Nanoporous Silicon as a Green, High-Tech Educational Tool
Abstract
:1. Introduction
1.1. Nanomaterial Education
1.2. Silicon and Nanoporous Silicon
2. Porous-Silicon Synthesis—The Need for Sustainable Approaches
2.1. Nanostructuring via Electrochemistry
2.2. Principles of Green Chemistry
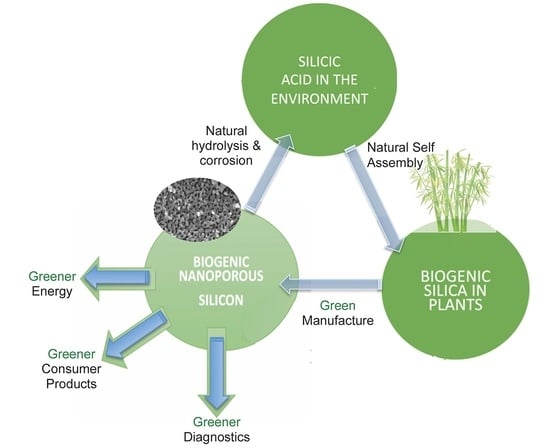
2.3. Biogenic Porous Silicon
3. Porous Silicon Properties—The Need for Novel Functionality and Tunability
3.1. Luminescent Silicon and Nanomaterial Physics
3.2. Biodegradable Silicon and Biomaterial Science
3.3. Silicon with Tunable Properties
4. Porous Silicon Uses—The Need for Product Safety, Reliability and Scalability
4.1. Diverse Applications, Their Associated Industries and Educational Topics
4.2. Medical Safety and Environmental Impact
5. Case Studies and Proposals on Education via Nanoporous Silicon
5.1. Undergraduate NanoPhysics Lecture Course at University of Birmingham, UK
5.2. Undergraduate Research Activities Featuring Plant-Derived pSi at Texas Christian University, US
5.3. Entrepreneurship in NST
5.3.1. Idea Generation and Translation
5.3.2. Porous Silicon IP as Patent Critiques
5.4. Interdisciplinary NST Schools and Conferences
6. Conclusions
7. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Supporting Information
References
- Albrecht, M.A.; Evans, C.W.; Rashton, C.L. Green chemistry and the health implications of nanoparticles. Green Chem. 2006, 8, 417–432. [Google Scholar] [CrossRef]
- Bar-Cohen, Y. Biomimetics—Using nature to inspire human innovation. Bioinsp. Biomim. 2006, 1, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Bhushan, B. Biomimetics- lessons from nature—An overview. Philos. Trans. R. Soc. 2009, A367, 1445–1486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clark, J.H. From waste to wealth using green chemistry: The way to long term stability. Curr. Opin. Green Sustain. Chem. 2013, 8, 10–11. [Google Scholar] [CrossRef]
- Porter, A.L.; Joutie, J. How interdisciplinary is nanotechnology? J. Nanopart. Res. 2009, 11, 1023–1041. [Google Scholar] [CrossRef] [Green Version]
- Roco, M.C. Converging science and technology at the nanoscale: Opportunities for education and training. Nat. Biotechnol. 2003, 21, 1247–1249. [Google Scholar] [CrossRef]
- Greenberg, A. Integrating nanoscience into the classroom: Perspectives on nanoscience education projects. ACS Nano 2009, 3, 762–769. [Google Scholar] [CrossRef]
- Jones, M.G.; Blonder, R.; Gardner, G.; Albe, V.; Falvo, M.; Chevrier, J. Nanotechnology and nanoscale science: Educational challenges. Int. J. Sci. Educ. 2013, 35, 1490–1512. [Google Scholar] [CrossRef]
- Yonai, E.; Blonder, R. Scientists suggest insertion of nanoscience and technology into middle school physics. Phys. Rev. Phys. Educ. Res. 2020, 16, 010110. [Google Scholar] [CrossRef] [Green Version]
- Kippeny, T.; Swafford, L.A.; Rosenthal, S.J. Semiconductor nanocrystals: A powerful visual aid for introducing the particle in a box. J. Chem. Educ. 2002, 79, 1094. [Google Scholar] [CrossRef]
- McFarland, A.D.; Haynes, C.L.; Mirkin, C.A.; Van Duyne, R.P.; Godwin, H.A. Color my nanoworld. J. Chem. Educ. 2004, 81, 544. [Google Scholar] [CrossRef]
- Mulfinger, L.; Solomon, S.D.; Bahadory, M.; Jeyarajasingam, A.V.; Rutkowsky, S.A.; Boritz, C. Synthesis and study of silver nanoparticles. J. Chem. Educ. 2007, 84, 322. [Google Scholar] [CrossRef]
- Oliver-Hoyo, M.; Gerber, R.W. From the research bench to the teaching laboratory: Gold nanoparticle layering. J. Chem. Educ. 2007, 84, 1174. [Google Scholar] [CrossRef]
- Guedens, W.J.; Reynders, M.; Van den Rul, H.; Elen, K.; Hardy, A.; Van Bael, M.K. ZnO-based sunscreen: The perfect example to introduce nanoparticles in an undergraduate or high school chemistry lab. J. Chem. Educ. 2013, 91, 259. [Google Scholar] [CrossRef]
- Sharma, R.K.; Gulati, S.; Mehta, S. Preparation of gold nanoparticles using tea: A green chemistry experiment. J. Chem. Educ. 2012, 89, 1396. [Google Scholar] [CrossRef]
- Sharma, R.K.; Yadav, S.; Gupta, R.; Arora, G. Synthesis of magnetic nanoparticles using potato extract for dye degradation: A green chemistry experiment. J. Chem. Educ. 2019, 96, 3038. [Google Scholar] [CrossRef]
- Divya, D.; Raj, K.G. From scrap to functional materials: Exploring green and sustainable chemistry approach in the undergraduate laboratory. J. Chem. Educ 2019, 96, 535. [Google Scholar] [CrossRef]
- Klobes, B.; Koch, C. Carbon nanoparticles from lactose and baking soda: Light absorption and fluorescence. J. Chem. Educ. 2020, 97, 143–146. [Google Scholar] [CrossRef]
- Uhlir, A., Jr. Electrolytic shaping of germanium and silicon. Bell Syst. Tech. J. 1956, 35, 333–347. [Google Scholar] [CrossRef]
- Parkhutik, V.; Canham, L.T. Porous silicon as an educational vehicle for introducing nanotechnology and interdisciplinary materials science. Phys. Status Solidi 2000, 182, 591–598. [Google Scholar] [CrossRef]
- Sakhnini, S.; Blonder, R. Nanotechnology applications as a context for teaching the essential concepts of NST. Int. J. Sci. Educ. 2016, 34, 521–538. [Google Scholar] [CrossRef]
- Andreani, L.C.; Bozzola, A.; Kowalczewski, P.; Liscidini, M.; Redorici, L. Silicon solar cells: Towards the efficiency limits. Adv. Phys. X 2009, 4, 1548305. [Google Scholar] [CrossRef] [Green Version]
- Williams, E.D.; Ayres, R.U.; Heller, M. The 1.7 kilogram microchip: Energy and material use in the production of semiconductor devices. Environm. Sci. Technol. 2002, 36, 5504–5510. [Google Scholar] [CrossRef]
- Oliver, J.Y.; Amirtharajah, R.; Akella, V.; Geyer, R.; Chong, F.T. Life cycle aware computing: Reusing silicon technology. Computer 2007, 40, 56–61. [Google Scholar] [CrossRef] [Green Version]
- Krishnan, N.; Boyd, S.; Somani, A.; Raoux, S.; Clark, D.; Dornfeld, D. A hybrid life cycle inventory of nanoscale semiconductor manufacturing. Environ. Sci. Technol. 2008, 42, 3069–3075. [Google Scholar] [CrossRef] [Green Version]
- Fthenakis, V.M.; Kim, H.C. Photovoltaics: Life cycle analyses. Sol. Energy 2011, 85, 1609–1628. [Google Scholar] [CrossRef]
- Wong, J.H.; Royapoor, M.; Chan, C.W. Review of life cycle analyses and embodied energy requirements of single crystalline and multi-crystalline silicon photovoltaic systems. Renew. Sustain. Energy Rev. 2016, 58, 608–618. [Google Scholar] [CrossRef]
- Canham, L.T. Properties of Porous Silicon; Inspec/IEE: London, UK, 1997. [Google Scholar]
- Koshida, N.; Kojima, A.; Migita, T.; Nakajima, Y. Multifunctional properties of nanocrystalline porous silicon as a quantum-confined material. Mater. Sci. Eng. 2002, C19, 285–289. [Google Scholar] [CrossRef]
- Canham, L.T. Handbook of Porous Silicon, 1st ed.; Springer International: Cham, Switzerland, 2014. [Google Scholar]
- Collings, P.J. Simple measurement of the bandgap of silicon and germanium. Am. J. Phys. 1980, 48, 197–199. [Google Scholar] [CrossRef]
- Vaidyanathan, M. Electronics from the bottom up: Strategies for teaching nanoelectronics at the undergraduate level. IEEE Trans. Educ. 2010, 54, 77–86. [Google Scholar] [CrossRef]
- Kammer, D.W.; Luddington, M.A. Laboratory experiments with silicon solar cells. Am. J. Phys. 1977, 45, 602–605. [Google Scholar] [CrossRef]
- Smith, R.P.; Hwang, A.A.-C.; Beetz, T.; Helgren, E. Introduction to semiconductor processing: Fabrication and characterization of p-n junction silicon solar cells. Am. J. Phys. 2018, 86, 740. [Google Scholar] [CrossRef]
- Dahle, R.; Rasel, R. 3D Printing as an effective educational tool for MEMS design and fabrication. IEEE Trans. Educ. 2016, 59, 210–215. [Google Scholar] [CrossRef]
- Siahmakoun, A. Laboratory training in silicon photonics for undergraduate and graduate students. In 15th Conference Education and Training in Optics/Photonics (ETOP 2019); SPIE Proceedings; OSA: Washington, DC, USA, 2019; Volume 11143, p. 59. [Google Scholar]
- Lehman, V. Electrochemistry of Silicon: Instrumentation, Science, Materials and Applications; Wiley-VCH: Weinheim, Germany, 2002; p. 286. [Google Scholar]
- Sailor, M.J. Porous Silicon in Practice; Wiley-VCH: Weinheim, Germany, 2011. [Google Scholar]
- Smith, R.L.; Collins, S.D. Porous silicon formation mechanisms. J. Appl. Phys. 1992, 71, R1–R22. [Google Scholar] [CrossRef]
- Qin, Z.; Joo, J.; Gu, L.; Sailor, M.J. Size control of porous silicon nanoparticles by electrochemical perforation etching. Part. Part. Syst. 2014, 31, 252–256. [Google Scholar] [CrossRef]
- Tasciotti, E.; Liu, X.; Bhavane, R.; Plant, K.; Leonard, A.D.; Price, B.K.; Cheng, M.M.-C.; Decuzzi, P.; Tour, J.M.; Robertson, F.; et al. Mesoporous silicon particles as a multistage delivery system for imaging and therapeutic applications. Nat. Nanotechnol. 2008, 3, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Weller, M.; Overton, T.; Rourke, J.; Armstrong, F. Green Chemistry in Inorganic Chemistry, 7th ed.; Oxford University Press: Boston, MA, USA, 2018; pp. 810–821. [Google Scholar]
- Nohira, T.; Yasuda, K.; Ito, Y. Pinpoint and bulk electrochemical reduction of insulating silicon dioxide to silicon. Nat. Mater. 2003, 2, 397–401. [Google Scholar] [CrossRef]
- Bao, Z.; Weatherspoon, M.R.; Shian, S.; Cai, Y.; Graham, P.D.; Allan, S.M.; Ahmad, G.; Dickerson, M.B.; Church, B.C.; Kang, Z.; et al. Chemical reduction of three-dimensional silica micro-assemblies into porous silicon replicas. Nature 2007, 446, 172–175. [Google Scholar] [CrossRef]
- Batchelor, L.; Loni, A.; Canham, L.T.; Hasan, M.; Coffer, J.L. Manufacture of porous silicon from living plants and agricultural waste: A scalable and environmentally friendly process. Silicon 2012, 4, 259–266. [Google Scholar] [CrossRef]
- Kalluri, J.R.; Gonzalez-Rodriguez, R.; Hartman, P.S.; Loni, A.; Canham, L.T.; Coffer, J.L. Single plant derived nanotechnology synergistic antibacterial therapies. PLoS ONE 2016, 11, e0163270. [Google Scholar] [CrossRef] [Green Version]
- Le, N.T.; Kalluri, J.R.; Loni, A.; Canham, L.T.; Coffer, J.L. Biogenic nanostructured porous silicon as a carrier for stabilization and delivery of natural therapeutic species. Mol. Therap. 2017, 14, 4509. [Google Scholar] [CrossRef]
- Kalluri, J.R.; West, J.; Akkaraju, G.R.; Canham, L.T.; Coffer, J.L. Plant-derived tandem drug mesoporous silicon microcarrier structures for anti-inflammatory therapy. ACS Omega 2019, 4, 8359–8364. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Huo, K.; McDowell, M.T.; Zhao, J.; Cui, Y. Rice husks as a sustainable source of nanostructured silicon for high performance Li ion battery anodes. Sci. Rep. 2013, 3, 1919. [Google Scholar] [CrossRef] [PubMed]
- Xing, A.; Tian, S.; Tang, H.; Losic, D.; Bao, Z. Mesoporous silicon engineered from the reduction of biosilica from rice husk as a high performance anode for Li ion batteries. RSC Adv. 2013, 3, 10145. [Google Scholar] [CrossRef]
- Liu, J.; Kopold, P.; van Aken, P.A.; Maier, J.; Yu, Y. Energy storage materials from nature through nanotechnology: A sustainable route from reed plants to a silicon anode for lithium ion batteries. Angew. Chem. 2015, 127, 9768–9772. [Google Scholar] [CrossRef]
- Zhu, G.; Luo, W.; Wang, L.; Jiang, W.; Yang, J. Silicon: Towards eco-friendly reduction techniques for lithium ion battery applications. J. Mater. Chem. A 2019, 7, 24175–24737. [Google Scholar] [CrossRef]
- Rehman, W.U.; Wang, H.; Manj, R.Z.A.; Luo, W.; Yang, J. When silicon materials meet natural sources: Opportunities and challenges for low cost lithium storage. Small 2019, 190458. [Google Scholar] [CrossRef]
- Kalidas, N.; Riikonen, J.; Xu, W.; Lahtinen, K.; Kallio, T.; Lehto, V.-P. Cascading use of barley rice husk ash to produce silicon for composite anodes of Li ion batteries. Mater. Chem. Phys. 2020, 245, 122736. [Google Scholar] [CrossRef]
- Canham, L. Origin and applications of efficient visible photoluminescence from silicon based nanostructures. Faraday Discuss 2020, 222, 10. [Google Scholar] [CrossRef] [PubMed]
- Canham, L.T. Silicon quantum wire array fabrication via electrochemical and chemical dissolution of wafers. Appl. Phys. Lett. 1990, 57, 1046–1048. [Google Scholar] [CrossRef]
- Schuppler, S.; Friedman, S.L.; Marcus, M.A.; Adler, D.L.; Xie, Y.; Ross, F.M.; Harris, T.D.; Brown, W.L.; Chabal, Y.J.; Brus, L.E.; et al. Dimensions of luminescent oxidized and porous silicon structures. Phys. Rev. Lett. 1994, 72, 2648–2651. [Google Scholar] [CrossRef]
- Zhang, Q.; Bayliss, S.C. The correlation of dimensionality with emitted wavelength and ordering of freshly produced porous silicon. J. Appl. Phys. 1996, 79, 1351–1356. [Google Scholar] [CrossRef]
- Von Behren, J.; Van Buuren, T.; Zacharias, M.; Chimowitz, E.H.; Fauchet, P.M. Quantum confinement in nanoscale silicon: The correlation of size with bandgap and luminescence. Solid State Commun. 1998, 105, 317–322. [Google Scholar] [CrossRef]
- Choi, J.; Wang, N.S.; Reipa, V. Photoassisted tuning of silicon nanocrystal photoluminescence. Langmuir 2007, 23, 3388–3394. [Google Scholar] [CrossRef]
- Yu, Y.; Fan, G.; Fermi, A.; Mazzaro, R.; Morandi, V.; Ceroni, P.; Smilgies, D.-M.; Korgel, B.A. Size-dependent photoluminescence efficiency of silicon nanocrystal quantum dots. J. Phys. Chem. C 2017, 121, 23240–23248. [Google Scholar] [CrossRef]
- Landry, M.L.; Morrell, T.E.; Karagounis, T.K.; Hsia, C.-H.; Wang, C.-Y. A safer, easier, faster synthesis of CdSe quantum dot nanocrystals. J. Chem. Educ. 2005, 82, 1698. [Google Scholar]
- Wang, Z.; Yu, J.; Zhang, X.; Li, N.; Liu, B.; Li, Y.; Wang, Y.; Wang, W.; Li, Y.; Zhang, L.; et al. Large scale and controllable graphene quantum dots from rice husk biomass: A comprehensive utilization strategy. ACS Appl. Mater. Interfaces 2016, 8, 1434–1439. [Google Scholar] [CrossRef]
- Wang, Z.; Zeng, S.; Li, Y.; Wang, W.; Zhang, Z.; Zeng, H.; Wang, W.; Sun, L. Luminescence mechanism of carbon-incorporated silica nanoparticles derived from rice husk biomass. Ind. Eng. Chem. Res. 2017, 56, 5906–5912. [Google Scholar] [CrossRef]
- Bose, S.; Ganayee, M.A.; Mondal, B.; Baidya, A.; Chennu, S.; Mohanty, J.S.; Pradeep, T. Synthesis of silicon nanoparticles from rice husk and their use as sustainable fluorophores for white light emission. ACS Sustain. Chem. Eng. 2018, 6, 6203–6210. [Google Scholar] [CrossRef]
- Canham, L.T. Bioactive silicon fabrication through nanoetching techniques. Adv. Mater. 1995, 7, 1033–1037. [Google Scholar] [CrossRef]
- Canham, L.T.; Newey, J.P.; Reeves, C.L.; Houlton, M.R.; Loni, A.; Simons, A.J. The Effects of dc electrical currents on the in-vitro calcification of bioactive silicon wafers. Adv. Mater. 1996, 8, 847–849. [Google Scholar] [CrossRef]
- Arroyo-Hernández, M.; Pérez-Rigueiro, J.; Manso-Silván, M.; Martínez-Duart, J.M. Bioactivity test for amine-based functionalized meso and macro porous silicon substrates. Mater. Sci. Eng. 2007, C27, 1211–1214. [Google Scholar]
- Sun, W.; Puzas, J.E.; Sheu, T.J.; Liu, X.; Fauchet, P.M. Nano- to microscale porous silicon as a cell interface for bone tissue engineering. Adv. Mater. 2007, 19, 921–924. [Google Scholar] [CrossRef]
- Whitehead, M.A.; Fan, D.; Mukherjee, P.; Akkaraju, G.R.; Canham, L.T.; Coffer, J.L. High porosity poly (caprolactone) /mesoporous silicon scaffolds: Calcium phosphate deposition and biological response to bone precursor cells. Tissue Eng. 2008, A14, 195–206. [Google Scholar]
- Henstock, J.R.; Ruktanonchai, U.R.; Canham, L.T.; Anderson, S.I. Porous silicon confers bioactivity to polycapralactone composites in-vitro. J. Mater. Sci. Mater. Med. 2014, 25, 1087–1097. [Google Scholar] [CrossRef]
- Anderson, S.H.C.; Elliott, H.; Wallis, D.J.; Canham, L.T.; Powell, J.J. Dissolution of partially porous silicon wafers under simulated physiological conditions. Phys. Status Solidi 2003, 197, 331–335. [Google Scholar] [CrossRef]
- Park, J.-H.; Gu, L.; von Maltzahn, G.; Ruoslahti, E.; Bhatia, S.N.; Sailor, M.J. Biodegradable luminescent porous silicon nanoparticles for in-vivo applications. Nat. Mater. 2009, 8, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Gongalsky, M.B.; Sviridov, A.P.; Bezsudnova, Y.I.; Osminkina, L.A. Biodegradation model of porous silicon nanoparticles. Colloid. Surf. 2020, B190, 110946. [Google Scholar] [CrossRef]
- Wong, K.K.W.; Liu, X.L. Nanomedicine: A primer for surgeons. Pediatric Surg. Int. 2012, 28, 943. [Google Scholar] [CrossRef] [Green Version]
- Ratner, B.D.; Hoffman, A.S.; Schoen, F.J.; Lemons, J.E. Biomaterials Science: An Introduction to Materials in Medicine, 3rd ed.; Elsevier Academic Press: New York, NY, USA, 2013. [Google Scholar]
- Canham, L.T. Porous silicon for medical use: From conception to clinical use. In Porous Silicon for Biomedical Applications; Chapter 1; Santos, H., Ed.; Elsevier Woodhead: Cambridge, UK, 2014. [Google Scholar]
- Sai, H.; Tan, K.W.; Hur, K.; Asenath-Smith, E.; Hovden, R.; Jiang, Y.; Riccio, M.; Muller, D.A.; Elser, V.; Estroff, L.A.; et al. Hierarchical porous polymer scaffolds from block copolymers. Science 2013, 341, 530–534. [Google Scholar] [CrossRef]
- Baino, F.; Fiorilli, S.; Vitale-Brovarone, C. Bioactive glass-based materials with hierarchical porosity for medical applications: Review of recent advances. Acta Biomater. 2016, 42, 18–32. [Google Scholar] [CrossRef] [PubMed]
- Song, T.; Yan, M.; Qian, M. The enabling role of dealloying in the creation of specific hierarchical porous metal structures. Corros. Sci. 2018, 134, 78–98. [Google Scholar] [CrossRef]
- Wang, M.-F.; Raghunathan, N.; Ziaie, B. A non-lithographic top-down electrochemical approach for creating hierarchical (micro to nano) superhydrophobic silicon surfaces. Langmuir 2007, 23, 2300–2303. [Google Scholar] [CrossRef]
- Mäkilä, E.; Anton Willmore, A.-M.; Yu, H.; Irri, M.; Aindow, M.; Teesalu, T.; Canham, L.T.; Kolasinski, K.W.; Salonen, J. Hierarchical nanostructuring of porous silicon with electrochemical and regenerative electroless etching. ACS Nano. 2019, 13, 13056–13064. [Google Scholar] [CrossRef]
- Jia, H.; Li, X.; Song, J.; Zhang, X.; Luo, L.; He, Y.; Li, B.; Cai, Y.; Hu, S.; Xiao, X.; et al. Hierarchical porous silicon structures with extraordinary mechanical strength as high performance lithium ion battery anodes. Nat. Commun. 2020, 11, 1474. [Google Scholar] [CrossRef] [Green Version]
- Canham, L.E.d. Handbook of Porous Silicon, 2nd ed.; Springer International: Cham, Switzerland, 2018. [Google Scholar]
- Magoariec, H.; Danescu, A. Modeling macroscopic elasticity of porous silicon. Phys. Status Solidi 2009, C6, 1680–1684. [Google Scholar] [CrossRef]
- Astrova, E.V.; Tolmachev, V.A. Effective refractive index and composition of oxidized porous silicon films. Mater. Sci. Eng. 2000, B69–70, 142–148. [Google Scholar] [CrossRef]
- Granitzer, P.; Rumpf, K. Porous silicon—A versatile host material. Materials 2010, 3, 943–948. [Google Scholar] [CrossRef]
- Muller, G.; Friedberger, A.; Knese, K. Porous silicon-based MEMS. Handbook of Silicon-Based MEMS Materials and Technologies; Elsevier: London, UK, 2010; pp. 481–502. [Google Scholar]
- Gautier, G. Porous silicon for electrical isolation in radio frequency devices: A review. Appl. Phys. Rev. 2014, 1, 011101. [Google Scholar] [CrossRef]
- Nassiopoulou, A.G. Thermal isolation with porous silicon. In Handbook of Porous Silicon; Springer International: Cham, Switzerland, 2014; pp. 753–765. [Google Scholar]
- Barillaro, G.; Strambini, L.M. Color tuning of light-emitting diodes by modulating the concentration of red-emitting silicon nanocrystal phophors. Appl. Phys. Lett. 2014, 104, 091102. [Google Scholar] [CrossRef] [Green Version]
- Gelloz, B. Electroluminescence of porous silicon. In Handbook of Porous Silicon, 1st ed.; Springer International: Cham, Switzerland, 2014; pp. 321–333. [Google Scholar]
- Harraz, F.A. Porous silicon chemical sensors and biosensors: A review. Sens. Actuators 2014, B202, 897–912. [Google Scholar] [CrossRef]
- Arshavsky-Graham, S.; Massad-Ivanir, N.; Segal, E.; Weiss, S. Porous silicon—based photonic biosensors: Current status and emerging applications. Anal. Chem. 2019, 91, 441–467. [Google Scholar] [CrossRef]
- Wei, J.; Buriak, J.M.; Siuzdak, G. Desorption-ionization mass spectrometry on porous silicon. Nature 1999, 399, 243–246. [Google Scholar] [CrossRef]
- Zhang, K.; Loong, S.L.E.; Connor, S.; Yu, S.W.K.; Tan, S.Y.; Ng, R.T.H.; Lee, K.M.; Canham, L.; Chow, P.K.H. Complete tumor response following intratumoral 32P BioSilicon on human hepatocellular and pancreatic carcinoma xenografts in nude mice. Clin. Cancer Res. 2006, 11, 7532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anglin, E.J.; Cheng, L.; Freeman, W.R.; Sailor, M.J. Porous silicon in drug delivery devices and materials. Adv. Drug Deliv. Rev. 2008, 60, 1266–1277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coffer, J.L. Porous silicon and tissue engineering scaffolds. In Handbook of Porous Silicon, 1st ed.; Springer International: Cham, Switzerland, 2014; pp. 921–927. [Google Scholar]
- Shabir, Q.; Skaria, C.; Brien, H.O.; Loni, A.; Barnett, C.; Canham, L.T. Taste and mouthfeel assessment of porous and non-porous silicon microparticles. Nano. Res. Lett. 2012, 7, 407. [Google Scholar] [CrossRef] [Green Version]
- Canham, L.T. Porous silicon and functional foods. In Handbook of Porous Silicon, 1st ed.; Springer International: Cham, Switzerland, 2014; pp. 985–997. [Google Scholar]
- Popov, A.P.; Priezzhev, A.V.; Lademann, J.; Myllyla, R. Alteration of skin-light scattering and absorption properties by application of sunscreen nanoparticles: A Monte Carlo study. J. Quant. Spectrosc. Rad. Transf. 2011, 112, 1891–1897. [Google Scholar] [CrossRef]
- Canham, L.T. Porous silicon for oral hygiene and cosmetics. In Handbook of Porous Silicon, 1st ed.; Springer International: Cham, Switzerland, 2014; pp. 999–1008. [Google Scholar]
- Li, X.; Gu, M.; Kennard, R.; Yan, P.; Chen, X.; Wang, C.; Sailor, M.J.; Zhang, J.G.; Liu, J. Mesoporous silicon sponge as an anti-pulverization structure for high performance lithium ion battery anodes. Nat. Commun. 2014, 5, 4105. [Google Scholar] [CrossRef] [Green Version]
- Dzhararov, T.; Bayramov, A. Porous silicon and solar cells. In Handbook of Porous Silicon, 2nd ed.; Springer International: Cham, Switzerland, 2018; pp. 1479–1492. [Google Scholar]
- Schierning, G. Silicon nanostructures for thermoelectric devices: A review of the current state of the art. Phys. Status Solidi 2014, 211, 1235–1249. [Google Scholar] [CrossRef]
- Shinoda, H.; Nakajima, T.; Ueno, K.; Koshida, N. Thermally induced ultrasonic emission from porous silicon. Nature 1999, 400, 853–855. [Google Scholar] [CrossRef]
- Du Plessis, M. A decade of porous silicon as a nano-explosive material. Propellants Explosives Pyrotech. 2014, 39, 348–364. [Google Scholar] [CrossRef] [Green Version]
- Santos, H. (Ed.) Porous Silicon for Biomedical Applications; Woodhead Publishing: Cambridge, UK, 2014. [Google Scholar]
- Andrady, A.L. Microplastics in the marine environment. Mar. Pollut. Bull 2011, 62, 1596–1605. [Google Scholar] [CrossRef]
- Albertsson, A.C.; Hakkarainen, M. Designed to degrade. Science 2017, 358, 872–873. [Google Scholar] [CrossRef]
- Struyf, E.; Smis, A.; Van Damme, S.; Meire, P.; Conley, D.J. The global biogeochemical silicon cycle. Silicon 2009, 1, 207–213. [Google Scholar] [CrossRef]
- Liang, Y.; Nikolic, M.; Belanger, R.; Gong, H.; Song, A. Silicon in Agriculture: From Theory to Practice; Springer: Berlin/Heidelberg, Germany, 2015; 253p. [Google Scholar]
- Howell, S.; Kalluri, J.; Loni, A.; Canham, L.T.; Coffer, J.L. Porous Silicon Morphologies Resulting from Different Components of the Silicon Accumulator Plant Equisetum Telmateia. Presented at the 2016 Porous Semiconductors: Science and Technology Conference, Tarragona, Spain, 6–11 March 2016. [Google Scholar]
- Kuratko, D.F. The emergence of entrepreneurship education: Development, trends and challenges. Entrepren. Theory Pract. 2005, 29, 577–597. [Google Scholar] [CrossRef]
- Demirel, P.; Li, Q.C.; Rentocchini, F.; Tamvada, J.P. Born to be green: New insights into the economics and management of green entrepreneurship. Small Bus. Econ. 2019, 52, 759–771. [Google Scholar] [CrossRef] [Green Version]
- Segal, G.; Borgia, D.; Schoenfeld, J. The motivation to become an entrepreneur. Int. J. Entrepr. Res. 2005, 11, 42–57. [Google Scholar] [CrossRef] [Green Version]
- Marvel, M.R.; Lumpkin, G.T. Technology entrepreneurs, human capital and its effect on innovation radicalness. Entrepr. Theory Pract. 2007, 31, 807–828. [Google Scholar] [CrossRef]
- Carsud, A.; Brannback, M. Entrepreneurial motivations: What do we still need to know? J. Small Bus. Manag. 2011, 49, 9–26. [Google Scholar] [CrossRef]
- Teran-Yepez, E.; Marin-Carrillo, G.M.; Casado-Belmonte, M.P.; Capobianco-Uriarte, M.M. Sustainable entrepreneurship: Review of its evolution and new trends. J. Clean. Prod. 2020, 252, 119742. [Google Scholar] [CrossRef]
- Bessant, J.; Tidd, J. Innovation and Entrepreneurship; Wiley & Sons: Weinheim, Germany, 2007. [Google Scholar]
- Kornish, L.J.; Hutchinson-Krupat, J. Research on idea generation and selection: Implications for management of technology. Prod. Oper. Manag. 2017, 26, 633–651. [Google Scholar] [CrossRef]
- Kirsch, D.; Goldfarb, B.; Gera, A. Form or substance: The role of business plans in venture capital decision making. Strat. Manag. J. 2009, 30, 481–515. [Google Scholar] [CrossRef]
- Blank, S. Why the lean start-up changes everything. Harv. Bus. Rev. 2013, 91, 63–72. [Google Scholar]
- Vaidhyanathan, S. Intellectual Property: A Very Short Introduction; Oxford University Press: Boston, MA, USA, 2017. [Google Scholar]
- Wright, M.; Siegel, D.S.; Mustar, P. An emerging ecosystem for student start-ups. J. Technol. Transf. 2017, 42, 909–922. [Google Scholar] [CrossRef]
- Chiesa, V.; Frattini, F. Commercializing technological innovation: Learning from failures in high-tech markets. J. Prod. Innov. Manag. 2011, 25, 437–454. [Google Scholar] [CrossRef]
- Phillips, R.G. Technology business incubators: How effective as technology transfer mechanisms? Technol. Soc. 2002, 24, 299–316. [Google Scholar] [CrossRef]
- Cohen, S. What do accelerators do? Insights from incubators and angels. Innovations 2013, 8, 19–25. [Google Scholar] [CrossRef]
- Walthoff-Borm, X.; Schwienbacher, A.; Vanacher, T. Equity crowdfunding: First resort or last resort? J. Bus. Ventur. 2018, 33, 513–533. [Google Scholar] [CrossRef]
- Amato, S.F.; Ezzell, R.M., Jr. Regulatory Affairs for biomaterials and medical devices. In Woodhead Series in Biomaterials; No 79; Woodhead: Cambridge, UK, 2014. [Google Scholar]
- Prestwich, G.D.; Bhatia, S.; Breurer, C.K.; Dahl, S.M.L.; Mason, C.; McFarland, R.; McQuillan, D.J.; Sackner-Bernstein, J.; Schox, J.; Tente, W.E.; et al. What is the greatest regulatory challenge in the translation of biomaterials to the clinic? Sci. Transl. Med. 2012, 4, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Nance, E. Careers in nanomedicine and drug delivery. Adv. Drug Deliv. Rev. 2019, 144, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Paull, R.; Wolfe, J.; Hebert, P.; Sinkula, M. Investing in nanotechnology. Nat. Biotechnol. 2003, 21, 144–1147. [Google Scholar] [CrossRef]
- Bawa, R. Patents and nanomedicine. Nanomedicine 2007, 2, 351–374. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Roco, M.C.; Li, X.; Lin, Y. Trends in nanotechnology patents. Nat. Nanotechnol. 2008, 3, 123–125. [Google Scholar] [CrossRef]
- Wu, L.; Zhu, H.; Chen, H.; Roco, M.C. Comparing nanotechnology landscapes in the US and China: A patent analysis perspective. J. Nanopart. Res. 2019, 21, 180. [Google Scholar] [CrossRef]
- Course. Available online: https://ocw.mit.edu/courses/electrical-engineering-and-computer-science/6-901-inventions-and-patents-fall-2005/ (accessed on 4 February 2021).
- Summer School. Available online: http://sailorgroup.ucsd.edu/courses/SummerSchool/ (accessed on 13 February 2021).
- Conference. Available online: http://www.psst2020.it/ (accessed on 13 February 2021).









| NST Concept | Concept Description | KEY NST EDUCATIONAL TOPICS LINKED TO NANOPOROUS SILICON |
|---|---|---|
| 1 | Size and scale classification | Nanopores, mesopores, and micropores. Hierarchical porosity. Nanocrystals and nanoparticles. Quantum wires and quantum dots. Biological building blocks. |
| 2 | Nanofabrication methods | Top-down vs. bottom-up fabrication. Nanotools. Control over size and shape at the nanoscale. Scalability. |
| 3 | Characterization at the nanoscale | Amorphous vs. nanocrystalline physical states. Nanometrology. Surface and interfacial chemistry. Size and shape distributions. |
| 4 | Size-dependent properties | Properties tunable by porosity (surface area/volume) Properties tunable by silicon skeleton dimensionality Size-dependent toxicity. Medical bioactivity and biodegradability. Size-tunable luminescence. |
| 5 | Functionality | New functionality enabled by nanostructuring bulk silicon. Extended functionality via size-dependent properties. |
| 6 | Multidisciplinary nature | Understanding and exploitation dependent on combined expertise and knowledge from many scientific disciplines and industries (see Table 2). Nanoelectronics. Nanomedicine. Solar Energy. |
| 7 | Environmental & societal impact | Societal benefits. Sustainable synthesis. Material life cycle assessments. Risk assessments. Biodurability. Implantable electronics. Nanobots. Ethical issues. |
| 1. Prevention |
| 2. Atom Economy |
| 3. Less Hazardous Chemical Syntheses |
| 4. Designing Safer Chemicals |
| 5. Safer Solvents and Auxiliaries |
| 6. Design for Energy Efficiency |
| 7. Use of Renewable Feedstocks |
| 9. Catalysis |
| 10. Design for Degradation |
| 11. Real-time analysis for Pollution Prevention |
| 12. Inherently Safer Chemistry for Accident Prevention |
| Property | Values for Crystalline Silicon | Ranges for Nanoporous Silicon |
|---|---|---|
| Density | 2.33 g/cm3 | 0.12–1.9 g/cm3 |
| Young’s Modulus | 160 GPa | 1–100 GPa |
| Optical bandgap | 1.1 eV | 1.1–3.2 eV |
| Refractive index | 3.5 | 1.1–3.0 |
| Electrical resistivity | 0.01–1000 ohm cm | 1000–1012 ohm cm |
| Thermal conductivity | 150 Wm−1 K−1 | 0.03–20 Wm−1 K−1 |
| Surface wettability (water contact angle) | 5°–96° | 0.5°–168° |
| Biodegradability kinetics | NA | Hours (nanoparticles) Days (microparticles) |
| Photoluminescence wavelength | 1100 nm | 400–1100 nm |
| Photoluminescence quantum efficiency | <0.001% | <32% (films) <60% (suspensions) |
| Burn rate | NA | 1–1840 m/s |
| Key Property of Nanoporous Silicon | Function | Industry | EDUCATIONAL TOPICS | References |
|---|---|---|---|---|
| Enhanced chemical reactivity VLSI compatibility | Sacrificial material for lithographic patterning | ELECTRONICS | Photolithography Micromachining Microsystem designMEMS | [88] Muller (2010) |
| Low AC conductivity Low dielectric constant | RF electrical isolation | ELECTRONICS | RF semiconductor devices and circuitry. | [89] Gautier (2014) |
| Low thermal conductivity | Thermal isolation | ELECTRONICS | Microsensor design Heat transport | [90] Nassiopoulou (2014) |
| Photo- luminescence | Down-converter in white LEDs | OPTO- ELECTRONICS | Radiative processes Phosphors Color rendering | [91] Barillaro (2014) |
| Electro- luminescence | Active layer in LED | OPTO- ELECTRONICS | Quantum confinement Excitons LED technology | [92] Gelloz (2014) |
| Tunable Refractive index | Micro-optical devices | OPTICS | Micro-optics Photonic communication | [86] Astrova (2000) |
| High Surface area | Matrix for Sensing | DIAGNOSTIC | Sensor technologies Surface chemistry | [93] Harraz (2014) [94] Arshavsky- Graham (2019) |
| High surface area | Matrix for adsorption- photodesorption | DIAGNOSTIC | Mass spectrometry Metabolomics Forensics | [95] Wei (1999) |
| Neutron transmutable | Host for medical radioisotope | MEDICAL | Targeted cancer therapy (Brachytherapy) | [96] Zhang (2006) |
| Biodegradabilty | Drug delivery | MEDICAL | Pharmacology Biomaterial testing | [97] Anglin (2008) |
| Biodegradability Photo- luminescence | Theranostics | MEDICAL | In vivo imaging Contrast agents | [73] Park (2009) |
| Bioactivity | Bone growth stimulation | MEDICAL | Orthopaedics Tissue engineering | [98] Coffer (2014) |
| Nanoporosity | Nutrient protection | FOOD | Organoleptic assessment Food additives & GRAS status | [99] Shabir (2012) [100] Canham (2014) |
| UV absorption | Sunscreen | COSMETIC | Light scattering Sun Protection Factors | [101] Popov (2011) [102] Canham (2014) |
| Tunable hardness | Toothpaste Abrasive | CONSUMER CARE | Abrasive testing Consumer acceptance | [102] Canham (2014) |
| Lithiation capacity | Anode in Li ion battery | ENERGY CONVERSION | Battery technology Mechanical properties | [103] Li (2014) |
| Tunable Refractive index | Antireflection coating in silicon photovoltaics | ENERGY CONVERSION | Solar-cell technology Light reflection | [104] Dzhafarov (2018) |
| Tunable thermal conductivity | Conversion of temperature gradients to electrical power | ENERGY CONVERSION | Energy scavenging Thermoelectric materials | [105] Schierning (2014) |
| Low thermal conductivity | Conversion of electrical power to sound | ENERGY CONVERSION | Ultrasonics Phonon Confinement | [106] Shinoda (1999) |
| Rapid oxidation | Rapid conversion of chemical energy to heat | ENERGY CONVERSION | Pyrotechnics. Explosives. Propellants | [107] Du Plessis (2014) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coffer, J.L.; Canham, L.T. Nanoporous Silicon as a Green, High-Tech Educational Tool. Nanomaterials 2021, 11, 553. https://doi.org/10.3390/nano11020553
Coffer JL, Canham LT. Nanoporous Silicon as a Green, High-Tech Educational Tool. Nanomaterials. 2021; 11(2):553. https://doi.org/10.3390/nano11020553
Chicago/Turabian StyleCoffer, Jeffery L., and Leigh T. Canham. 2021. "Nanoporous Silicon as a Green, High-Tech Educational Tool" Nanomaterials 11, no. 2: 553. https://doi.org/10.3390/nano11020553
APA StyleCoffer, J. L., & Canham, L. T. (2021). Nanoporous Silicon as a Green, High-Tech Educational Tool. Nanomaterials, 11(2), 553. https://doi.org/10.3390/nano11020553