong Sun, and Jiyan Shi. 2021. "Nanoscale Zero-Valent Iron Modified by Bentonite with Enhanced Cr(VI) Removal Efficiency, Improved Mobility, and Reduced Toxicity" Nanomaterials 11, no. 10: 2580. https://doi.org/10.3390/nano11102580
APA StyleYe, J., Luo, Y., Sun, J., & Shi, J. (2021). Nanoscale Zero-Valent Iron Modified by Bentonite with Enhanced Cr(VI) Removal Efficiency, Improved Mobility, and Reduced Toxicity. Nanomaterials, 11(10), 2580. https://doi.org/10.3390/nano11102580