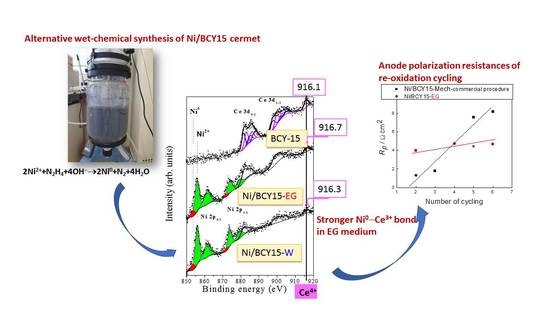
New Insights on the Nickel State Deposited by Hydrazine Wet-Chemical Synthesis Route in the Ni/BCY15 Proton-Conducting SOFC Anode
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis
2.3. Catalyst Characterization
2.3.1. Standard Characterization
2.3.2. Scanning Electron Microscopy (SEM)
2.3.3. Electron Paramagnetic Resonance (EPR) Spectroscopy
2.3.4. X-ray Photoelectron Spectroscopy
2.4. Electrochemical Measurements
3. Results and Discussion
3.1. X-ray Powder Diffraction
3.2. N2 Physisorption
3.3. Structural and Morphological Characterization
3.4. Electron Paramagnetic Resonance
3.5. X-ray Photoelectron Spectroscopy
3.6. Electrochemical Performance
4. Discussion and Summary
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marchenko, O.V.; Solomin, S.V. The future energy: Hydrogen versus electricity. Int. J. Hydrogen Energ. 2015, 40, 3801–3805. [Google Scholar] [CrossRef]
- Staffell, I.; Scamman, D.; Abad, A.V.; Balcombe, P.; Dodds, P.E.; Ekins, P.; Shah, N.; Ward, K.R. The role of hydrogen and fuel cells in the global energy system. Energy Environ. Sci. 2019, 12, 463–491. [Google Scholar] [CrossRef] [Green Version]
- Stambouli, A.; Traversa, E. Solid oxide fuel cells (SOFCs): A review of an environmentally clean and efficient source of energy. Renew. Sust. Energ. Rev. 2002, 6, 433–455. [Google Scholar] [CrossRef]
- Fabbri, E.; Pergolesi, D.; Traversa, E. Materials challenges toward proton-conducting oxide fuel cells: A critical review. E. Chem. Soc. Rev. 2010, 39, 4355–4369. [Google Scholar] [CrossRef]
- Tong, J.; Clark, D.; Bernau, L.; Subramaniyan, A.; O’Hayre, R. Proton-conducting yttrium-doped barium cerate ceramics synthesized by a cost-effective solid-state reactive sintering method. Solid State Ionics 2010, 181, 1486–1498. [Google Scholar] [CrossRef]
- Sawant, P.; Varma, S.; Gonal, M.R.; Wani, B.N.; Prakash, D.; Bharadwaj, S.R. Effect of Ni Concentration on Phase Stability, Microstructure and Electrical Properties of BaCe0.8Y0.2O3-Ni Cermet SOFC Anode and its application in proton conducting ITSOFC. Electrochim. Acta 2014, 120, 80–85. [Google Scholar] [CrossRef]
- Feng, W.; Wu, W.; Jin, C.; Zhou, M.; Bian, W.; Tang, W.; Gomez, J.; Boardman, R.; Ding, D. Exploring the structural uniformity and integrity of protonic ceramic thin film electrolyte using wet powder spraying. J. Power Sources Adv. 2021, 11, 100067. [Google Scholar] [CrossRef]
- Hussain, S.; Yangping, L. Review of solid oxide fuel cell materials: Cathode, anode, and electrolyte. Energy Transit. 2020, 4, 113–126. [Google Scholar] [CrossRef]
- Cellule de Pile a Combustible Haute Temperature a Conduction Mixte Anionique et Protonique. Patent No. 0550696000, 7 March 2005.
- Thorel, A.; Chesnaud, A.; Viviani, M.; Barbucci, A.; Presto, S.; Piccardo, P.; Ilhan, Z.; Vladikova, D.; Stoynov, Z. IDEAL-Cell, a high temperature innovative dual membrane fuel-cell. Chapter 3, Cell designs, processing and performance. In Proceedings of the Solid Oxide Fuel Cells 11 (SOFCXI), Vienna, Austria, 4–9 October 2009; Singhal, S.C., Yokokawa, H., Eds.; The Electrochemical Society, ECS Transactions: Pennington, NJ, USA, 2009; Volume 25, pp. 753–762. [Google Scholar]
- Vladikova, D.; Stoynov, Z.; Raikova, G.; Thorel, A.; Chesnaud, A.; Abreu, J.; Viviani, M.; Barbucci, A.; Presto, S.; Carpanese, P. Impedance spectroscopy studies of dual membrane fuel cell. Electrochim. Acta 2011, 56, 7955–7962. [Google Scholar] [CrossRef]
- Vladikova, D.; Stoynov, Z.; Raikova, G.; Krapchanska, M.; Thorel, A.; Chesnaud, A. Dual membrane fuel cell—Impedance approach for proof of concept. Bulg. Chem. Commun. 2012, 44, 364–370. [Google Scholar]
- Thorel, A.S.; Abreu, J.; Ansar, S.A.; Barbucci, A.; Brylewski, T.; Chesnaud, A.; Ilhan, Z.; Piccardo, P.; Prazuch, J.; Presto, S.; et al. Proof of Concept for the Dual Membrane Cell: I. Fabrication and Electrochemical Testing of First Prototypes. J. Electrochem. Soc. 2013, 160, F360–F366. [Google Scholar] [CrossRef]
- Masson, D.; Perrozzi, F.; Piccardo, P.; Viviani, M.; Pilot, C.; Stoynov, Z.; Vladikova, D.; Chesnaud, A.; Thorel, A. Shaping of a dual membrane SOFC and first electrochemical tests in a dedicated 3-chamber set-up. Chapter 7, Cell designs, fabrication, performance and durability. In Proceedings of the Solid Oxide Fuel Cells 14 (SOFC-XIV), Glasgow, Scotland, 26–31 July 2015; Singhal, S.C., Eguchi, K., Eds.; The Electrochemical Society, ECS Transactions: Pennington, NJ, USA, 2015; Volume 68, pp. 1969–1978. [Google Scholar]
- Raikova, G.; Krapchanska, M.; Genov, I.; Caboche, G.; Combemale, L.; Thorel, A.; Chesnaud, A.; Vladikova, D.; Stoynov, Z. Impedance investigation of BaCe0.85Y0.15O3−δ properties for hydrogen conductor in fuel cells. Bulg. Chem. Commun. 2012, 44, 389–394. [Google Scholar]
- Vladikova, D.; Stoynov, Z.; Chesnaud, A.; Thorel, A.; Viviani, M.; Barbucci, A.; Raikova, G.; Carpanese, P.; Krapchanska, M.; Mladenova, E. Application of yttrium doped barium cerate for improvement of the dual membrane SOFC design. Int. J. Hydrogen Energ. 2014, 39, 21561–21568. [Google Scholar] [CrossRef] [Green Version]
- Krezhov, K.; Vladikova, D.; Raikova, G.; Malakova, T.; Genov, I.; Nonova, T.; Svab, E.; Fabian, M. BaCe0.85Y0.15O3−Δ based materials for solid oxide fuel cells: Room-temperature neutron diffraction study. Neutron and Heavy Ion Radiations. In Proceedings of the International Conference on Radiation and Applications in Various Fields of Research, Niš, Serbia, 23–27 May 2016; Ristić, G.S., Ed.; RAD Centre: Niš, Serbia, 2016; Volume 1, pp. 117–123. [Google Scholar]
- Raikova, G.; Krezhov, K.; Genov, I.; Thorel, A.; Chesnaud, A.; Malakova, T.; Vladikova, D.; Stoynov, Z. Structural and electrochemical characterization of yttrium doped barium cerate BaCe0.85Y0.15O3-α for applications in solid oxide fuel cells. Bulg. Chem. Commun. 2017, 49, 162–170. [Google Scholar]
- Miller, H.A.; Bouzek, K.; Hnat, J.; Loos, S.; Bernaecker, C.; Weissgaerber, T.; Rontzsch, L.; Meier-Haack, J. Green hydrogen from anion exchange membrane water electrolysis: A review of recent developments in critical materials and operating conditions. Sustain. Energ. Fuels 2020, 4, 2114–2133. [Google Scholar] [CrossRef]
- Safari, A.; Panda, R.K.; Janas, V.F. Ferroelectricity. Materials, characteristics, and applications. In Key Engineering Materials Advanced Ceramic Materials; Mostaghaci, H., Ed.; Trans Tech Publications, Ltd.: Bäch, Switzerland, 1996; Volumes 122–124, pp. 35–70. [Google Scholar]
- Liu, Y.; Shao, Z.; Mori, T.; Jiang, S.P. Development of nickel based cermet anode materials in solid oxide fuel cells—Now and future. MRE 2021, 1, 100003. [Google Scholar] [CrossRef]
- Caldes, M.T.; Kravchyk, K.V.; Benamira, M.; Besnard, N.; Gunes, V.; Bohnke, O.; Joubert, O. Metallic Nanoparticles and Proton Conductivity: Improving Proton Conductivity of BaCe0.9Y0.1O3−δ Using a Catalytic Approach. Chem. Mater. 2012, 24, 4641–4646. [Google Scholar] [CrossRef]
- Costa, R.; Grünbaum, N.; Berger, M.-H.; Dessemond, L.; Thorel, A. On the use of NiO as sintering additive for BaCe0.9Y0.1O3−α. Solid State Ionics 2009, 180, 891–895. [Google Scholar] [CrossRef]
- Fua, Q.; Tietz, F.; Sebold, D.; Tao, S.; Irvine, J.T.S. An efficient ceramic-based anode for solid oxide fuel cells. J. Power Sources 2007, 171, 663–669. [Google Scholar] [CrossRef]
- Essoumhi, A.; Taillades, G.; Taillades-Jacquin, M.; Jones, D.J.; Rozière, J. Synthesis and characterization of Ni-cermet/proton conducting thin film electrolyte symmetrical assemblies. Solid State Ionics 2008, 179, 2155–2159. [Google Scholar] [CrossRef]
- Chesnaud, A.; Thorel, A.; Valy, J.; Vladikova, D.; Stoynov, Z.; Raikova, G. Optimisation of a BCY15/Ni composite as an anode for a dual membrane fuel cell. Preparation and DC resistivity measuremen. In Proceedings of the Internat. Workshop “Advances and Innovations in SOFCs 2. From Materials to the Systems”, Katarino, Bulgaria, 11–16 September 2011; Vladikova, D., Stoynov, Z., Eds.; Acad. Evgeni Budevski Institute of Electrochemistry and Energy Systems: Sofia, Bulgaria; Bulgarian Academy of Sciences: Sofia, Bulgaria, 2011; pp. 45–52. [Google Scholar]
- Raikova, G.; Caboche, G.; Combemale, L.; Thorel, A.; Chesnaud, A.; Abreu, J.; Vladikova, D.; Stoynov, Z. Optimization of the anode compartment of a dual membrane fuel cell. In Proceedings of the Internat. Workshop “Advances and Innovations in SOFCs 2. From Materials to the Systems”, Katarino, Bulgaria, 11–16 September 2011; Vladikova, D., Stoynov, Z., Eds.; Acad. Evgeni Budevski Institute of Electrochemistry and Energy Systems: Sofia, Bulgaria; Bulgarian Academy of Sciences: Sofia, Bulgaria, 2011; pp. 37–44. [Google Scholar]
- Young, J.L.; Molero, H.; Birss, V.I. The effect of pre-oxidation treatments on the oxidation tolerance of Ni-yttria-stabilized zirconia anodes in solid oxide fuel cells. J. Power Sources 2014, 271, 538–547. [Google Scholar] [CrossRef]
- Wojcieszak, R.; Monteverdi, S.; Bettahar, M.M. Ni/CeO2 catalysts prepared by aqueous hydrazine reduction. Colloids Surf. A Physicochem. Eng. Asp. 2008, 317, 116–122. [Google Scholar] [CrossRef]
- Gabrovska, M.; Nikolova, D.; Mladenova, E.; Vladikova, D.; Rakovsky, S.; Stoynov, Z. Ni incorporation in pSOFC anode ceramic matrix: Part I. Wet chemical reduction in an aqueous medium. Bulg. Chem. Commun. 2017, 49, 171–178. [Google Scholar]
- Goia, D.V. Preparation and formation mechanisms of uniform metallic particles in homogeneous solutions. J. Mater. Chem. 2004, 14, 451–458. [Google Scholar] [CrossRef]
- Park, J.; Chae, E.; Kim, S.; Lee, J.; Kim, J.; Yoon, S.; Choi, J.-Y. Preparation of fine Ni powders from nickel hydrazine complex. Mater. Chem. Phys. 2006, 97, 371–378. [Google Scholar] [CrossRef]
- Wang, D.P.; Sun, D.-B.; Yu, H.-Y.; Meng, H.-M. Morphology controllable synthesis of nickel nanopowders by chemical reduction process. J. Cryst. Growth 2008, 310, 1195–1201. [Google Scholar] [CrossRef]
- Tanner, C.W.; Virkar, A.V. InstabiIiiy of BaCeO3 in H2O-Containing Atmospheres. J. Electrochem. Soc. 1996, 143, 1386–1389. [Google Scholar] [CrossRef]
- Gabrovska, M.; Nikolova, D.; Mladenova, E.; Vladikova, D.; Rakovsky, S.; Stoynov, Z. Ni incorporation in pSOFC anode ceramic matrix: Part II. Wet chemical reduction in an anhydrous medium. Bulg. Chem. Commun. 2018, 50, 119–126. [Google Scholar]
- Kurihara, L.K.; Chow, G.M.; Schoen, P.E. Nanocrystalline metallic powders and films produced by the polyol method. Nanostruct. Mater. 1995, 5, 607–613. [Google Scholar] [CrossRef]
- Huang, G.-Y.; Xu, S.-M.; Xu, G.; Li, L.-Y.; Zhang, L.-F. Preparation of fine nickel powders via reduction of nickel hydrazine complex precursors. Trans. Nonferrous Met. Soc. China 2009, 19, 389–393. [Google Scholar] [CrossRef]
- Filotti, L.; Bensalem, A.; Bozon-Verduraz, F.; Shafeev, G.A.; Voronov, V.V. A comparative study of partial reduction of ceria via laser ablation in air and soft chemical route. Appl. Surf. Sci. 1997, 109–110, 249–252. [Google Scholar] [CrossRef]
- Vladikova, D.E.; Stoynov, Z.B.; Wuillemin, Z.; Montinaro, D.; Piccardo, P.; Genova, I.; Rolland, M. Impedance studies of the reduction process in NiO-YSZ SOFC anodes. Chapter 7, Cell designs, fabrication, performance and durability. In Proceedings of the Solid Oxide Fuel Cells 14 (SOFC-XIV), Glasgow, Scotland, 26–31 July 2015; Singhal, S.C., Eguchi, K., Eds.; The Electrochemical Society, ECS Transactions: Pennington, NJ, USA, 2015; Volume 68, pp. 1161–1168. [Google Scholar]
- Zakowsky, N.; Williamson, S.; Irwine, J.T.S. Elaboration of CO2 tolerance limits of BaCe0.9Y0.1O3–d electrolytes for fuel cells and other applications. Solid State Ionics 2005, 176, 3019–3026. [Google Scholar] [CrossRef]
- Groen, J.C.; Peffer, L.A.A.; Pérez-Ramırez, J. Pore size determination in modified micro- and mesoporous materials. Pitfalls and limitations in gas adsorption data analysis. Microporous Mesoporous Mater. 2003, 60, 1–17. [Google Scholar] [CrossRef]
- Groen, J.C.; Pérez-Ramırez, J. Critical appraisal of mesopore characterization by adsorption analysis. J. Appl. Catal. A Gen. 2004, 268, 121–125. [Google Scholar] [CrossRef]
- Rouquerol, F.; Rouquerol, J.; Sing, K.S.W.; Llewellyn, P.; Maurin, G. Adsorption by Powders and Porous Solids Principles, Methodology and Applications, 2nd ed.; Academic Press: Cambridge, MA, USA; Elsevier Ltd.: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Thommes, M.M.; Kaneko, K.; Niemark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Lecloux, A.; Pirard, J.P. The Importance of Standard Isotherms in the Analysis of Adsorption Isotherms for Determining the Porous Texture of Solids. J. Colloid Interface Sci. 1979, 70, 265–281. [Google Scholar] [CrossRef]
- Serwicka, E.M. Surface area and porosity, X-ray diffraction and chemical analyses. Catal. Today 2000, 56, 335–346. [Google Scholar] [CrossRef]
- Lia, L.; Lin, X. Solid solubility and transport properties of Ce1−xNdxO2−d nanocrystalline solid solutions by a sol-gel route. J. Mater. Res. 2001, 16, 3207–3213. [Google Scholar] [CrossRef]
- Bensalem, A.; Shafeev, G.; Bozon-Verduraz, F. Application of electroless procedures to the preparation of palladium catalysts. Catal. Lett. 1993, 18, 165–171. [Google Scholar] [CrossRef]
- Abi-Aad, E.; Bennani, A.; Bonnelle, J.-P.; Aboukaïs, A. Transition-metal ion dimers formed in CeO2: An EPR study. J. Chem. Soc. Faraday Trans. 1995, 91, 99–104. [Google Scholar] [CrossRef]
- Wang, J.B.; Tai, Y.-L.; Dow, W.-P.; Huang, T.-J. Study of ceria-supported nickel catalyst and effect of yttria doping on carbon dioxide reforming of methane. Appl. Catal. A Gen. 2001, 218, 69–79. [Google Scholar] [CrossRef]
- Kim, K.S.; Winograd, N. X-ray photoelectron spectroscopic studies of nickel-oxygen surfaces using oxygen and argon ion-bombardment. Surf. Sci. 1974, 43, 625–643. [Google Scholar] [CrossRef]
- Löchel, B.P.; Strehblow, H.H. Breakdown of Passivity of Nickel by Fluoride: II. Surface Analytical Studies. J. Electrochem. Soc. 1884, 131, 713–723. [Google Scholar] [CrossRef]
- Grosvenor, A.P.; Biesinger, M.C.; Smart, R.S.C.; McIntyre, N.S. New interpretations of XPS spectra of nickel metal and oxides. Surf. Sci. 2006, 600, 1771–1779. [Google Scholar] [CrossRef]
- Carley, A.F.; Jackson, S.D.; O’Shea, J.N.; Roberts, M.W. The formation and characterisation of Ni3+—An X-ray photoelectron spectroscopic investigation of potassium-doped Ni(110)–O. Surf. Sci. 1999, 440, L868–L874. [Google Scholar] [CrossRef]
- Liu, Z.; Grinter, D.C.; Lustemberg, P.G.; Nguyen-Phan, T.-D.; Zhou, Y.; Luo, S.; Waluyo, I.; Crumlin, E.J.; Stacchiola, J.D.; Zhou, J.; et al. Dry Reforming of Methane on a Highly-Active Ni-CeO2 Catalyst: Effects of Metal-Support Interactions on C–H Bond Breaking. Angew. Chem. Int. Ed. 2016, 55, 7455–7459. [Google Scholar] [CrossRef] [Green Version]
- Beche, E.; Charvin, P.; Perarnau, D.; Abanades, S.; Flamant, G. Ce 3D XPS investigation of cerium oxides and mixed cerium oxide (CexTiyOz). Surf. Interface Anal. 2008, 40, 264–267. [Google Scholar] [CrossRef]
- Wang, L.; Meng, F. Oxygen vacancy and Ce3+ ion dependent magnetism of monocrystal CeO2 nanopoles synthesized by a facile hydrothermal method. Mater. Res. Bull. 2013, 48, 3492–3498. [Google Scholar] [CrossRef]
- Shyu, J.Z.; Otto, K.; Watkins, W.L.H.; Graham, G.W. Characterization of Pd/γ-alumina catalysts containing ceria. J. Catal. 1988, 114, 23–33. [Google Scholar] [CrossRef]
- Ingo, G.M.; Paparazzo, E.; Bagnarelli, O.; Zacchetti, N. XPS studies on cerium, zirconium and yttrium valence states in plasma-sprayed coatings. Surf. Interface Anal. 1990, 16, 515–519. [Google Scholar] [CrossRef]
- Laachir, A.; Perrichon, V.; Badri, A.; Lamotte, J.; Catherine, E.; Lavalley, J.C.; El Fallah, J.; Hilaire, L.; Le Normand, F.; Quéméré, E.; et al. Reduction of CeO2 by hydrogen. Magnetic susceptibility and Fourier-transform infrared, ultraviolet and X-ray photoelectron spectroscopy measurements. J. Chem. Soc. Faraday Trans. 1991, 87, 1601–1609. [Google Scholar] [CrossRef]
- Deshpande, S.; Patil, S.; Kuchibhatla, S.V.N.T.; Seal, S. Size dependency variation in lattice parameter and valency states in nanocrystalline cerium oxide. Appl. Phys. Lett. 2005, 87, 133113. [Google Scholar] [CrossRef]
- Zhou, Y.; Perket, J.M.; Crooks, A.B.; Zhou, J. Effect of Ceria Support on the Structure of Ni Nanoparticles. J. Phys. Chem. Lett. 2010, 1, 1447–1453. [Google Scholar] [CrossRef]
- Praline, G.; Koel, B.E.; Hance, R.L.; Lee, H.-I.; White, J.M. X-ray photoelectron study of the reaction of oxygen with cerium. J. Electron Spectrosc. Relat. Phenom. 1980, 21, 17–30. [Google Scholar] [CrossRef]
- Pereira, A.; Blouin, M.; Pillonnet, A.; Guay, D. Structure and valence properties of ceria films synthesized by laser ablation under reducing atmosphere. Mater. Res. Express 2014, 1, 015704. [Google Scholar] [CrossRef]
- Caballero, A.; Holgado, J.P.; Gonzalez-delaCruz, V.M.; Habas, S.E.; Herranz, T.; Salmeron, M. In situ spectroscopic detection of SMSI effect in a Ni/CeO2 system: Hydrogen-induced burial and dig out of metallic nickel. Chem. Commun. 2010, 46, 1097–1099. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Wang, X.; Di, Q.; Zhao, H.; Liang, B.; Yang, J. Mutual Effects of Fluorine Dopant and Oxygen Vacancies on Structural and Luminescence Characteristics of F Doped SnO2 Nanoparticles. J. Mater. 2017, 10, 1398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tu, Y.; Chen, S.; Li, X.; Gorbaciova, J.; Gillin, W.P.; Krause, S.; Briscoe, J. Control of oxygen vacancies in ZnO nanorods by annealing and their influence on ZnO/PEDOT: PSS diode behavior. J. Mater. Chem. C 2018, 6, 1815–1821. [Google Scholar] [CrossRef] [Green Version]
- Saini, S.; Shah, J.; Kotnala, R.K.; Yadav, K.L. Nickel substituted oxygen deficient nanoporous lithium ferrite based green energy device hydroelectric cell. J. Alloys Compd. 2020, 827, 154334. [Google Scholar] [CrossRef]
- Choi, M.; Ibrahim, I.A.M.; Kim, K.; Koo, J.Y.; Kim, S.J.; Son, J.-W.; Han, J.W.; Lee, W. Engineering of Charged Defects at Perovskite Oxide Surfaces for Exceptionally Stable Solid Oxide Fuel Cell Electrodes. ACS Appl. Mater. Interfaces 2020, 12, 21494–21504. [Google Scholar] [CrossRef] [PubMed]
- Gauzzi, A.; Mathieu, H.J.; James, J.H.; Kellett, B. AES, XPS and SIMS characterization of YBa2Cu3O7 superconducting high Tc thin films. Vacuum 1990, 41, 870–874. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, Y.; Tu, B.; Dong, Y.; Cheng, M. Electrochemical impedance investigation of the redox behaviour of a Ni–YSZ anode. J. Power Sources 2007, 165, 114–119. [Google Scholar] [CrossRef]
- Lustemberg, P.G.; Ramírez, P.J.; Liu, Z.; Gutiérrez, R.A.; Grinter, D.G.; Carrasco, J.; Senanayake, S.D.; Rodriguez, J.A.; Ganduglia-Pirovano, M.V. Room-Temperature Activation of Methane and Dry Re-forming with CO2 on Ni-CeO2(111) Surfaces: Effect of Ce3+ Sites and Metal–Support Interactions on C–H Bond Cleavage. ACS Catal. 2016, 6, 8184–8191. [Google Scholar]







| Oxidation | Reduction | ||||
|---|---|---|---|---|---|
| Duration | N2 | Air | Duration | N2 | H2 |
| (Min) | (mL/Min/cm2) | (mL/Min/cm2) | (Min) | (mL/Min/cm2) | (mL/Min/cm2) |
| 2 | 3.97 | 3.97 | 6 | 3.97 | 3.97 |
| 6 | 3.97 | 0 | 10 | 22.28 | 0 |
| 5 | 3.97 | 0 | |||
| Sample | Phase | Phase Quantity, wt.% | LNi(111) nm | SBET m2/g | dav nm | Constant C |
|---|---|---|---|---|---|---|
| BCY15 | BaCeO3 | − | 3 | 14 | 90 | |
| Ni/BCY15-W | Ni0 | 40 | 15.7 | 8 | 11.5 | 97 |
| − | − | |||||
| BaCO3 | 60 | |||||
| Ni/BCY15-EG | Ni0 | 80 | 13.0 | 11 | 9.1 | 82 |
| BaCeO3 | 16 | |||||
| BaCO3 | 4 |
| Stage | Ni/BCY15-W | Ni/BCY15-EG | ||
|---|---|---|---|---|
| Phase | Phase Quantity, wt.% | Phase | Phase Quantity, wt.% | |
| Sintered anode tablets | NiO | 40 | NiO | 68 |
| BaCeO3 | 54 | BaCeO3 | 30 | |
| BaNiO2.36 | 3 | Y2BaNiO5 | 2 | |
| Y0.10Ce0.90O1.95 | 3 | |||
| Reduced anode tablets | Ni0 | 80 | Ni0 | 92 |
| BaCeO3 | 17 | BaCeO3 | 8 | |
| BaNiO2.36 | 2 | |||
| Y0.10Ce0.90O1.95 | 1 | |||
| Sample | Binding Energy (eV) | ||||
|---|---|---|---|---|---|
| Ni2p | Ce3d | O1s | |||
| Ni0 | Ni2+ | Ce4+ Satellite | I | II | |
| BCY15 | − | − | 916.1 | 528.8 | 531.5 |
| Ni/BCY15-W | 853.4 | 856.0 | 916.3 | 529.4 | 531.7 |
| Ni/BCY15-EG | 853.4 | 856.0 | 916.7 | 529.4 | 531.5 |
| Sample | Ni2+/Ni0 Ratio | Contribution, % | Ni/(Ba+Ce+Y) | Ba/(Ce+Y) | |
|---|---|---|---|---|---|
| Ni0 | Ce4+ | ||||
| BCY15 | − | − | 62 | − | 0.63 |
| Ni/BCY15-W | 10.4 | 8.8 | 84 | 1.82 | 1.52 |
| Ni/BCY15-EG | 9.9 | 9.2 | 65 | 1.94 | 0.35 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nikolova, D.; Gabrovska, M.; Raikova, G.; Mladenova, E.; Vladikova, D.; Kostov, K.L.; Karakirova, Y. New Insights on the Nickel State Deposited by Hydrazine Wet-Chemical Synthesis Route in the Ni/BCY15 Proton-Conducting SOFC Anode. Nanomaterials 2021, 11, 3224. https://doi.org/10.3390/nano11123224
Nikolova D, Gabrovska M, Raikova G, Mladenova E, Vladikova D, Kostov KL, Karakirova Y. New Insights on the Nickel State Deposited by Hydrazine Wet-Chemical Synthesis Route in the Ni/BCY15 Proton-Conducting SOFC Anode. Nanomaterials. 2021; 11(12):3224. https://doi.org/10.3390/nano11123224
Chicago/Turabian StyleNikolova, Dimitrinka, Margarita Gabrovska, Gergana Raikova, Emiliya Mladenova, Daria Vladikova, Krassimir L. Kostov, and Yordanka Karakirova. 2021. "New Insights on the Nickel State Deposited by Hydrazine Wet-Chemical Synthesis Route in the Ni/BCY15 Proton-Conducting SOFC Anode" Nanomaterials 11, no. 12: 3224. https://doi.org/10.3390/nano11123224
APA StyleNikolova, D., Gabrovska, M., Raikova, G., Mladenova, E., Vladikova, D., Kostov, K. L., & Karakirova, Y. (2021). New Insights on the Nickel State Deposited by Hydrazine Wet-Chemical Synthesis Route in the Ni/BCY15 Proton-Conducting SOFC Anode. Nanomaterials, 11(12), 3224. https://doi.org/10.3390/nano11123224