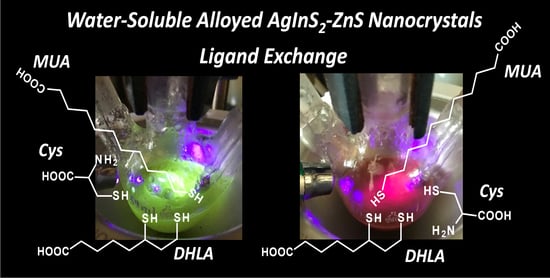
Organic-to-Aqueous Phase Transfer of Alloyed AgInS2-ZnS Nanocrystals Using Simple Hydrophilic Ligands: Comparison of 11-Mercaptoundecanoic Acid, Dihydrolipoic Acid and Cysteine
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of AgInS2-ZnS Nanocrystals (R-“Red” and G-“Green” Types)
2.3. Primary Ligand Exchange for 11-Mercaptoundecanoic Acid (MUA)
2.4. Primary Ligand Exchange for Dihydrolipoic Acid (DHLA)
2.5. Primary Ligand Exchange for L-Cysteine (Cys)
2.6. Characterization
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Talapin, D.V.; Lee, J.-S.; Kovalenko, M.V.; Shevchenko, E.V. Prospects of Colloidal Nanocrystals for Electronic and Optoelectronic Applications. Chem. Rev. 2010, 110, 389–458. [Google Scholar] [CrossRef]
- Aldakov, D.; Lefrançois, A.; Reiss, P. Ternary and Quaternary Metal Chalcogenide Nanocrystals: Synthesis, Properties and Applications. J. Mater. Chem. C 2013, 1, 3756–3776. [Google Scholar] [CrossRef]
- Reiss, P.; Carrière, M.; Lincheneau, C.; Vaure, L.; Tamang, S. Synthesis of Semiconductor Nanocrystals, Focusing on Nontoxic and Earth-Abundant Materials. Chem. Rev. 2016, 18, 10731–10819. [Google Scholar] [CrossRef]
- Jing, L.; Kershaw, S.V.; Li, Y.; Huang, X.; Li, Y.; Rogach, A.L.; Gao, M. Aqueous Based Semiconductor Nanocrystals. Chem. Rev. 2016, 116, 10623–10730. [Google Scholar] [CrossRef] [PubMed]
- Bujak, P. Core and Surface Engineering in Binary, Ternary and Quaternary Semiconductor Nanocrystals – A Critical Review. Synth. Met. 2016, 222, 93–114. [Google Scholar] [CrossRef]
- Coughlan, C.; Ibáñez, M.; Dobrozhan, O.; Singh, A.; Cabot, A.; Ryan, K.M. Compound Copper Chalcogenide Nanocrystals. Chem. Rev. 2017, 117, 5865–6109. [Google Scholar] [CrossRef]
- Girma, W.M.; Fahmi, M.Z.; Permadi, A.; Abate, M.A.; Chang, J.-Y. Synthetic Strategies and Biomedical Applications of I-III-VI Ternary Quantum Dots. J. Mater. Chem. B 2017, 5, 6193–6216. [Google Scholar] [CrossRef]
- Sobiech, M.; Bujak, P.; Luliński, P.; Pron, A. Semiconductor Nanocrystal-Polymer Hybrid Nanomaterials and their Application in Molecular Imprinting. Nanoscale 2019, 11, 12030–12074. [Google Scholar] [CrossRef] [PubMed]
- Moodelly, D.; Kowalik, P.; Bujak, P.; Pron, A.; Reiss, P. Synthesis, Photophysical Properties and Surface Chemistry of Chalcopyrite-Type Semiconductor Nanocrystals. J. Mater. Chem. C 2019, 7, 11665–11709. [Google Scholar] [CrossRef]
- Palui, G.; Aldeek, F.; Wang, W.; Mattoussi, H. Strategies for Interfacing Inorganic Nanocrystals with Biological Systems Based on Polymer-Coating. Chem. Soc. Rev. 2015, 44, 193–227. [Google Scholar] [CrossRef] [PubMed]
- Reiss, P.; Protière, M.; Li, L. Core/Shell Semiconductor Nanocrystals. Small 2009, 5, 154–168. [Google Scholar] [CrossRef]
- Derfus, A.M.; Chan, W.C.W.; Bhatia, S.N. Probing the Cytotoxicity of Semiconductor Quantum Dots. Nano Lett. 2004, 4, 11–18. [Google Scholar] [CrossRef]
- Kirchner, C.; Liedl, T.; Kudera, S.; Pellegrino, T.; Javier, A.M.; Gaub, H.E.; Stölzle, S.; Fertig, N.; Parak, W.J. Cytotoxicity of Colloidal CdSe and CdSe/ZnS Nanoparticles. Nano Lett. 2005, 5, 331–338. [Google Scholar] [CrossRef]
- Ye, L.; Yong, K.-T.; Liu, L.; Roy, I.; Hu, R.; Zhu, J.; Cai, H.; Law, W.-C.; Liu, J.; Wang, K.; et al. A Pilot Study in Non-Human Primates Shows No Adverse Response to Intravenous Injection of Quantum Dots. Nat. Nanotechnol. 2012, 7, 453–458. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Ma, C.; Zhang, W.; Yang, S.; Wang, S.; Lv, L.; Zhu, L.; Xia, R.; Xu, X. Tumor Cell-Targeted Zn3In2S6 and Ag-In-Zn-S Quantum Dots for Color Adjustable Luminophores. J. Mater. Chem. B 2016, 4, 7909–7918. [Google Scholar] [CrossRef]
- Song, J.; Ma, C.; Zhang, W.; Li, X.; Zhang, W.; Wu, R.; Cheng, X.; Ali, A.; Yang, M.; Zhu, L.; et al. Bandgap and Structure Engineering via Cation Exchange: From Binary Ag2S to Ternary AgInS2, Quaternary AgZnInS alloy and AgZnInS/ZnS Core/Shell Fluorescent Nanocrystals for Bioimaging. ACS Appl. Mater. Interfaces 2016, 8, 24826–24836. [Google Scholar] [CrossRef] [PubMed]
- Matysiak-Brynda, E.; Bujak, P.; Augustin, E.; Kowalczyk, A.; Mazerska, Z.; Pron, A.; Nowicka, A.M. Stable Nanoconjugates of Transferrin with Alloyed Quaternary Nanocrystals Ag-In-Zn-S as a Biological Entity for Tumor Recognition. Nanoscale 2018, 10, 1286–1296. [Google Scholar] [CrossRef]
- Pilch, J.; Matysiak-Brynda, E.; Kowalczyk, A.; Bujak, P.; Mazerska, Z.; Nowicka, A.M.; Augustin, E. New Unsymmetrical Bisacridine Derivatives Noncovalently Attached to Quaternary Quantum Dots Improve Cancer Therapy by Enhancing Cytotoxicity toward Cancer Cells and Protecting Normal Cells. ACS Appl. Mater. Interfaces 2020, 12, 17276–17289. [Google Scholar] [CrossRef]
- Delices, A.; Moodelly, D.; Hurot, C.; Hou, Y.; Ling, W.L.; Saint-Pierre, C.; Gasparutto, D.; Nogues, G.; Reiss, P.; Kheng, K. Aqueous Synthesis of DNA-Functionalized Near-Infrared AgInS2/ZnS Core/Shell Quantum Dots. ACS Appl. Mater. Interfaces 2020, 12, 44026–44038. [Google Scholar] [CrossRef]
- Pilch, J.; Kowalik, P.; Bujak, P.; Nowicka, A.M.; Augustin, E. Quantum Dots as a Good Carriers of Unsymmetrical Bisacridines for Modulating Cellular Uptake and the Biological Response in Lung and Colon Cancer Cells. Nanomaterials 2021, 11, 462. [Google Scholar] [CrossRef] [PubMed]
- Ruzycka-Ayoush, M.; Kowalik, P.; Kowalczyk, A.; Bujak, P.; Nowicka, A.M.; Wojewodzka, M.; Kruszewski, M.; Grudzinski, I.P. Quantum Dots as Targeted Doxorubicin Drug Delivery Nanosystems. Cancer Nanotechnol. 2021, 12, 8. [Google Scholar] [CrossRef]
- Gabka, G.; Bujak, P.; Giedyk, K.; Ostrowski, A.; Malinowska, K.; Herbich, J.; Golec, B.; Wielgus, I.; Pron, A. A Simple Route to Alloyed Quaternary Nanocrystals Ag-In-Zn-S with Shape and Size Control. Inorg. Chem. 2014, 53, 5002–5012. [Google Scholar] [CrossRef] [PubMed]
- Gabka, G.; Bujak, P.; Kotwica, K.; Ostrowski, A.; Lisowski, W.; Sobczak, J.W.; Pron, A. Luminophores of Tunable Colors from Ternary Ag-In-S and Quaternary Ag-In-Zn-S Nanocrystals Covering the Visible to Near-Infrared Spectral Range. Phys. Chem. Chem. Phys. 2017, 19, 1217–1228. [Google Scholar] [CrossRef]
- Bujak, P.; Wróbel, Z.; Penkala, M.; Kotwica, K.; Kmita, A.; Gajewska, M.; Ostrowski, A.; Kowalik, P.; Pron, A. Highly Luminescent Ag-In-Zn-S Quaternary Nanocrystals: Growth Mechanism and Surface Chemistry Elucidation. Inorg. Chem. 2019, 58, 1358–1370. [Google Scholar] [CrossRef] [PubMed]
- Kowalik, P.; Penkala, M.; Bujak, P.; Kmita, A.; Gajewska, M.; Ostrowski, A.; Slodek, A.; Pron, A. From Ag2S to Luminescent Ag-In-S Nanocrystals via an Ultrasonic Method—An in situ Synthesis Study in an NMR Tube. J. Mater. Chem. C 2020, 8, 8942–8952. [Google Scholar] [CrossRef]
- Gabka, G.; Bujak, P.; Gryszel, M.; Kotwica, K.; Pron, A. Anchor Groups Effect on Spectroscopic and Electrochemical Properties of Quaternary Nanocrystals Cu-In-Zn-S Capped with Arylamine Derivatives. J. Phys. Chem. C 2015, 119, 9656–9664. [Google Scholar] [CrossRef]
- Tamang, S.; Beaune, G.; Texier, I.; Reiss, P. Aqueous Phase Transfer of InP/ZnS Nanocrystals Conserving Fluorescence and High Colloidal Stability. ACS Nano 2011, 9392–9402. [Google Scholar] [CrossRef]
- Zhang, W.; Zhong, X. Facile Synthesis of ZnS-CuInS2-Alloyed Nanocrystals for a Color-Tunable Fluorchrome and Photocatalyst. Inorg. Chem. 2011, 50, 4065–4072. [Google Scholar] [CrossRef] [PubMed]
- Mattoussi, H.; Mauro, J.M.; Goldman, E.R.; Anderson, G.P.; Sundar, V.C.; Mikulec, F.V.; Bawendi, M.G. Self-Assembly of CdSe-ZnS Quantum Dot Bioconjugates Using an Engineered Recombinant Protein. J. Am. Chem. Soc. 2000, 122, 12142–12150. [Google Scholar] [CrossRef]
- Fang, Z.; Liu, L.; Xu, L.L.; Yin, X.G.; Zhong, X.H. Synthesis of Highly Stable Dihydrolipoic Acid Capped Water-Soluble CdTe Nanocrystals. Nanotechnology 2008, 19, 235603. [Google Scholar] [CrossRef]
- Uyeda, H.T.; Medintz, I.L.; Jaiswal, J.K.; Simon, S.M.; Mattoussi, H. Synthesis of Compact Multidentate Ligands to Prepare Stable Hydrophilic Quantum Dot Fluorophores. J. Am. Chem. Soc. 2005, 127, 3870–3878. [Google Scholar] [CrossRef]
- Susumu, K.; Uyeda, H.T.; Medintz, I.L.; Pons, T.; Delehanty, J.B.; Mattoussi, H. Enhancing the Stability and Biological Functionalities of Quantum Dots via Compact Multifunctional Ligands. J. Am. Chem. Soc. 2007, 129, 13987–13996. [Google Scholar] [CrossRef]
- Liu, W.; Howarth, M.; Greytak, A.B.; Zheng, Y.; Nocera, D.G.; Ting, A.Y.; Bawendi, M.G. Compact Biocompatible Quantum Dots Functionalized for Cellular Imaging. J. Am. Chem. Soc. 2008, 130, 1274–1284. [Google Scholar] [CrossRef]
- Mei, B.C.; Susumu, K.; Medintz, I.L.; Delehanty, J.B.; Mountziaris, T.J.; Mattoussi, H. Modular Poly(ethylene glycol) Ligands for Biocompatible Semiconductor and Gold Nanocrystals with Extended pH and Ionic Stability. J. Mater. Chem. 2008, 18, 4949–4958. [Google Scholar] [CrossRef]
- Stewart, M.H.; Susumu, K.; Mei, B.C.; Medintz, I.L.; Delehanty, J.B.; Blanco-Canosa, J.B.; Dawson, P.E.; Mattoussi, H. Multidentate Poly(ethylene glycol) Ligands Provide Colloidal Stability to Semiconductor and Metallic Nanocrystals in Extreme Conditions. J. Am. Chem. Soc. 2010, 132, 9804–9813. [Google Scholar] [CrossRef] [PubMed]
- Palui, G.; Avellini, T.; Zhan, N.; Pan, F.; Gray, D.; Alabugin, I.; Mattoussi, H. Photoinduced Phase Transfer of Luminescent Quantum Dots to Polar and Aqueous Media. J. Am. Chem. Soc. 2012, 134, 16370–16378. [Google Scholar] [CrossRef] [PubMed]
- Zhan, N.; Palui, G.; Mattoussi, H. Preparation of Compact Biocompatible Quantum Dots Using Multicoordinating Molecular-Scale Ligands Based on a Zwitterionic Hydrophilic Motif and Lipoic Acid Anchors. Nat. Protoc. 2015, 10, 859–874. [Google Scholar] [CrossRef]
- Gunsalus, I.C.; Barton, L.S.; Gruber, W. Biosynthesis and Structure of Lipoic Acid Derivatives. J. Am. Chem. Soc. 1956, 78, 1763–1766. [Google Scholar] [CrossRef]
- Roux, S.; Garcia, B.; Bridot, J.-L.; Salomé, M.; Marquette, C.; Lemelle, L.; Gillet, P.; Blum, L.; Perriat, P.; Tillement, O. Synthesis, Characterization of Dihydrolipoic Acid Capped Gold Nanoparticles, and Functionalization by the Electroluminescent Luminol. Langmuir 2005, 21, 2526–2536. [Google Scholar] [CrossRef]
- Kowalik, P.; Bujak, P.; Wróbel, Z.; Penkala, M.; Kotwica, K.; Maroń, A.; Pron, A. From Red to Green Luminescence via Surface Functionalization. Effect of 2-(5-Mercaptothien-2-yl)-8(thien-2-yl)-5-hexylthieno[3,4-c]pyrrole-4,6-dione Ligands on the Photoluminescence of Alloyed Ag-In-Zn-S Nanocrystals. Inorg. Chem. 2020, 59, 14594–14604. [Google Scholar] [CrossRef] [PubMed]






| Ligand | Ag/In/Zn/S(S) | %wt. C | %wt. S | %wt. N | Size (nm) | PL (nm) | Q.Y.(%) | |
|---|---|---|---|---|---|---|---|---|
| Red (R) | Initial | 1.00/2.80/1.30/4.00(6.00) | 57.6 | 5.1 | - | 6.2 ± 0.9 | 720 | 67.0 |
| MUA | 1.00/1.00/1.10/3.50(3.10) | 42.9 | 14.0 | - | 5.6 ± 0.8 | 730 | 30.0 | |
| DHLA | 1.00/1.00/1.00/3.30(3.00) | 8.7 | 23.0 | - | 4.6 ± 0.6 | 672 | 40.0 | |
| Cys | 1.00/1.00/0.80/19.40(2.80) | 26.7 | 22.5 | 6.4 | 4.3 ± 0.7 | 707 | 28.0 | |
| Green (G) | Initial | 1.00/1.50/7.80/17.0(10.50) | 55.3 | 11.5 | - | 4.2 ± 0.6 | 543 | 48.0 |
| MUA | 1.00/1.20/5.60/9.40(7.90) | 50.2 | 13.2 | - | 3.7 ± 0.6 | 576 | 25.0 | |
| DHLA | 1.00/1.00/8.80/25.10(10.80) | 20.9 | 20.7 | - | 3.6 ± 0.7 | 540 | 28.0 | |
| Cys | 1.00/5.80/12.10/126.2(21.3) | 24.6 | 20.9 | 6.3 | 3.7 ± 1.0 | 524 | 16.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kowalik, P.; Bujak, P.; Penkala, M.; Pron, A. Organic-to-Aqueous Phase Transfer of Alloyed AgInS2-ZnS Nanocrystals Using Simple Hydrophilic Ligands: Comparison of 11-Mercaptoundecanoic Acid, Dihydrolipoic Acid and Cysteine. Nanomaterials 2021, 11, 843. https://doi.org/10.3390/nano11040843
Kowalik P, Bujak P, Penkala M, Pron A. Organic-to-Aqueous Phase Transfer of Alloyed AgInS2-ZnS Nanocrystals Using Simple Hydrophilic Ligands: Comparison of 11-Mercaptoundecanoic Acid, Dihydrolipoic Acid and Cysteine. Nanomaterials. 2021; 11(4):843. https://doi.org/10.3390/nano11040843
Chicago/Turabian StyleKowalik, Patrycja, Piotr Bujak, Mateusz Penkala, and Adam Pron. 2021. "Organic-to-Aqueous Phase Transfer of Alloyed AgInS2-ZnS Nanocrystals Using Simple Hydrophilic Ligands: Comparison of 11-Mercaptoundecanoic Acid, Dihydrolipoic Acid and Cysteine" Nanomaterials 11, no. 4: 843. https://doi.org/10.3390/nano11040843
APA StyleKowalik, P., Bujak, P., Penkala, M., & Pron, A. (2021). Organic-to-Aqueous Phase Transfer of Alloyed AgInS2-ZnS Nanocrystals Using Simple Hydrophilic Ligands: Comparison of 11-Mercaptoundecanoic Acid, Dihydrolipoic Acid and Cysteine. Nanomaterials, 11(4), 843. https://doi.org/10.3390/nano11040843