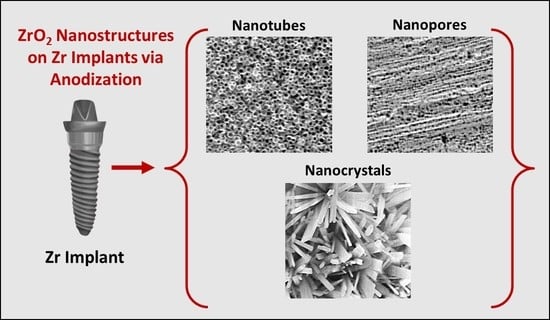
Towards Clinical Translation: Optimized Fabrication of Controlled Nanostructures on Implant-Relevant Curved Zirconium Surfaces
Abstract
:1. Introduction
- Fabrication optimization has only been restricted to planar Zr flat foil that is easy to manage. However, clinically used orthopaedic and dental implants are based on curved surfaces and edges, thereby limiting the clinical translation of conventional anodized Zr flat foil.
- Dental implants generally use microscale roughness which, to date, is regarded as a ‘gold standard’ for ensuring osseointegration. Thus, preserving rather than removal of this micro-roughness (which is routinely performed to fabricate nanotubes) is needed along with superimposition of nanostructures (dual micro–nano).
2. Experimental Section
2.1. Materials and Chemicals
2.2. Electrochemical Anodization (EA)
2.3. Surface Characterization
3. Results and Discussion
- (1)
- Curved substrate: radial/perpendicular growth of nanotubes outwards [29].
- (2)
- Internal stresses: due to uneven electric field distribution.
- (3)
- Mechanical stress: due to volume expansion and limited space for growth.
- (4)
- Weak spots: electrolyte penetration resulting in unstable/fragile anodic layers [35].
- (5)
- Substrate: micro-roughness further exacerbates the stresses/weak spots [36].
- (6)
- Nanotube collapse (or bundling): especially for longer tubes.
- (1)
- (2)
- Polishing the substrate prior to anodization using mechanical, chemical or electropolishing treatments (will reduce/remove micro-roughness) [37].
- (3)
- Reducing water content, voltage/current, or anodization time (may reduce diameter/length of anodized nanostructures due to reduced growth rates).
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhang, Y.; Chen, H.-X.; Duan, L.; Fan, J.-B. The Electronic Structures, Elastic Constants, Dielectric Permittivity, Phonon Spectra, Thermal Properties and Optical Response of Monolayer Zirconium Dioxide: A First-Principles Study. Thin Solid Film. 2021, 721, 138549. [Google Scholar] [CrossRef]
- Schünemann, F.H.; Galárraga-Vinueza, M.E.; Magini, R.; Fredel, M.; Silva, F.; Souza, J.C.M.; Zhang, Y.; Henriques, B. Zirconia Surface Modifications for Implant Dentistry. Mater. Sci. Eng. C 2019, 98, 1294–1305. [Google Scholar] [CrossRef]
- Rupp, F.; Liang, L.; Geis-Gerstorfer, J.; Scheideler, L.; Hüttig, F. Surface Characteristics of Dental Implants: A Review. Dent. Mater. 2018, 34, 40–57. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Gulati, K.; Arora, H.; Han, P.; Fournier, B.; Ivanovski, S. Race to Invade: Understanding Soft Tissue Integration at the Transmucosal Region of Titanium Dental Implants. Dent. Mater. 2021, in press. [Google Scholar] [CrossRef] [PubMed]
- Tuna, T.; Wein, M.; Swain, M.; Fischer, J.; Att, W. Influence of Ultraviolet Photofunctionalization on the Surface Characteristics of Zirconia-Based Dental Implant Materials. Dent. Mater. 2015, 31, e14–e24. [Google Scholar] [CrossRef] [PubMed]
- Honda, J.; Komine, F.; Kusaba, K.; Kitani, J.; Matsushima, K.; Matsumura, H. Fracture Loads of Screw-Retained Implant-Supported Zirconia Prostheses after Thermal and Mechanical Stress. J. Prosthodont. Res. 2020, 64, 313–318. [Google Scholar] [CrossRef] [PubMed]
- AlFarraj, A.A.; Aldosari, A.; Sukumaran, A.; Al Amri, M.D.; van Oirschot, A.J.A.B.; Jansen, J.A. A Comparative Study of the Bone Contact to Zirconium and Titanium Implants after 8 Weeks of Implantation in Rabbit Femoral Condyles. Odontology 2018, 106, 37–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sivaraman, K.; Chopra, A.; Narayan, A.I.; Balakrishnan, D. Is Zirconia a Viable Alternative to Titanium for Oral Implant? A Critical Review. J. Prosthodont. Res. 2018, 62, 121–133. [Google Scholar] [CrossRef] [PubMed]
- Patil, N.A.; Kandasubramanian, B. Biological and mechanical enhancement of zirconium dioxide for medical applications. Ceram. Int. 2020, 46, 4041–4057. [Google Scholar] [CrossRef]
- Gomez, S.A.; Schreiner, W.; Duffó, G.; Ceré, A.S. Surface Characterization of Anodized Zirconium for Biomedical Applications. Appl. Surf. Sci. 2011, 257, 6397–6405. [Google Scholar]
- Wang, Y.B.; Zheng, Y.F.; Wei, S.C.; Li, M. In Vitro Study on Zr-Based Bulk Metallic Glasses as Potential Biomaterials. J. Biomed. Mater. Res. Part B Appl. Biomater. 2011, 96, 34–46. [Google Scholar] [CrossRef]
- Hobbs, L.W.; Rosen, V.B.; Mangin, S.P.; Treska, M.; Hunter, G. Oxidation Microstructures and Interfaces in the Oxidized Zirconium Knee. Int. J. Appl. Ceram. Technol. 2005, 2, 221–246. [Google Scholar] [CrossRef]
- Uchida, M.; Kim, H.M.; Miyaji, F.; Kokubo, T.; Nakamura, T. Apatite Formation on Zirconium Metal Treated with Aqueous Naoh. Biomaterials 2002, 23, 313–317. [Google Scholar] [CrossRef]
- Gomez, S.A.; Ballarre, J.; Orellano, J.C.; Duffó, G.; Cere, S. Surface Modification of Zirconium by Anodisation as Material for Permanent Implants: In Vitro and in Vivo Study. J. Mater. Sci. Mater. Med. 2013, 24, 161–169. [Google Scholar] [CrossRef]
- Jović, V.D.; Jović, B.M. The Influence of the Conditions of the ZrO2 Passive Film Formation on Its Properties in 1 M NaOH. Corros. Sci. 2008, 50, 3063–3069. [Google Scholar] [CrossRef]
- Gulati, K.; Hamlet, S.M.; Ivanovski, S. Tailoring the Immuno-Responsiveness of Anodized Nano-Engineered Titanium Implants. J. Mater. Chem. B 2018, 6, 2677–2689. [Google Scholar] [CrossRef]
- de la Hoz, M.F.T.; Katunar, M.R.; González, A.; Sanchez, A.G.; Díaz, A.O.; Ceré, S. Effect of Anodized Zirconium Implants on Early Osseointegration Process in Adult Rats: A Histological and Histomorphometric Study. Prog. Biomater. 2019, 8, 249–260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bacchelli, B.; Giavaresi, G.; Franchi, M.; Martini, D.; de Pasquale, V.; Trirè, A.; Fini, M.; Giardino, R.; Ruggeri, A. Influence of a Zirconia Sandblasting Treated Surface on Peri-Implant Bone Healing: An Experimental Study in Sheep. Acta Biomater. 2009, 5, 2246–2257. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhu, S.; Han, Y.; Xiao, C.; Tang, W. Formation and Bioactivity of Ha Nanorods on Micro-Arc Oxidized Zirconium. Mater. Sci. Eng. C 2014, 43, 86–91. [Google Scholar] [CrossRef]
- Quan, R.; Yang, D.; Yan, J.; Li, W.; Wu, X.; Wang, H. Preparation of Graded Zirconia–Cap Composite and Studies of Its Effects on Rat Osteoblast Cells in Vitro. Mater. Sci. Eng. C 2009, 29, 253–260. [Google Scholar] [CrossRef]
- Li, X.; Deng, J.; Lu, Y.; Zhang, L.; Sun, J.; Wu, F. Tribological Behavior of ZrO2/Ws2 Coating Surfaces with Biomimetic Shark-Skin Structure. Ceram. Int. 2019, 45, 21759–21767. [Google Scholar] [CrossRef]
- Gulati, K.; Kogawa, M.; Maher, S.; Atkins, G.; Findlay, D.; Losic, D. Titania Nanotubes for Local Drug Delivery from Implant Surfaces. In Electrochemically Engineered Nanoporous Materials; Springer: Berlin/Heidelberg, Germany, 2015; pp. 307–355. [Google Scholar]
- Tsuchiya, H.; Macak, j.; Taveira, L.; Schmuki, P. Fabrication and Characterization of Smooth High Aspect Ratio Zirconia Nanotubes. Chem. Phys. Lett. 2005, 410, 188–191. [Google Scholar] [CrossRef]
- Katunar, M.R.; Sanchez, A.G.; Coquillat, A.S.; Civantos, A.; Campos, E.M.; Ballarre, J.; Vico, T.; Baca, M.; Ramos, V.; Cere, S. In vitro and in vivo characterization of anodised zirconium as a potential material for biomedical applications. Mater. Sci. Eng. C 2017, 75, 957–968. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Xu, R.; Wang, X.; Li, Y. In Situ Synthesis of Zirconia Nanotube Crystallines by Direct Anodization. Corros. Sci. 2008, 50, 1593–1597. [Google Scholar] [CrossRef]
- Guo, L.; Zhao, J.; Wang, X.; Xu, R.; Lu, Z.; Li, Y. Bioactivity of Zirconia Nanotube Arrays Fabricated by Electrochemical Anodization. Mater. Sci. Eng. C 2009, 29, 1174–1177. [Google Scholar] [CrossRef]
- Frandsen, C.J.; Brammer, K.S.; Noh, K.; Connelly, L.S.; Oh, S.; Chen, L.H.; Jin, S. Zirconium Oxide Nanotube Surface Prompts Increased Osteoblast Functionality and Mineralization. Mater. Sci. Eng. C 2011, 31, 1716–1722. [Google Scholar] [CrossRef]
- Zhang, L.; Han, Y. Enhanced Bioactivity of Self-Organized ZrO2 Nanotube Layer by Annealing and Uv Irradiation. Mater. Sci. Eng. C 2011, 31, 1104–1110. [Google Scholar] [CrossRef]
- Gulati, K.; Santos, A.; Findlay, D.; Losic, D. Optimizing Anodization Conditions for the Growth of Titania Nanotubes on Curved Surfaces. J. Phys. Chem. C 2015, 119, 16033–16045. [Google Scholar] [CrossRef]
- Gulati, K.; Maher, S.; Chandrasekaran, S.; Findlay, D.M.; Losic, D. Conversion of Titania (TiO2) into Conductive Titanium (Ti) Nanotube Arrays for Combined Drug-Delivery and Electrical Stimulation Therapy. J. Mater. Chem. B 2016, 4, 371–375. [Google Scholar] [CrossRef]
- Gulati, K.; Ivanovski, S. Dental Implants Modified with Drug Releasing Titania Nanotubes: Therapeutic Potential and Developmental Challenges. Expert Opin. Drug Deliv. 2017, 14, 1009–1024. [Google Scholar] [CrossRef]
- Gulati, K.; Li, T.; Ivanovski, S. Consume or Conserve: Microroughness of Titanium Implants toward Fabrication of Dual Micro–Nanotopography. ACS Biomater. Sci. Eng. 2018, 4, 3125–3131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, J.; Wang, X.; Xu, R.; Meng, F.; Guo, L.; Li, Y. Fabrication of High Aspect Ratio Zirconia Nanotube Arrays by Anodization of Zirconium Foils. Mater. Lett. 2008, 62, 4428–4430. [Google Scholar] [CrossRef]
- Kaur, G.; Willsmore, T.; Gulati, K.; Zinonos, I.; Wang, Y.; Kurian, M.; Hay, S.; Losic, D.; Evdokiou, A. Titanium Wire Implants with Nanotube Arrays: A Study Model for Localized Cancer Treatment. Biomaterials 2016, 101, 176–188. [Google Scholar] [CrossRef] [PubMed]
- Proost, J.; Vanhumbeeck, J.; van Overmeere, Q. Instability of Anodically Formed TiO2 Layers (Revisited). Electrochim. Acta 2009, 55, 350–357. [Google Scholar] [CrossRef]
- Fan, M.; la Mantia, F. Effect of Surface Topography on the Anodization of Titanium. Electrochem. Commun. 2013, 37, 91–95. [Google Scholar] [CrossRef]
- Li, T.; Gulati, K.; Wang, N.; Zhang, Z.; Ivanovski, S. Understanding and Augmenting the Stability of Therapeutic Nanotubes on Anodized Titanium Implants. Mater. Sci. Eng. C 2018, 88, 182–195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muratore, F.; Hashimoto, T.; Skeldon, P.; Thompson, G.E. Effect of Ageing in the Electrolyte and Water on Porous Anodic Films on Zirconium. Corros. Sci. 2011, 53, 2299–2305. [Google Scholar] [CrossRef]
- Guo, T.; Oztug, N.A.K.; Han, P.; Ivanovski, S.; Gulati, K. Old Is Gold: Electrolyte Aging Influences the Topography, Chemistry, and Bioactivity of Anodized TiO2 Nanopores. ACS Appl. Mater. Interfaces 2021, 13, 7897–7912. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Gulati, K.; Wang, N.; Zhang, Z.; Ivanovski, S. Bridging the Gap: Optimized Fabrication of Robust Titania Nanostructures on Complex Implant Geometries Towards Clinical Translation. J. Colloid Interface Sci. 2018, 529, 452–463. [Google Scholar] [CrossRef] [Green Version]
- Pilling, N.B. The Oxidation of Metals at High Temperature. J. Inst. Met. 1923, 29, 529–582. [Google Scholar]
- Gulati, K.; Kogawa, M.; Prideaux, M.; Findlay, D.M.; Atkins, G.J.; Losic, D. Drug-Releasing Nano-Engineered Titanium Implants: Therapeutic Efficacy in 3D Cell Culture Model, Controlled Release and Stability. Mater. Sci. Eng. C 2016, 69, 831–840. [Google Scholar] [CrossRef]
- Rahman, S.; Gulati, K.; Kogawa, M.; Atkins, G.J.; Pivonka, P.; Findlay, D.M.; Losic, D. Drug Diffusion, Integration, and Stability of Nanoengineered Drug-Releasing Implants in Bone Ex-Vivo. J. Biomed. Mater. Res. Part A 2016, 104, 714–725. [Google Scholar] [CrossRef] [Green Version]
- Gulati, K.; Moon, H.G.; Kumar, P.T.S.; Han, P.; Ivanovski, S. Anodized Anisotropic Titanium Surfaces for Enhanced Guidance of Gingival Fibroblasts. Mater. Sci. Eng. C 2020, 112, 110860. [Google Scholar] [CrossRef]
- Gulati, K.; Moon, H.G.; Li, T.; Kumar, P.T.S.; Ivanovski, S. Titania Nanopores with Dual Micro-/Nano-Topography for Selective Cellular Bioactivity. Mater. Sci. Eng. C 2018, 91, 624–630. [Google Scholar] [CrossRef]
- Ismail, S.; Ahmad, Z.A.; Berenov, A.; Lockman, Z. Effect of Applied Voltage and Fluoride Ion Content on the Formation of Zirconia Nanotube Arrays by Anodic Oxidation of Zirconium. Corros. Sci. 2011, 53, 1156–1164. [Google Scholar] [CrossRef]
- Zhou, X.; Nguyen, N.T.; Özkan, S.; Schmuki, P. Anodic Tio2 Nanotube Layers: Why Does Self-Organized Growth Occur—A Mini Review. Electrochem. Commun. 2014, 46, 157–162. [Google Scholar] [CrossRef] [Green Version]
- Jessensky, O.; Müller, F.; Gösele, U. Self-Organized Formation of Hexagonal Pore Arrays in Anodic Alumina. Appl. Phys. Lett. 1998, 72, 1173–1175. [Google Scholar] [CrossRef] [Green Version]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chopra, D.; Gulati, K.; Ivanovski, S. Towards Clinical Translation: Optimized Fabrication of Controlled Nanostructures on Implant-Relevant Curved Zirconium Surfaces. Nanomaterials 2021, 11, 868. https://doi.org/10.3390/nano11040868
Chopra D, Gulati K, Ivanovski S. Towards Clinical Translation: Optimized Fabrication of Controlled Nanostructures on Implant-Relevant Curved Zirconium Surfaces. Nanomaterials. 2021; 11(4):868. https://doi.org/10.3390/nano11040868
Chicago/Turabian StyleChopra, Divya, Karan Gulati, and Sašo Ivanovski. 2021. "Towards Clinical Translation: Optimized Fabrication of Controlled Nanostructures on Implant-Relevant Curved Zirconium Surfaces" Nanomaterials 11, no. 4: 868. https://doi.org/10.3390/nano11040868
APA StyleChopra, D., Gulati, K., & Ivanovski, S. (2021). Towards Clinical Translation: Optimized Fabrication of Controlled Nanostructures on Implant-Relevant Curved Zirconium Surfaces. Nanomaterials, 11(4), 868. https://doi.org/10.3390/nano11040868