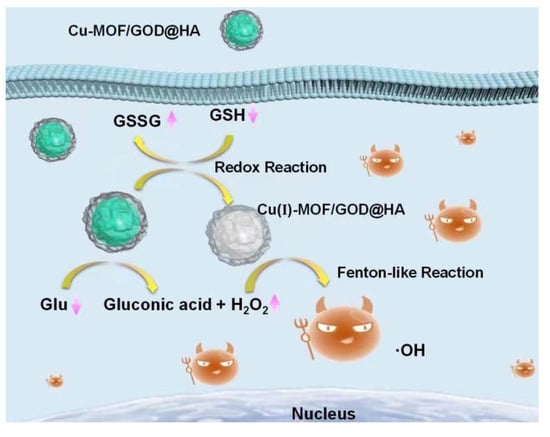
Construction of Novel Nanocomposites (Cu-MOF/GOD@HA) for Chemodynamic Therapy
Abstract
:1. Introduction
2. Materials and Methods
2.1. The Preparation of Cu-MOF/GOD@HA Nanocomposites
2.1.1. Cu-MOF/GOD
2.1.2. Cu-MOF/GOD@HA
2.2. Extracellular ·OH Generation under Catalysis by Cu-MOF
2.2.1. Reaction between Cu-MOF and GSH
2.2.2. Extracellular ·OH Generation under Catalysis by Cu-MOF
2.2.3. Gluconic Acid Generation under Catalysis by GOD
2.2.4. The H2O2 Generation under Catalysis by GOD
2.3. Cell Experiments
2.3.1. Cytotoxicity Assay
2.3.2. Intracellular ·OH Generation Capability of Cu-MOF/GOD@HA
3. Results
3.1. Preparation and Characterization of Cu-MOF/GOD@HA
3.2. Depletion of GSH and Generation of H2O2
3.3. Cell Experiment
3.4. In Vivo Antitumor Efficacy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hao, Y.N.; Zhang, W.X.; Gao, Y.R.; Wei, Y.N.; Shu, Y.; Wang, J.H. State-of-the-art advances of copper-based nanostructures in the enhancement of chemodynamic therapy. J. Mater. Chem. B 2021, 9, 250–266. [Google Scholar] [CrossRef] [PubMed]
- Tian, Q.; Xue, F.; Wang, Y.; Cheng, Y.; An, L.; Yang, S.; Chen, X.; Huang, G. Recent advances in enhanced chemodynamic therapy strategies. Nano Today 2021, 39. [Google Scholar] [CrossRef]
- Li, S.; Jiang, P.; Jiang, F.; Liu, Y. Recent advances in nanomaterial-based nanoplatforms for chemodynamic cancer therapy. Adv. Funct. Mater. 2021, 31. [Google Scholar] [CrossRef]
- Lin, L.-S.; Huang, T.; Song, J.; Ou, X.-Y.; Wang, Z.; Deng, H.; Tian, R.; Liu, Y.; Wang, J.-F.; Liu, Y.; et al. Synthesis of copper peroxide nanodots for H2O2 self-supplying chemodynamic therapy. J. Am. Chem. Soc. 2019, 141, 9937–9945. [Google Scholar] [CrossRef]
- Chang, M.; Wang, M.; Wang, M.; Shu, M.; Ding, B.; Li, C.; Pang, M.; Cui, S.; Hou, Z.; Lin, J. A multifunctional cascade bioreactor based on hollow-structured Cu2MoS4 for synergetic cancer chemo-dynamic therapy/starvation therapy/phototherapy/immunotherapy with remarkably enhanced efficacy. Adv. Mater. 2019, 31. [Google Scholar] [CrossRef]
- Fu, L.-H.; Hu, Y.-R.; Qi, C.; He, T.; Jiang, S.; Jiang, C.; He, J.; Qu, J.; Lin, J.; Huang, P. Biodegradable manganese-doped calcium phosphate nanotheranostics for traceable cascade reaction-enhanced anti-tumor therapy. ACS Nano 2019, 13, 13985–13994. [Google Scholar] [CrossRef]
- Tang, Z.; Liu, Y.; He, M.; Bu, W. Chemodynamic therapy: Tumour microenvironment-mediated Fenton and Fenton-like reactions. Angew. Chem. Int. Ed. 2018, 58, 946–956. [Google Scholar] [CrossRef]
- Chudal, L.; Pandey, N.K.; Phan, J.; Johnson, O.; Lin, L.; Yu, H.; Shu, Y.; Huang, Z.; Xing, M.; Liu, J.P.; et al. Copper-cysteamine nanoparticles as a heterogeneous Fenton-like catalyst for highly selective cancer treatment. ACS Appl. Bio Mater. 2020, 3, 1804–1814. [Google Scholar] [CrossRef]
- Zeng, L.; Cao, Y.; He, L.; Ding, S.; Bian, X.W.; Tian, G. Metal-ligand coordination nanomaterials for radiotherapy: Emerging synergistic cancer therapy. J. Mater. Chem. B 2021, 9, 208–227. [Google Scholar] [CrossRef]
- Zhang, C.; Bu, W.; Ni, D.; Zhang, S.; Li, Q.; Yao, Z.; Zhang, J.; Yao, H.; Wang, Z.; Shi, J. Synthesis of iron nanometallic glasses and their application in cancer therapy by a localized Fenton reaction. Angew. Chem. 2016, 128, 2141–2146. [Google Scholar] [CrossRef]
- Bokare, A.D.; Choi, W. Review of iron-free Fenton-like systems for activating H2O2 in advanced oxidation processes. J. Hazard. Mater. 2014, 275, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Chudal, L.; Pandey, N.K.; Phan, J.; Johnson, O.; Li, X.; Chen, W. Investigation of PPIX-Lipo-MnO2 to enhance photodynamic therapy by improving tumor hypoxia. Mater. Sci. Eng. 2019, 104. [Google Scholar] [CrossRef] [PubMed]
- Yao, M.; Ma, L.; Li, L.; Zhang, J.; Lim, R.X.; Chen, W.; Zhang, Y. A new modality for cancer treatment--nanoparticle mediated microwave induced photodynamic therapy. J. Biomed. Nanotechnol. 2016, 12, 1835–1851. [Google Scholar] [CrossRef]
- Fu, L.; Wan, Y.; Qi, C.; He, J.; Li, C.; Yang, C.; Xu, H.; Lin, J.; Huang, P. Nanocatalytic theranostics with glutathione depletion and enhanced reactive oxygen species generation for efficient cancer therapy. Adv. Mater. 2021, 33. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Liang, C.; Sun, X.; Chen, J.; Yang, Z.; Zhao, H.; Feng, L.; Liu, Z. H2O2-responsive liposomal nanoprobe for photoacoustic inflammation imaging and tumor theranostics via in vivo chromogenic assay. Proc. Natl. Acad. Sci. USA 2017, 114, 5343. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Wu, J.; Jin, Y.; Zhen, W.; Wang, Y.; Liu, J.; Jin, L.; Zhang, S.; Zhao, Y.; Song, S.; et al. Copper(I) phosphide nanocrystals for in situ self-generation magnetic resonance imaging-guided photothermal-enhanced chemodynamic synergetic therapy resisting deep-seated tumor. Adv. Funct. Mater. 2019, 29. [Google Scholar] [CrossRef]
- Wang, Y.; Song, M. pH-responsive cascaded nanocatalyst for synergistic like-starvation and chemodynamic therapy. Colloids Surf. B 2020, 192. [Google Scholar] [CrossRef]
- Fan, W.; Lu, N.; Huang, P.; Liu, Y.; Yang, Z.; Wang, S.; Yu, G.; Liu, Y.; Hu, J.; He, Q.; et al. Glucose-responsive sequential generation of hydrogen peroxide and nitric oxide for synergistic cancer starving-like/gas therapy. Angew. Chem. Int. Ed. 2016, 56, 1229–1233. [Google Scholar] [CrossRef]
- Wang, H.; Chen, Y.; Wang, H.; Liu, X.; Zhou, X.; Wang, F. DNAzyme-loaded, metal-organic frameworks (MOFs) for self-sufficient gene therapy. Angew. Chem. Int. Ed. 2019, 58, 7380–7384. [Google Scholar] [CrossRef]
- Zhang, K.; Meng, X.; Yang, Z.; Dong, H.; Zhang, X. Enhanced cancer therapy by hypoxia-responsive copper metal-organic frameworks nanosystem. Biomaterials 2020, 258. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, W.; Mao, D.; Teh, C.; Wang, B.; Liu, B. Metal-organic framework assisted and tumor microenvironment modulated synergistic image-guided photo-chemo therapy. Adv. Funct. Mater. 2020, 30. [Google Scholar] [CrossRef]
- Ding, S.-S.; He, L.; Bian, X.-W.; Tian, G. Metal-organic frameworks-based nanozymes for combined cancer therapy. Nano Today 2020, 35. [Google Scholar] [CrossRef]
- Zhou, J.; Li, M.; Hou, Y.; Luo, Z.; Chen, Q.; Cao, H.; Huo, R.; Xu, C.; Sutrisno, C.; Hao, L.; et al. Engineering of a nanosized biocatalyst for combined tumor starvation and low-temperature photothermal therapy. ACS Nano 2018, 12, 2858–2872. [Google Scholar] [CrossRef] [PubMed]
- Phua, S.Z.F.; Yang, G.; Lim, W.Q.; Verma, A.; Chen, H.; Thanabalu, T.; Zhao, Y. Catalase-integrated hyaluronic acid as nanocarriers for enhanced photodynamic therapy in solid tumor. ACS Nano 2019, 13, 4742–4751. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.R.; Zhu, Y.X.; Liu, X.; Pan, G.Y.; Gao, G.; Sun, W.; Zhang, X.; Jiang, Y.W.; Wu, F.G. Construction of dually responsive nanotransformers with nanosphere-nanofiber-nanosphere transition for overcoming the size paradox of anticancer nanodrugs. ACS Nano 2019, 13, 11781–11792. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; He, F.; Xiang, K.; Zhang, J.; Xu, M.; Long, P.; Su, H.; Gan, Z.; Yu, Q. CD44-targeted facile enzymatic activatable chitosan nanoparticles for efficient antitumor therapy and reversal of multidrug resistance. Biomacromolecules 2018, 19, 883–895. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.Y.; Chung, H.; Min, K.H.; Yoon, H.Y.; Kim, K.; Park, J.H.; Kwon, I.C.; Jeong, S.Y. Self-assembled hyaluronic acid nanoparticles for active tumor targeting. Biomaterials 2010, 31, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.Y.; Yoon, H.Y.; Kim, J.H.; Bae, S.M.; Park, R.W.; Kang, Y.M.; Kim, I.S.; Kwon, I.C.; Choi, K.; Jeong, S.Y.; et al. Smart nanocarrier based on PEGylated hyaluronic acid for cancer therapy. ACS Nano 2011, 5, 8591–8599. [Google Scholar] [CrossRef]
- Lv, Y.; Xu, C.; Zhao, X.; Lin, C.; Yang, X.; Xin, X.; Zhang, L.; Qin, C.; Han, X.; Yang, L.; et al. Nanoplatform assembled from a CD44-targeted prodrug and smart liposomes for dual targeting of tumor microenvironment and cancer cells. ACS Nano 2018, 12, 1519–1536. [Google Scholar] [CrossRef]
- Mu, J.; Lin, J.; Huang, P.; Chen, X. Development of endogenous enzyme-responsive nanomaterials for theranostics. Chem. Soc. Rev. 2018, 47, 5554–5573. [Google Scholar] [CrossRef]
- Li, Y.; Li, X.; Guan, Q.; Zhang, C.; Xu, T.; Dong, Y.; Bai, X.; Zhang, W. Strategy for chemotherapeutic delivery using a nanosized porous metal-organic framework with a central composite design. Int. J. Nanomed. 2017, 12, 1465–1474. [Google Scholar] [CrossRef] [Green Version]
- Ming, J.; Zhu, T.; Yang, W.; Shi, Y.; Huang, D.; Li, J.; Xiang, S.; Wang, J.; Chen, X.; Zheng, N. Pd@Pt-GOx/HA as a novel enzymatic cascade nanoreactor for high-efficiency starving-enhanced chemodynamic cancer therapy. ACS Appl. Mater. Interfaces 2020, 12, 51249–51262. [Google Scholar] [CrossRef] [PubMed]
- Špadina, M.; Gourdin-Bertin, S.; Dražić, G.; Selmani, A.; Dufrêche, J.F.; Bohinc, K. Charge properties of TiO2 nanotubes in NaNO3 aqueous solution. ACS Appl. Mater. Interfaces 2018, 10, 13130–13142. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Wang, S.; Deng, H.; Yang, W.; Rao, L.; Tian, R.; Liu, Y.; Yu, G.; Zhou, Z.; Song, J.; et al. Endogenous labileiron pool-mediated free radical generation for cancer chemodynamic therapy. J. Am. Chem. Soc. 2020, 142, 15320–15330. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Wang, X.; Cheng, L.; Tang, Y.; Zhan, G.; Gong, F.; Zhang, R.; Hu, J.; Liu, Z.; Yang, X. GSH-depleted PtCu3 nanocages for chemodynamic-enhanced sonodynamic cancer therapy. Adv. Funct. Mater. 2019, 30. [Google Scholar] [CrossRef]
- Morlock, L.K.; Böttcher, D.; Bornscheuer, U.T. Simultaneous detection of NADPH consumption and H2O2 production using the Ampliflu™ Red assay for screening of P450 activities and uncoupling. Appl. Microbiol. Biotechnol. 2017, 102, 985–994. [Google Scholar] [CrossRef]
- Pandey, N.K.; Xiong, W.; Wang, L.; Chen, W.; Bui, B.; Yang, J.; Amador, E.; Chen, M.; Xing, C.; Athavale, A.A.; et al. Aggregation-induced emission luminogens for highly effective microwave dynamic therapy. Bioact. Mater. 2021. [Google Scholar] [CrossRef]
- Chen, X.; Liu, J.; Li, Y.; Pandey, N.K.; Chen, T.; Wang, L.; Amador, E.H.; Chen, W.; Liu, F.; Xiao, E.; et al. Study of copper-cysteamine based X-ray induced photodynamic therapy and its effects on cancer cell proliferation and migration in a clinical mimic setting. Bioact. Mater. 2021. [Google Scholar] [CrossRef]
- Wang, Y.; Alkhaldi, N.; Pandey, N.; Chudal, L.; Wang, L.; Lin, L.; Zhang, M.; Yong, Y.; Amador, E.; Huda, M.; et al. A new type of cuprous-cysteamine sensitizers: Synthesis, optical properties and potential applications. Mater. Today Phys. 2021. [Google Scholar] [CrossRef]
- Zhang, Q.; Guo, X.; Cheng, Y.; Chudal, L.; Pandey, N.K.; Zhang, J.; Ma, L.; Xi, Q.; Yang, G.; Chen, Y.; et al. Use of copper-cysteamine nanoparticles to simultaneously enable radiotherapy, oxidative therapy and immunotherapy for melanoma treatment. Signal Transduct. Target. Ther. (Nat.) 2020, 5. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hao, Y.-N.; Qu, C.-C.; Shu, Y.; Wang, J.-H.; Chen, W. Construction of Novel Nanocomposites (Cu-MOF/GOD@HA) for Chemodynamic Therapy. Nanomaterials 2021, 11, 1843. https://doi.org/10.3390/nano11071843
Hao Y-N, Qu C-C, Shu Y, Wang J-H, Chen W. Construction of Novel Nanocomposites (Cu-MOF/GOD@HA) for Chemodynamic Therapy. Nanomaterials. 2021; 11(7):1843. https://doi.org/10.3390/nano11071843
Chicago/Turabian StyleHao, Ya-Nan, Cong-Cong Qu, Yang Shu, Jian-Hua Wang, and Wei Chen. 2021. "Construction of Novel Nanocomposites (Cu-MOF/GOD@HA) for Chemodynamic Therapy" Nanomaterials 11, no. 7: 1843. https://doi.org/10.3390/nano11071843
APA StyleHao, Y. -N., Qu, C. -C., Shu, Y., Wang, J. -H., & Chen, W. (2021). Construction of Novel Nanocomposites (Cu-MOF/GOD@HA) for Chemodynamic Therapy. Nanomaterials, 11(7), 1843. https://doi.org/10.3390/nano11071843