Methionine-Functionalized Graphene Oxide/Sodium Alginate Bio-Polymer Nanocomposite Hydrogel Beads: Synthesis, Isotherm and Kinetic Studies for an Adsorptive Removal of Fluoroquinolone Antibiotics
Abstract
:1. Introduction
2. Experimental Segments
2.1. Materials and Instruments
2.2. Synthesis of Met-GO/SA Polymer Nanocomposite Hydrogel Beads and Individual Components
2.2.1. Synthesis of GO
2.2.2. Synthesis of Met-GO
2.2.3. Synthesis of Met-GO/SA Beads
2.3. Adsorption Studies
3. Results and Discussion
3.1. Characterization of Met-GO/SA
3.1.1. FTIR Analysis
3.1.2. XRD Analysis
3.1.3. HR-TEM Analysis
3.1.4. FE-SEM Analysis
3.1.5. TGA/DTG Analysis
3.2. Adsorption Tests
3.2.1. Effect of Solution pH
3.2.2. Effect of Adsorbent Dosage
3.2.3. Effect of Contact Time
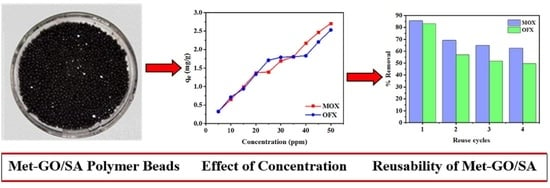
3.2.4. Effect of Initial Concentration
3.3. Adsorption Isotherm Studies
3.4. Adsorption Kinetic Studies
3.5. Adsorption Thermodynamics Studies
3.6. Effect of Ionic Strength of Solution
3.7. Comparison with Other Adsorbents for FQs Antibiotics Removal
3.8. Regeneration and Reusability of Met-GO/SA
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Martin, J.; Camacho-Muñoz, D.; Santos, J.L.; Aparicio, I.; Alonso, E. Occurrence of pharmaceutical compounds in wastewater and sludge from wastewater treatment plants: Removal and ecotoxicological impact of wastewater discharges and sludge disposal. J. Hazard. Mater. 2012, 239, 40–47. [Google Scholar] [CrossRef]
- Gaffney, V.J.; Almeida, C.M.M.; Rodrigues, A.; Ferreira, E.; Benoliel, M.J.; Cardoso, V.V. Occurrence of pharmaceuticals in a water supply system and related human health risk assessment. Water Res. 2015, 72, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.; Singh, A.K.; Carabineiro, S.A.C. Assessing the photocatalytic degradation of fluoroquinolone norfloxacin by Mn:ZnS quantum dots: Kinetic study, degradation pathway and influencing factors. Nanomaterials 2020, 10, 964. [Google Scholar] [CrossRef]
- He, K.; Soares, A.D.; Adejumo, H.; Diarmid, M.M.; Squibb, K.; Blaney, L. Detection of a wide variety of human and veterinary fluoroquinolone antibiotics in municipal wastewater and wastewater-impacted surface water. J. Pharm. Biomed. Anal. 2015, 106, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Brown, S. Fluoroquinolones in animal health. J. Vet. Pharmacol. Ther. 1996, 19, 1–14. [Google Scholar] [CrossRef]
- Bryan, L.E.; Bedard, J.; Wong, S.; Chamberland, S. Quinolone antimicrobial agents: Mechanism of action and resistance development. Clin. Investig. Med. 1989, 12, 14–19. [Google Scholar]
- Peng, H.; Pan, B.; Wu, M.; Liu, R.; Zhang, D.; Wu, D.; Xing, B. Adsorption of ofloxacin on carbon nanotubes: Solubility, pH and cosolvent effects. J. Hazard. Mater. 2012, 211, 342–348. [Google Scholar] [CrossRef]
- Wahab, M.; Zahoor, M.; Salman, S.M. A novel approach to remove ofloxacin antibiotic from industrial effluent using magnetic carbon nanocomposite prepared from sawdust of Dalbergia sissoo by batch and membrane hybrid technology. Desalin. Water Treat. 2019, 165, 83–96. [Google Scholar] [CrossRef]
- Ye, Z.; Weinberg, H.S.; Meyer, M.T. Occurrence of antibiotics in drinking water. Anal. Bioanal. Chem. 2007, 387, 1365–1377. [Google Scholar]
- Prutthiwanasan, B.; Phechkrajang, C.; Suntornsuk, L. Fluorescent labeling of ciprofloxacin and norfloxacin and its application for residues analysis in surface water. Talanta 2016, 159, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Sturini, M.; Speltini, A.; Maraschi, F.; Pretali, L.; Ferri, E.N.; Profumo, A. Sunlight induced degradation of fluoroquinolones in wastewater effluent: Photoproducts identification and toxicity. Chemosphere 2015, 134, 313–318. [Google Scholar] [CrossRef]
- Xing, X.; Feng, J.; Lv, G.; Song, K.; Mei, L.; Liao, L.; Wang, X.; Xu, B. Adsorption Mechanism of Ciprofloxacin from Water by Synthesized Birnessite. Adv. Mater. Sci. Eng. 2015, 2015, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Jain, B.; Singh, A.K.; Banchhor, S.; Jonnalagadda, S.B.; Susan, A.B.H. Treatment of pharmaceutical wastewater by heterogeneous Fenton process: An innovative approach. Nanotechnol. Environ. Eng. 2020, 5, 1–13. [Google Scholar] [CrossRef]
- Li, B.; Zhang, T. Biodegradation and Adsorption of Antibiotics in the Activated Sludge Process. Environ. Sci. Technol. 2010, 44, 3468–3473. [Google Scholar] [CrossRef]
- Fei, Y.; Yong, L.; Sheng, H.; Jie, M. Adsorptive removal of ciprofloxacin by sodium alginate/graphene oxide composite beads from aqueous solution. J. Colloid Interf. Sci. 2016, 484, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Lu, M.; Wan, J.; Sun, Y.; Lan, H.; Deng, X. Effects of pH, dissolved humic acid and Cu2+ on the adsorption of norfloxacin on montmorillonite-biochar composite derived from wheat straw. Biochem. Eng. J. 2018, 130, 104–112. [Google Scholar] [CrossRef]
- Huang, B.; Liu, Y.; Li, B.; Liu, S.; Zeng, G.; Zeng, Z.; Wang, X.; Ning, Q.; Zheng, B.; Yang, C. Effect of Cu(II) ions on the enhancement of tetracycline adsorption by Fe3O4@SiO2-chitosan/graphene oxide nanocomposite. Carbohydr. Polym. 2017, 157, 576–585. [Google Scholar] [CrossRef]
- Li, S.; Gong, Y.; Yang, Y.; He, C.; Hu, L.; Zhu, L.; Sun, L.; Shu, D. Recyclable CNTs/Fe3O4 magnetic nanocomposites as adsorbents to remove bisphenol A from water and their regeneration. Chem. Eng. J. 2015, 260, 231–239. [Google Scholar] [CrossRef]
- Shao, L.; Ren, Z.; Zhang, G.; Chen, L. Facile synthesis, characterization of a MnFe2O4/activated carbon magnetic composite and its effectiveness in tetracycline removal. Mater. Chem. Phys. 2012, 135, 16–24. [Google Scholar] [CrossRef]
- Tan, F.; Sun, D.; Gao, J.; Zhao, Q.; Wang, X.; Teng, F.; Quan, X.; Chen, J. Preparation of molecularly imprinted polymer nanoparticles for selective removal of fluoroquinolone antibiotics in aqueous solution. J. Hazard. Mater. 2013, 244, 750–757. [Google Scholar] [CrossRef]
- El Bekkali, C.; Bouyarmane, H.; Laasri, S.; el Karbane, M.; Saoiabi, A.; Laghzizil, A. Sorption and photocatalytic degradation of ciprofloxacin and ofloxacin in aqueous suspensions of TiO2 and ZnO catalysts. J. Mater. Environ. Sci. 2017, 8, 4902–4906. [Google Scholar]
- Nurchi, V.M.; Alonso, M.C.; Pilo, M.I.; Spano, N.; Sanna, G.; Toniolo, R. Sorption of ofloxacin and chrysoidine by grape stalk. A representative case of biomass removal of emerging pollutants from wastewater. Arab. J. Chem. 2019, 12, 1141–1147. [Google Scholar] [CrossRef]
- Zhu, H.; Chen, T.; Liu, J.; Li, D. Adsorption of tetracycline antibiotics from an aqueous solution onto graphene oxide/calcium alginate composite fibers. RSC Adv. 2018, 8, 2616–2621. [Google Scholar] [CrossRef] [Green Version]
- Hashmi, A.; Singh, A.K.; Jain, B.; Carabineiro, S.A.C. Chloramine-T/N Bromosuccinimide/FeCl3/KIO3 Decorated Graphene Oxide Nanosheets and Their Antibacterial Activity. Nanomaterials 2020, 10, 105. [Google Scholar] [CrossRef] [Green Version]
- Zhen, M.M.; Guo, S.Q.; Gao, G.D.; Zhou, Z.; Liu, L. TiO2–B nanorods on reduced graphene oxide as anode materials for Li ion batteries. Chem. Commun. 2015, 51, 507–510. [Google Scholar] [CrossRef]
- Pan, L.; Wang, Z.; Yang, Q.; Huang, R. Efficient Removal of Lead, Copper and Cadmium Ions from Water by a Porous Calcium Alginate/Graphene Oxide Composite Aerogel. Nanomaterials 2018, 8, 957. [Google Scholar] [CrossRef] [Green Version]
- Mallakpour, S.; Abdolmaleki, A.; Borandeh, S. Covalently functionalized graphene sheets with biocompatible natural amino acids. Appl. Surf. Sci. 2014, 307, 533–542. [Google Scholar] [CrossRef]
- Xiao, J.; Lv, W.; Xie, Z.; Song, Y.; Zheng, Q. L-cysteine-reduced graphene oxide/poly(vinyl alcohol) ultralight aerogel as a broad-spectrum adsorbent for anionic and cationic dyes. J. Mater. Sci. 2017, 52, 5807–5821. [Google Scholar] [CrossRef]
- Yang, X.; Zhou, T.; Ren, B.; Hursthouse, A.; Zhang, Y. Removal of Mn (II) by Sodium Alginate/Graphene Oxide Composite Double-Network Hydrogel Beads from Aqueous Solutions. Sci. Rep. 2018, 8, 10717–10733. [Google Scholar] [CrossRef] [Green Version]
- AbouTaleb, M.F.; Mohamed, S.K.; Alkahtani, A. Radiation synthesis and characterization of sodium alginate/chitosan/hydroxyapatite nanocomposite hydrogels: A drug delivery system for liver cancer. Polym. Bull. 2015, 72, 725–742. [Google Scholar] [CrossRef]
- Mandal, B.; Ray, S.K. Synthesis of interpenetrating network hydrogel from poly (acrylic acid-co-hydroxyethyl methacrylate) and sodium alginate: Modeling and kinetics study for removal of synthetic dyes from water. Carbohydr. Polym. 2013, 98, 257–269. [Google Scholar] [CrossRef]
- Attallah, O.A.; Al-Ghobashy, M.A.; Nebsen, M.; Salem, M.Y. Adsorptive Removal of Fluoroquinolones from Water by Pectin-Functionalized Magnetic Nanoparticles: Process Optimization Using a Spectrofluorimetric Assay. ACS Sustain. Chem. Eng. 2017, 5, 133–145. [Google Scholar] [CrossRef]
- Cao, W.Q.; Song, J.; Yang, G.P. An adsorption and thermodynamic study of ofloxacin on marine sediments. Environ. Chem. 2017, 14, 350–360. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Ji, Z.; Wu, J.; Shen, X.; Zhou, H.; Xi, H. Preparation and characterization of graphene/NiO nanocomposites. J. Mater. Sci. 2011, 46, 1190–1195. [Google Scholar] [CrossRef]
- Asthana, A.; Verma, R.; Singh, A.K.; Susan, M.A.B.H. Glycine functionalized magnetic nanoparticle entrapped calcium alginate beads: A promising adsorbent for removal of Cu(II) ions. J. Environ. Chem. Eng. 2016, 4, 1985–1995. [Google Scholar] [CrossRef]
- Yadav, S.; Asthana, A.; Chakraborty, R.; Jain, B.; Singh, A.K.; Carabineiro, S.A.C.; Susan, M.A.B.H. Cationic Dye Removal Using Novel Magnetic/Activated Charcoal/β-Cyclodextrin/Alginate Polymer Nanocomposite. Nanomaterials 2020, 10, 170. [Google Scholar] [CrossRef] [Green Version]
- Ding, Y.; Tian, Z.; Li, H.; Wang, X. Efficient removal of organic dyes using a three-dimensional graphene aerogel with excellent. New Carbon Mater. 2019, 34, 315–324. [Google Scholar] [CrossRef]
- Orth, E.S.; Fonsaca, J.E.S.; Domingues, S.H.; Mehl, H.; Oliveira, M.M.; Zarbin, A.J.G. Targeted Thiolation of Graphene Oxide and its Utilization as Precursor for Graphene/Silver Nanoparticles Composites. Carbon 2013, 61, 543–550. [Google Scholar] [CrossRef]
- Deng, M.; Huang, Y.; Zhang, X.; Feng, Z.; Gou, J.; Sun, B. Preparation of a Novel Chelating Resin Bearing amidino thiourea Moieties and its Removal Properties for Hg(II) Ions in Aqueous Solution. Sep. Sci. Technol. 2016, 51, 1499–1508. [Google Scholar]
- Rambabu, G.; Bhat, S.D. Amino acid functionalized graphene oxide based nanocomposite membrane electrolytes for direct methanol fuel cells. J. Membr. Sci. 2018, 551, 1–11. [Google Scholar] [CrossRef]
- Chandraker, K.; Nagwanshi, R.; Jadhav, S.K.; Ghosh, K.K.; Satnami, M.L. Antibacterial properties of amino acid functionalized silver nanoparticles decorated on graphene oxide sheets. Spectrochim. Acta Part A 2017, 181, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Yap, P.L.; Kabiri, S.; Tran, D.N.H.; Losic, D. Multifunctional Binding Chemistry on Modified Graphene Composite for Selective and Highly Efficient Adsorption of Mercury. ACS Appl. Mater. Inter. 2019, 11, 6350–6362. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Zhang, Y.; Chu, W.; Chen, Z.; Wang, J. Adsorptive removal of antibiotics from water using peanut shells from agricultural waste. RSC Adv. 2018, 8, 13546–13555. [Google Scholar] [CrossRef] [Green Version]
- Ehtesabia, H.; Bagheria, Z.; Avini, M.Y. Application of three-dimensional graphene hydrogels for removal of ofloxacin from aqueous solutions. Environ. Nanotechnol. Monitor. Manag. 2019, 12, 100274. [Google Scholar] [CrossRef]
- Xu, Y.; Bai, H.; Lu, G.; Li, C.; Shi, G. Flexible graphene film via the filtration of water-soluble noncovalent functionalized graphene sheets. J. Am. Chem. Soc. 2008, 130, 5856–5857. [Google Scholar] [CrossRef]
- Verma, S.; Mungse, H.P.; Kumar, N.; Choudhary, S.; Jain, S.L.; Sain, B.; Khatri, O.P. Graphene oxide: An efficient and reusable carbocatalyst for Aza-Michael addition of amines to activated alkenes. Chem. Commun. 2011, 47, 12673–12675. [Google Scholar] [CrossRef] [PubMed]
- Rishi, K.; Rana, N. Particle size and shape analysis using image J with customized tool for segmentation of particles. Int. J. Comput. Sci. Commun. Netw. 2015, 4, 23–28. [Google Scholar]
- Qiusheng, Z.; Xiaoyan, L.; Jin, Q.; Jing, W.; Xuegang, L. Porous zirconium alginate beads adsorbent for fluoride adsorption from aqueous solutions. RSC Adv. 2015, 5, 2100–2112. [Google Scholar] [CrossRef]
- Yadav, S.; Goel, N.; Kumar, V.; Tikoo, K.; Singhal, S. Removal of fluoroquinolone from aqueous solution using graphene oxide: Experimental and computational elucidation. Environ. Sci. Pollut. Res. 2018, 25, 2942–2957. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Lang, Y.; Liu, B.; Zhao, H. Ofloxacin adsorption on chitosan/biochar composite: Kinetics, isotherms, and effects of solution chemistry. Polycycl. Aromat. Compd. 2019, 39, 287–297. [Google Scholar] [CrossRef]
- Tang, Y.; Guo, H.; Xiao, L.; Yu, S.; Gao, N.; Wang, Y. Synthesis of reduced graphene oxide/magnetite composites and investigation of their adsorption performance of fluoroquinolone antibiotics. Colloids Surf. A 2013, 424, 74–80. [Google Scholar] [CrossRef]
- Bangari, R.S.; Sinha, N. Adsorption of tetracycline, ofloxacin and cephalexin antibiotics on boron nitride nanosheets from aqueous solution. J. Mol. Liq. 2019, 293, 111376–111388. [Google Scholar] [CrossRef]
- Nogueira, J.; António, M.; Mikhalev, S.M.; Fateixa, S.; Trindade, T.; Silva, A.L.D. Porous Carrageenan-Derived Carbons for Efficient Ciprofloxacin Removal from Water. Nanomaterials 2018, 8, 1004. [Google Scholar] [CrossRef] [Green Version]
- Futalan, C.M.; Tsai, W.C.; Lin, S.S.; Hsien, K.J.; Dalida, M.L.; Wan, M.W. Copper, nickel and lead adsorption from aqueous solution using chitosan-immobilized on bentonite in ternary system. Sustain. Environ. Res. 2012, 22, 345–355. [Google Scholar]
- Langmuir, I. The constitution and fundamental properties of solids and liquids. Part I. Solids. J. Am. Chem. Soc. 1916, 38, 2221–2295. [Google Scholar] [CrossRef] [Green Version]
- Qureshi, T.; Memon, N.; Memon, S.Q.; Ashraf, M.A. Decontamination of ofloxacin: Optimization of removal process onto sawdust using response surface methodology. Desalin. Water Treat. 2016, 57, 221–229. [Google Scholar] [CrossRef]
- Freundlich, H. Uber die adsorption in losungen. Z. Phys. Chem. 1906, 57, 85–470. [Google Scholar] [CrossRef]
- Brasquet, C.; Subrenat, E.; Cloirec, P. Selective adsorption on fibrous activated carbon of organics from aqueous solution: Correlation between adsorption and molecular structure. Water Sci. Technol. 1997, 35, 251–259. [Google Scholar] [CrossRef]
- Legergren, S. About the Theory of so-Called Adsorption of Soluble Substances. K. Sven. Vetenskapsakad. Handl. Band 1898, 24, 1–39. [Google Scholar]
- Ho, Y.S.; McKay, G. Pseudo-Second Order Model for Sorption Processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Weber, W.J.; Morris, J.C. Kinetics of Adsorption on Carbon from Solution. J. San. Eng. Div. ASCE 1963, 89, 31–59. [Google Scholar] [CrossRef]
- Ji, L.L.; Chen, W.; Duan, L.; Zhu, D.Q. Mechanisms for strong adsorption of tetracycline to carbon nanotubes: A comparative study using activated carbon and graphite as adsorbents. Environ. Sci. Technol. 2009, 7, 2322–2327. [Google Scholar] [CrossRef]
- Ofomaja, A.E.; Naidoo, E.B.; Modise, S.J. Kinetic and pseudo-second-order modelling of lead biosorption onto pine cone powder. Ind. Eng. Chem. Res. 2010, 49, 2562–2572. [Google Scholar] [CrossRef]
- Liu, H.; Liu, W.; Zhang, J.; Zhang, C.; Ren, L.; Li, Y. Removal of cephalexin from aqueous solutions by original and Cu(II)/Fe(III) impregnated activated carbons developed from lotus stalks Kinetics and equilibrium studies. J. Hazard. Mater. 2011, 185, 1528–1535. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Li, P.; Yang, L.; Wu, L.; He, L.; Gao, F.; Qi, X.; Zhang, Z. Iron/zinc and phosphoric acid modified sludge biochar as an efficient adsorbent for fluoroquinolones antibiotics removal. Ecotoxicol. Environ. Saf. 2020, 196, 110550–110560. [Google Scholar] [CrossRef] [PubMed]
- Wuana, R.A.; Sha’Ato, R.; Iorhen, S. Aqueous phase removal of ofloxacin using adsorbents from Moringa oleifera pod husks. Adv. Environ. Res. 2015, 4, 49–68. [Google Scholar] [CrossRef]
- Sengil, I.A.; Ozacar, M. Biosorption of Cu(II) from aqueous solutions by mimosa tannin gel. J. Hazard. Mater. 2008, 157, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Kong, Q.; He, X.; Shu, L.; Miao, M.S. Ofloxacin adsorption by activated carbon derived from luffa sponge: Kinetic, isotherm, and thermodynamic analyses. Process Saf. Environ. Prot. 2017, 112, 254–264. [Google Scholar] [CrossRef]
- Ma, J.; Sun, Y.; Yu, F. Efficient removal of tetracycline with KOH-activated graphene from aqueous solution. R. Soc. Open Sci. 2017, 4, 170731–170742. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Gao, B.; Li, H. Removal of sulfamethoxazole and ciprofloxacin from aqueous solutions by graphene oxide. J. Hazard. Mater. 2015, 282, 201–207. [Google Scholar] [CrossRef]
- Wang, Y.; Lu, J.; Wu, J.; Liu, Q.; Zhang, H.; Jin, S. Adsorptive Removal of Fluoroquinolone Antibiotics Using Bamboo Biochar. Sustainability 2015, 7, 12947–12957. [Google Scholar] [CrossRef] [Green Version]
- Rubashvili, I.; Eprikashvili, L.; Kordzakhia, T.; Zautashvili, M.; Pirtskhalava, N.; Dzagania, M. Adsorptive Removal Study of the Frequently Used Fluoroquinolone Antibiotics—Moxifloxacin and Norfloxacin from Wastewaters using Natural Zeolites. Mediterr. J. Chem. 2019, 9, 142–154. [Google Scholar] [CrossRef] [Green Version]
- Pouretedal, H.R.; Sadegh, N. Effective removal of Amoxicillin, Cephalexin, Tetracycline and Penicillin G from aqueous solutions using activated carbon nanoparticles prepared from vine wood. J. Water Process Eng. 2014, 1, 64–73. [Google Scholar] [CrossRef]
- Elhussien, M.E.; Lraheem, M.A.A.; Hussein, R.M.; Elsaim, M.H. Removal of Ciprofloxacin Hydrochloride from Aqueous Solution by Pomegranate Peel Grown in Alziedab Agricultural Scheme—River Nile State, Sudan. Adv. Biochem. 2017, 5, 89–96. [Google Scholar] [CrossRef]
- Zhang, C.L.; Qiao, G.L.; Zhao, F.; Wang, Y. Thermodynamic and kinetic parameters of ciprofloxacin adsorption onto modified coal fly ash from aqueous solution. J. Mol. Liq. 2011, 163, 53–56. [Google Scholar] [CrossRef]
- Li, S.; Fang, L.; Ye, M.; Zhang, Y. Enhanced adsorption of norfloxacin on modified TiO2 particles prepared via surface molecular imprinting technique. Desalin. Water Treat. 2016, 57, 408–418. [Google Scholar]
- Chen, Y.; Lan, T.; Duan, L.; Wang, F.; Zhao, B.; Zhang, S.; Wei, W. Adsorptive Removal and Adsorption Kinetics of Fluoroquinolone by Nano-Hydroxyapatite. PLoS ONE. 2015, 10, e0145025. [Google Scholar] [CrossRef]
- Yadav, S.; Asthana, A.; Singh, A.K.; Chakraborty, R.; Vidya, S.S.; Susan, M.A.B.H.; Carabineiro, S.A.C. Adsorption of cationic dyes, drugs and metal from aqueous solutions using a polymer composite of magnetic/β-cyclodextrin/activated charcoal/Na alginate: Isotherm, kinetics and regeneration studies. J. Hazard. Mater. 2021, 409, 124840–124861. [Google Scholar] [CrossRef]
- Hassan, S.A.; Ali, F.J. Assessement of the Ofloxacin (Novecin) Adsorption from aqueous solutions by Two Agricultural Wastes. Int. J. Adv. Sci. Tech. Res. 2014, 2, 950–965. [Google Scholar]
- Goyne, K.W.; Chorover, J.; Kubicki, J.D.; Zimmerman, A.R.; Brantley, S.L. Sorption of the antibiotic ofloxacin to mesoporous and nonporous alumina and silica. J. Colloid Interf. Sci. 2005, 283, 160–170. [Google Scholar] [CrossRef]
- Yu, Z.; Hu, C.; Dichiara, A.B.; Jiang, W.; Gu, J. Cellulose Nanofibril/Carbon Nanomaterial Hybrid Aerogels for Adsorption Removal of Cationic and Anionic Organic Dyes. Nanomaterials 2020, 10, 169. [Google Scholar] [CrossRef] [Green Version]
- Ahammad, N.A.; Zulkifli, M.A.; Ahmad, M.A.; Hameed, B.H.; Din, A.T.M. Desorption of chloramphenicol from ordered mesoporous carbon-alginate beads: Effects of operating parameters, and isotherm, kinetics, and regeneration studies. J. Environ. Chem. Eng. 2021, 9, 105015–105026. [Google Scholar] [CrossRef]
















| FQ Antibiotic | Structure | Formula | Weight (g mol−1) | λmax (nm) | Ref. |
|---|---|---|---|---|---|
| Moxifloxacin |  | C21H24FN3O4 | 401.431 | 290 | [32] |
| Ofloxacin |  | C18H20FN3O4 | 361.368 | 288 | [33] |
| Materials | 2θ (°) | FWHM | d-Spacing (Å) | Size (nm) |
|---|---|---|---|---|
| GO | 10.26 20.13 42.51 | 1.15 7.27 5.02 | 10.40 | 3.29 |
| Met-GO | 9.21 18.51 21.74 42.48 | 0.92 4.98 3.91 2.38 | 10.71 | 3.92 |
| Met-GO/SA | 10.05 20.92 42.59 | 7.13 6.46 6.38 | 10.43 | 1.19 |
| Isotherm Models | Moxifloxacin (MOX) | Ofloxacin (OFX) | |||
|---|---|---|---|---|---|
| GO/SA | Met-GO/SA | GO/SA | Met-GO/SA | ||
| Langmuir model | qm (mg/g) | 2.00 | 4.115 | 1.798 | 3.436 |
| KL (L/mg) | 0.157 | 0.101 | 0.108 | 0.147 | |
| RL | 0.241 | 0.331 | 0.316 | 0.253 | |
| R2 | 0.963 | 0.965 | 0.979 | 0.827 | |
| Freundlich model | KF (mg/g)(mg/L)n | 1.640 | 2.844 | 1.132 | 5.675 |
| n | 1.655 | 1.644 | 1.589 | 2.00 | |
| R2 | 0.947 | 0.905 | 0.967 | 0.754 | |
| Kinetic Models | Parameters | Moxifloxacin (MOX) | Ofloxacin (OFX) | ||
|---|---|---|---|---|---|
| GO/SA | Met-GO/SA | GO/SA | Met-GO/SA | ||
| Pseudo-first order | k1 | 0.021 | 0.037 | 0.024 | 0.014 |
| qe (mg/g) | 1.595 | 1.208 | 1.493 | 1.203 | |
| R2 | 0.879 | 0.992 | 0.893 | 0.954 | |
| Pseudo-second order | k2 | 0.057 | 0.040 | 0.004 | 0.026 |
| qe (mg/g) | 0.854 | 1.552 | 1.254 | 1.414 | |
| R2 | 0.996 | 0.998 | 0.986 | 0.977 | |
| Intraparticle diffusion model | kid (mg/g/min) | 0.028 | 0.067 | 0.058 | 0.072 |
| C | 0.400 | 0.620 | 0.115 | 0.415 | |
| R2 | 0.962 | 0.942 | 0.990 | 0.954 | |
| Adsorbate | ΔH° (kJ/mol) | ΔS° (J/mol/K) | ΔG° (kJ/mol) | ||
|---|---|---|---|---|---|
| 308 K | 318 K | 328 K | |||
| MOX | −24.19 | −62.59 | −4.573 | −3.923 | −3.283 |
| OFX | −15.38 | −35.63 | −4.094 | −4.013 | −3.351 |
| Adsorbent | FQ Antibiotic | qmax (mg/g) | Reference |
|---|---|---|---|
| Natural Clinoptilolite Clinoptilolite H-Form | Moxifloxacin Moxifloxacin | 1.72 2.71 | [73] |
| Activated carbon nanoparticles (AC) | Tetracycline | 1.98 | [74] |
| AC(PPZ)KOH | Ciprofloxacin | 2.353 | [75] |
| Modified coal fly ash | Ciprofloxacin | 1.547 | [76] |
| MIPs | Norfloxacin | 2.99 | [77] |
| Nano- Hydroxyapatite | Norfloxacin Ciprofloxacin | 1.486 1.271 | [78] |
| Fe3O4/CD/AC/SA | Norfloxacin Ciprofloxacin | 2.551 3.125 | [79] |
| Spent black tea leaves (SBTL) | Ofloxacin | −3.356 | [80] |
| Nonporous SiO2 | Ofloxacin | 2.1 | [81] |
| GO/SA | Moxifloxacin Ofloxacin | 2.0 1.798 | This study |
| Met-GO/SA | Moxifloxacin Ofloxacin | 4.115 3.436 | This study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yadav, S.; Asthana, A.; Singh, A.K.; Chakraborty, R.; Vidya, S.S.; Singh, A.; Carabineiro, S.A.C. Methionine-Functionalized Graphene Oxide/Sodium Alginate Bio-Polymer Nanocomposite Hydrogel Beads: Synthesis, Isotherm and Kinetic Studies for an Adsorptive Removal of Fluoroquinolone Antibiotics. Nanomaterials 2021, 11, 568. https://doi.org/10.3390/nano11030568
Yadav S, Asthana A, Singh AK, Chakraborty R, Vidya SS, Singh A, Carabineiro SAC. Methionine-Functionalized Graphene Oxide/Sodium Alginate Bio-Polymer Nanocomposite Hydrogel Beads: Synthesis, Isotherm and Kinetic Studies for an Adsorptive Removal of Fluoroquinolone Antibiotics. Nanomaterials. 2021; 11(3):568. https://doi.org/10.3390/nano11030568
Chicago/Turabian StyleYadav, Sushma, Anupama Asthana, Ajaya Kumar Singh, Rupa Chakraborty, S. Sree Vidya, Ambrish Singh, and Sónia A. C. Carabineiro. 2021. "Methionine-Functionalized Graphene Oxide/Sodium Alginate Bio-Polymer Nanocomposite Hydrogel Beads: Synthesis, Isotherm and Kinetic Studies for an Adsorptive Removal of Fluoroquinolone Antibiotics" Nanomaterials 11, no. 3: 568. https://doi.org/10.3390/nano11030568
APA StyleYadav, S., Asthana, A., Singh, A. K., Chakraborty, R., Vidya, S. S., Singh, A., & Carabineiro, S. A. C. (2021). Methionine-Functionalized Graphene Oxide/Sodium Alginate Bio-Polymer Nanocomposite Hydrogel Beads: Synthesis, Isotherm and Kinetic Studies for an Adsorptive Removal of Fluoroquinolone Antibiotics. Nanomaterials, 11(3), 568. https://doi.org/10.3390/nano11030568