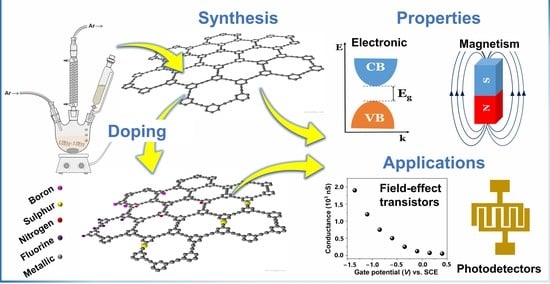
Developments in Synthesis and Potential Electronic and Magnetic Applications of Pristine and Doped Graphynes
Abstract
:1. Introduction
2. From Atomic Structure Suggestion to Experimental Appearance
2.1. Mechanochemical Synthesis
2.2. The Vapor–Liquid–Solid Growth
2.3. Thermal Treatment
2.4. “On-Surface” Synthesis under an Ultra-High Vacuum
2.5. “On-Surface” Synthesis by Chemical Vapor Deposition
2.6. Wet Chemical Synthesis
2.6.1. Developments of Coupling Reactions (All Reactants in Solution Phase)

2.6.2. Two-Phase Methods (Interfacial Synthesis Utilizing Two Immiscible Liquids)
2.6.3. Developments of Heterogeneous Coupling Reactions at Liquid/Solid Interfaces on Diverse Substrates
3. Electronic Properties
3.1. Dirac Cone
3.2. Electronic Band Structure
3.2.1. The Electronic Band Structure of GYs

3.2.2. The Electronic Structure of GDYs

3.2.3. The Electronic Band Structure of GY Nanoribbons
3.2.4. The Electronic Band Structure of Bulky GYs and GDYs
3.3. Electronic Transport
3.4. Optoelectronic Properties
4. Magnetism of Pure and Doped Graphyne-Like Materials
4.1. Theoretically Investigated Magnetic Properties
4.1.1. Metal-Doped GYs
4.1.2. Non-Metal Doped GYs and GDYs

4.2. Experimentally Investigated Magnetic Properties
5. Mechanical Properties of Graphynes
6. Conclusions and Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Szczurek, A.; Amaral-Labat, G.; Fierro, V.; Pizzi, A.; Masson, E.; Celzard, A. The use of tannin to prepare carbon gels. Part I: Carbon aerogels. Carbon 2011, 49, 2773–2784. [Google Scholar] [CrossRef]
- Szczurek, A.; Fierro, V.; Medjahdi, G.; Celzard, A. Carbon aerogels prepared by autocondensation of flavonoid tannin. Carbon Resour. Convers. 2019, 2, 72–84. [Google Scholar] [CrossRef]
- Szczurek, A.; Fierro, V.; Pizzi, A.; Celzard, A. Mayonnaise, whipped cream and meringue, a new carbon cuisine. Carbon 2013, 58, 245–248. [Google Scholar] [CrossRef]
- Celzard, A.; Fierro, V. “Green”, innovative, versatile and efficient carbon materials from polyphenolic plant extracts. Carbon 2020, 167, 792–815. [Google Scholar] [CrossRef]
- Sharma, S. Glassy Carbon: A Promising Material for Micro- and Nanomanufacturing. Materials 2018, 11, 1857–1908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szczurek, A.; Fierro, V.; Plyushch, A.; Macutkevic, J.; Kuzhir, P.; Celzard, A. Structure and Electromagnetic Properties of Cellular Glassy Carbon Monoliths with Controlled Cell Size. Materials 2018, 11, 709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chalifoux, W.; Tykwinski, R. Synthesis of polyynes to model the sp-carbon allotrope carbyne. Nat. Chem. 2010, 2, 967–971. [Google Scholar] [CrossRef]
- Heidari, A. Computational Study on Molecular Structures of C20, C60, C240, C540, C960, C2160 and C3840 Fullerene Nano Molecules under Synchrotron Radiations Using Fuzzy Logic. J. Mater. Sci. Eng. 2016, 5, 2169-0022. [Google Scholar] [CrossRef] [Green Version]
- Rietmeijer, F.J.M.; Rotundi, A.; Heymann, D. C60 and Giant Fullerenes in Soot Condensed in Vapors with Variable C/H2 Ratio. Fuller. Nanotub. Carbon Nanostruct. 2004, 12, 659–668. [Google Scholar] [CrossRef]
- Hersam, M.C. Progress towards monodisperse single-walled carbon nanotubes. Nat. Nanotech. 2008, 3, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Homayoun, A.; Bannister, C.W.; Yum, K. Single-walled carbon nanotubes as near-infrared optical biosensors for life sciences and biomedicine. Biotechnol. J. 2015, 10, 447–459. [Google Scholar] [CrossRef] [PubMed]
- Ahlawat, J.; Asil, S.M.; Barroso, G.G.; Nurunnabi, M.; Narayan, M. Application of carbon nano onions in the biomedical field: Recent advances and challenges. Biomater. Sci. 2021, 9, 626–644. [Google Scholar] [CrossRef]
- Yin, M.; Cohen, M.L. Will diamond transform under megabar pressures? Phys. Rev. Lett. 1983, 50, 2006. [Google Scholar] [CrossRef]
- Yin, M. Si-III (BC-8) crystal phase of Si and C: Structural properties, phase stabilities, and phase transitions. Phys. Rev. B 1984, 30, 1773. [Google Scholar] [CrossRef]
- Yang, X.; Wang, Y.; Xiao, R.; Liu, H.; Bing, Z.; Zhang, Y.; Yao, X. A new two-dimensional semiconducting carbon allotrope with direct band gap: A first-principles prediction. J. Phys. Condens. Matter 2020, 33, 045502. [Google Scholar] [CrossRef]
- Höhne, R.; Esquinazi, P. Can Carbon Be Ferromagnetic? Adv. Mater. 2002, 14, 753–756. [Google Scholar] [CrossRef]
- Maździarz, M.; Mrozek, A.; Kuś, W.; Burczyński, T. Anisotropic-cyclicgraphene: A new two-dimensional semiconducting carbon allotrope. Materials 2018, 11, 432. [Google Scholar] [CrossRef] [Green Version]
- Kaiser, K.; Scriven, L.M.; Schulz, F.; Gawel, P.; Gross, L.; Anderson, H.L. An sp-hybridized molecular carbon allotrope, cyclo [18] carbon. Science 2019, 365, 1299–1301. [Google Scholar] [CrossRef] [Green Version]
- Hoffmann, R.; Kabanov, A.A.; Golov, A.A.; Proserpio, D.M. Homo Citans and Carbon Allotropes: For an Ethics of Citation. Angew. Chem. Int. Ed. 2016, 55, 10962–10976. [Google Scholar] [CrossRef]
- Correa, A.A.; Bonev, S.A.; Galli, G. Carbon under extreme conditions: Phase boundaries and electronic properties from first-principles theory. Proc. Natl. Acad. Sci. USA 2006, 103, 1204–1208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grochala, W. Diamond: Electronic ground state of carbon at temperatures approaching 0 K. Angew. Chem. Int. Ed. 2014, 53, 3680–3683. [Google Scholar] [CrossRef] [PubMed]
- White, M.A.; Kahwaji, S.; Freitas, V.L.S.; Siewert, R.; Weatherby, J.A.; Ribeiro da Silva, M.D.M.C.; Verevkin, S.P.; Johnson, E.R.; Zwanziger, J.W. The Relative Thermodynamic Stability of Diamond and Graphite. Angew. Chem. Int. Ed. 2020, 60, 1546–1549. [Google Scholar] [CrossRef]
- Németh, P.; Garvie, L.A.; Aoki, T.; Dubrovinskaia, N.; Dubrovinsky, L.; Buseck, P.R. Lonsdaleite is faulted and twinned cubic diamond and does not exist as a discrete material. Nat. Commun. 2014, 5, 1–5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCulloch, D.G.; Wong, S.; Shiell, T.B.; Haberl, B.; Cook, B.A.; Huang, X.; Boehler, R.; McKenzie, D.R.; Bradby, J.E. Investigation of Room Temperature Formation of the Ultra-Hard Nanocarbons Diamond and Lonsdaleite. Small 2020, 16, 2004695. [Google Scholar] [CrossRef]
- Tiwari, S.K.; Mishra, R.K.; Ha, S.K.; Huczko, A. Evolution of graphene oxide and graphene: From imagination to industrialization. ChemNanoMat 2018, 4, 598–620. [Google Scholar] [CrossRef]
- Tiwari, S.K.; Kumar, V.; Huczko, A.; Oraon, R.; Adhikari, A.D.; Nayak, G. Magical allotropes of carbon: Prospects and applications. Crit. Rev. Solid State Mater. Sci. 2016, 41, 257–317. [Google Scholar] [CrossRef]
- Abdi, G.; Alizadeh, A.; Khodaei, M.M. Highly carboxyl-decorated graphene oxide sheets as metal-free catalytic system for chemoselective oxidation of sulfides to sulfones. Mater. Chem. Phys. 2017, 201, 323–330. [Google Scholar] [CrossRef]
- Huang, C.S.; Li, Y.J.; Wang, N.; Xue, Y.R.; Zuo, Z.C.; Liu, H.B.; Li, Y.L. Progress in Research into 2D Graphdiyne-Based Materials. Chem. Rev. 2018, 118, 7744–7803. [Google Scholar] [CrossRef]
- Boukhvalov, D. Stable antiferromagnetic graphone. Phys. E 2010, 43, 199–201. [Google Scholar] [CrossRef] [Green Version]
- Lebegue, S.; Klintenberg, M.; Eriksson, O.; Katsnelson, M. Accurate electronic band gap of pure and functionalized graphane from GW calculations. Phys. Rev. B 2009, 79, 245117. [Google Scholar] [CrossRef] [Green Version]
- Feng, L.; Zhang, W. The structure and magnetism of graphone. AIP Adv. 2012, 2, 042138. [Google Scholar] [CrossRef]
- Peng, Q.; Dearden, A.K.; Crean, J.; Han, L.; Liu, S.; Wen, X.; De, S. New materials graphyne, graphdiyne, graphone, and graphane: Review of properties, synthesis, and application in nanotechnology. Nanotechnol. Sci. Appl. 2014, 7, 1. [Google Scholar] [CrossRef] [Green Version]
- Baughman, R.; Eckhardt, H.; Kertesz, M. Structure-property predictions for new planar forms of carbon: Layered phases containing sp 2 and sp atoms. J. Chem. Phys. 1987, 87, 6687–6699. [Google Scholar] [CrossRef]
- Solis, D.; Woellner, C.F.; Borges, D.D.; Galvao, D.S. Mechanical and Thermal Stability of Graphyne and Graphdiyne Nanoscrolls. MRS Adv. 2017, 2, 129–134. [Google Scholar] [CrossRef] [Green Version]
- Li, G.; Li, Y.; Liu, H.; Guo, Y.; Li, Y.; Zhu, D. Architecture of graphdiyne nanoscale films. Chem. Commun. 2010, 46, 3256–3258. [Google Scholar] [CrossRef]
- Majidi, R. Electronic properties of porous graphene, α-graphyne, graphene-like, and graphyne-like BN sheets. Can. J. Phys. 2016, 94, 305–309. [Google Scholar] [CrossRef]
- Xie, C.; Wang, N.; Li, X.; Xu, G.; Huang, C. Research on the Preparation of Graphdiyne and Its Derivatives. Chem. Eur. J. 2020, 26, 569–583. [Google Scholar] [CrossRef]
- Li, Y.; Xu, L.; Liu, H.; Li, Y. Graphdiyne and graphyne: From theoretical predictions to practical construction. Chem. Soc. Rev. 2014, 43, 2572–2586. [Google Scholar] [CrossRef] [PubMed]
- Puigdollers, A.R.; Alonso, G.; Gamallo, P. First-principles study of structural, elastic and electronic properties of alpha-, beta- and gamma-graphyne. Carbon 2016, 96, 879–887. [Google Scholar] [CrossRef]
- Haley, M.M.; Brand, S.C.; Pak, J.J. Carbon networks based on dehydrobenzoannulenes: Synthesis of graphdiyne substructures. Angew. Chem. Int. Ed. 1997, 36, 836–838. [Google Scholar] [CrossRef]
- Serafini, P.; Milani, A.A.; Proserpio, D.M.; Casari, C.S. Designing all graphdiyne materials as graphene derivatives: Topologically driven modulation of electronic properties. arXiv 2021, arXiv:2103.13085. [Google Scholar]
- Zhou, J.; Li, J.; Liu, Z.; Zhang, J. Exploring Approaches for the Synthesis of Few-Layered Graphdiyne. Adv. Mater. 2019, 31, 1803758. [Google Scholar] [CrossRef] [PubMed]
- Glaser, C. Untersuchungen über einige Derivate der Zimmtsäure. Justus Liebigs Ann. Chem. 1870, 154, 137–171. [Google Scholar] [CrossRef] [Green Version]
- Eglinton, G.; Galbraith, A. 182. Macrocyclic acetylenic compounds. Part I. Cyclo tetradeca-1: 3-diyne and related compounds. J. Chem. Soc. 1959, 182, 889–896. [Google Scholar] [CrossRef]
- Hay, A.S. Oxidative coupling of acetylenes. III. J. Org. Chem. 1962, 27, 3320–3321. [Google Scholar] [CrossRef]
- King, A.O.; Okukado, N.; Negishi, E.-I. Highly general stereo-, regio-, and chemo-selective synthesis of terminal and internal conjugated enynes by the Pd-catalysed reaction of alkynylzinc reagents with alkenyl halides. J. Chem. Soc. Chem. Commun. 1977, 19, 683–684. [Google Scholar] [CrossRef]
- Hatanaka, Y.; Hiyama, T. Cross-coupling of organosilanes with organic halides mediated by a palladium catalyst and tris(diethylamino)sulfonium difluorotrimethylsilicate. J. Org. Chem. 1988, 53, 918–920. [Google Scholar] [CrossRef]
- Sonogashira, K. Development of Pd–Cu catalyzed cross-coupling of terminal acetylenes with sp2-carbon halides. J. Organomet. Chem. 2002, 653, 46–49. [Google Scholar] [CrossRef]
- Vollhardt, K.P.C. Cobalt-Mediated [2 + 2 + 2]-Cycloadditions: A Maturing Synthetic Strategy [New Synthetic Methods (43)]. Angew. Chem. Int. Ed. 1984, 23, 539–556. [Google Scholar] [CrossRef] [Green Version]
- Rubin, Y.; Knobler, C.B.; Diederich, F. Tetraethynylethene. Angew. Chem. Int. Ed. 1991, 30, 698–700. [Google Scholar] [CrossRef]
- Diederich, F.; Rubin, Y. Synthetic Approaches toward Molecular and Polymeric Carbon Allotropes. Angew. Chem. Int. Ed. 1992, 31, 1101–1123. [Google Scholar] [CrossRef]
- Diederich, F. Carbon scaffolding: Building acetylenic all-carbon and carbon-rich compounds. Nature 1994, 369, 199–207. [Google Scholar] [CrossRef]
- Wan, W.B.; Brand, S.C.; Pak, J.J.; Haley, M.M. Synthesis of Expanded Graphdiyne Substructures. Chem. Eur. J. 2000, 6, 2044–2052. [Google Scholar] [CrossRef]
- Tahara, K.; Yamamoto, Y.; Gross, D.E.; Kozuma, H.; Arikuma, Y.; Ohta, K.; Koizumi, Y.; Gao, Y.; Shimizu, Y.; Seki, S.; et al. Syntheses and Properties of Graphyne Fragments: Trigonally Expanded Dehydrobenzo 12 annulenes. Chem. Eur. J. 2013, 19, 11251–11260. [Google Scholar] [CrossRef]
- Iyoda, M.; Yamakawa, J.; Rahman, M.J. Conjugated Macrocycles: Concepts and Applications. Angew. Chem. Int. Ed. 2011, 50, 10522–10553. [Google Scholar] [CrossRef]
- Diederich, F.; Kivala, M. All-carbon scaffolds by rational design. Adv. Mater. 2010, 22, 803–812. [Google Scholar] [CrossRef]
- Haley, M.M. Synthesis and properties of annulenic subunits of graphyne and graphdiyne nanoarchitectures. Pure Appl. Chem. 2008, 80, 519–532. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Q.; Li, W.; Lu, Y.; Meng, H.; Li, C. Efficient destruction of hexachlorobenzene by calcium carbide through mechanochemical reaction in a planetary ball mill. Chemosphere 2017, 166, 275–280. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Q.; Li, W.; Meng, H.; Lu, Y.; Li, C. Synthesis and Supercapacitor Application of Alkynyl Carbon Materials Derived from CaC2 and Polyhalogenated Hydrocarbons by Interfacial Mechanochemical Reactions. ACS Appl. Mater. Interfaces 2017, 9, 3895–3901. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Li, Y.; Tang, J.; Cui, X. Synthesis of hydrogen substituted graphyne through mechanochemistry and its electrocatalytic properties. Acta Phys. Chim. Sin. 2018, 34, 1080–1087. [Google Scholar] [CrossRef]
- Li, Q.; Li, Y.; Chen, Y.; Wu, L.; Yang, C.; Cui, X. Synthesis of γ-graphyne by mechanochemistry and its electronic structure. Carbon 2018, 136, 248–254. [Google Scholar] [CrossRef]
- Qian, X.; Liu, H.; Huang, C.; Chen, S.; Zhang, L.; Li, Y.; Wang, J.; Li, Y. Self-catalyzed growth of large-area nanofilms of two-dimensional carbon. Sci. Rep. 2015, 5, 1–7. [Google Scholar] [CrossRef]
- Zuo, Z.; Shang, H.; Chen, Y.; Li, J.; Liu, H.; Li, Y.; Li, Y. A facile approach for graphdiyne preparation under atmosphere for an advanced battery anode. Chem. Commun. 2017, 53, 8074–8077. [Google Scholar] [CrossRef] [PubMed]
- Shang, H.; Zuo, Z.; Zheng, H.; Li, K.; Tu, Z.; Yi, Y.; Liu, H.; Li, Y.; Li, Y. N-doped graphdiyne for high-performance electrochemical electrodes. Nano Energy 2018, 44, 144–154. [Google Scholar] [CrossRef]
- Zhang, S.; Du, H.; He, J.; Huang, C.; Liu, H.; Cui, G.; Li, Y. Nitrogen-doped graphdiyne applied for lithium-ion storage. ACS Appl. Mater. Interfaces 2016, 8, 8467–8473. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Li, X.; Zhao, F.; Wang, N.; Xie, C.; He, J.; Si, W.; Yi, Y.; Yang, Z.; Li, X. Preparation and structure study of phosphorus-doped porous graphdiyne and its efficient lithium storage application. 2D Mater. 2019, 6, 035020. [Google Scholar] [CrossRef]
- Shen, X.; Yang, Z.; Wang, K.; Wang, N.; He, J.; Du, H.; Huang, C. Nitrogen-Doped Graphdiyne as High-Capacity Electrode Materials for Both Lithium-Ion and Sodium-Ion Capacitors. ChemElectroChem 2018, 5, 1435–1443. [Google Scholar] [CrossRef]
- Yang, Z.; Cui, W.; Wang, K.; Song, Y.; Zhao, F.; Wang, N.; Long, Y.; Wang, H.; Huang, C. Chemical Modification of the sp-Hybridized Carbon Atoms of Graphdiyne by Using Organic Sulfur. Chem. Eur. J. 2019, 25, 5643–5647. [Google Scholar] [CrossRef]
- Du, H.; Zhang, Z.; He, J.; Cui, Z.; Chai, J.; Ma, J.; Yang, Z.; Huang, C.; Cui, G. A Delicately Designed Sulfide Graphdiyne Compatible Cathode for High-Performance Lithium/Magnesium–Sulfur Batteries. Small 2017, 13, 1702277. [Google Scholar] [CrossRef]
- Zhang, S.; Cai, Y.; He, H.; Zhang, Y.; Liu, R.; Cao, H.; Wang, M.; Liu, J.; Zhang, G.; Li, Y. Heteroatom doped graphdiyne as efficient metal-free electrocatalyst for oxygen reduction reaction in alkaline medium. J. Mater. Chem. A 2016, 4, 4738–4744. [Google Scholar] [CrossRef] [Green Version]
- Abyazisani, M.; Jayalatharachchi, V.; MacLeod, J. Directed on-surface growth of covalently-bonded molecular nanostructures. In Comprehensive Nanoscience and Nanotechnology, 2nd ed.; Andrews, D., Nann, T., Lipson, R., Eds.; Academic Press: Cambridge, MA, USA, 2019; Volume 2, pp. 299–326. [Google Scholar]
- Schultz, M.J.; Zhang, X.; Unarunotai, S.; Khang, D.-Y.; Cao, Q.; Wang, C.; Lei, C.; MacLaren, S.; Soares, J.A.; Petrov, I. Synthesis of linked carbon monolayers: Films, balloons, tubes, and pleated sheets. Proc. Natl. Acad. Sci. USA 2008, 105, 7353–7358. [Google Scholar] [CrossRef] [Green Version]
- Kang, F.; Xu, W. On-Surface Synthesis of One-Dimensional Carbon-Based Nanostructures via C−X and C−H Activation Reactions. ChemPhysChem 2019, 20, 2251–2261. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-Q.; Kepčija, N.; Kleinschrodt, M.; Diller, K.; Fischer, S.; Papageorgiou, A.C.; Allegretti, F.; Björk, J.; Klyatskaya, S.; Klappenberger, F. Homo-coupling of terminal alkynes on a noble metal surface. Nat. Commun. 2012, 3, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chen, Q.; Xiao, L.; Shang, J.; Zhou, X.; Zhang, Y.; Wang, Y.; Shao, X.; Li, J.; Chen, W.; et al. Lattice-Directed Formation of Covalent and Organometallic Molecular Wires by Terminal Alkynes on Ag Surfaces. ACS Nano 2015, 9, 6305–6314. [Google Scholar] [CrossRef] [PubMed]
- Klappenberger, F.; Zhang, Y.-Q.; Björk, J.; Klyatskaya, S.; Ruben, M.; Barth, J.V. On-surface synthesis of carbon-based scaffolds and nanomaterials using terminal alkynes. Acc. Chem. Res. 2015, 48, 2140–2150. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chen, Q.; He, Q.; Zhang, Y.; Fu, X.; Wang, Y.; Zhao, D.; Chen, W.; Xu, G.Q.; Wu, K. Bromine adatom promoted C–H bond activation in terminal alkynes at room temperature on Ag(111). Phys. Chem. Chem. Phys. 2018, 20, 11081–11088. [Google Scholar] [CrossRef]
- Sun, Q.; Yu, X.; Bao, M.; Liu, M.; Pan, J.; Zha, Z.; Cai, L.; Ma, H.; Yuan, C.; Qiu, X. Direct formation of C−C triple-bonded structural motifs by on-surface dehalogenative homocouplings of tribromomethyl-substituted arenes. Angew. Chem. Int. Ed. 2018, 57, 4035–4038. [Google Scholar] [CrossRef]
- Yu, X.; Cai, L.; Bao, M.; Sun, Q.; Ma, H.; Yuan, C.; Xu, W. On-surface synthesis of graphyne nanowires through stepwise reactions. Chem. Commun. 2020, 56, 1685–1688. [Google Scholar] [CrossRef]
- Sun, Q.; Cai, L.; Ma, H.; Yuan, C.; Xu, W. Dehalogenative homocoupling of terminal alkynyl bromides on Au (111): Incorporation of acetylenic scaffolding into surface nanostructures. ACS Nano 2016, 10, 7023–7030. [Google Scholar] [CrossRef]
- Gao, H.Y.; Wagner, H.; Zhong, D.; Franke, J.H.; Studer, A.; Fuchs, H. Glaser coupling at metal surfaces. Angew. Chem. Int. Ed. 2013, 52, 4024–4028. [Google Scholar] [CrossRef]
- Zhou, H.; Liu, J.; Du, S.; Zhang, L.; Li, G.; Zhang, Y.; Tang, B.Z.; Gao, H.-J. Direct visualization of surface-assisted two-dimensional diyne polycyclotrimerization. J. Am. Chem. Soc. 2014, 136, 5567–5570. [Google Scholar] [CrossRef]
- Gao, H.-Y.; Franke, J.R.-H.; Wagner, H.; Zhong, D.; Held, P.-A.; Studer, A.; Fuchs, H. Effect of metal surfaces in on-surface glaser coupling. J. Phys. Chem. C 2013, 117, 18595–18602. [Google Scholar] [CrossRef]
- Liu, J.; Ruffieux, P.; Feng, X.; Müllen, K.; Fasel, R. Cyclotrimerization of arylalkynes on Au (111). Chem. Commun. 2014, 50, 11200–11203. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Lv, H.; Feng, L.; Tao, Z.; Huang, J.; Fan, Q.; Wu, X.; Zhu, J. Unravelling the Mechanism of Glaser Coupling Reaction on Ag(111) and Cu(111) Surfaces: A Case for Halogen Substituted Terminal Alkyne. J. Phys. Chem. C 2018, 122, 14537–14545. [Google Scholar] [CrossRef]
- Liu, R.; Gao, X.; Zhou, J.; Xu, H.; Li, Z.; Zhang, S.; Xie, Z.; Zhang, J.; Liu, Z. Chemical vapor deposition growth of linked carbon monolayers with acetylenic scaffoldings on silver foil. Adv. Mater. 2017, 29, 1604665. [Google Scholar] [CrossRef] [PubMed]
- Moroni, M.; Le Moigne, J.; Luzzati, S. Rigid rod conjugated polymers for nonlinear optics: 1. Characterization and linear optical properties of poly(aryleneethynylene) derivatives. Macromolecules 1994, 27, 562–571. [Google Scholar] [CrossRef]
- Petrosyan, A.; Ehlers, P.; Surkus, A.-E.; Ghochikyan, T.V.; Saghyan, A.S.; Lochbrunner, S.; Langer, P. Straightforward synthesis of tetraalkynylpyrazines and their photophysical properties. Org. Biomol. Chem. 2016, 14, 1442–1449. [Google Scholar] [CrossRef] [PubMed]
- Sonoda, M.; Inaba, A.; Itahashi, K.; Tobe, Y. Synthesis of Differentially Substituted Hexaethynylbenzenes Based on Tandem Sonogashira and Negishi Cross-Coupling Reactions. Org. Lett. 2001, 3, 2419–2421. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.X.; Su, F.; Trewin, A.; Wood, C.D.; Campbell, N.L.; Niu, H.; Dickinson, C.; Ganin, A.Y.; Rosseinsky, M.J.; Khimyak, Y.Z. Conjugated microporous poly (aryleneethynylene) networks. Angew. Chem. Int. Ed. 2007, 46, 8574–8578. [Google Scholar] [CrossRef]
- Dawson, R.; Laybourn, A.; Khimyak, Y.Z.; Adams, D.J.; Cooper, A.I. High surface area conjugated microporous polymers: The importance of reaction solvent choice. Macromolecules 2010, 43, 8524–8530. [Google Scholar] [CrossRef]
- Tan, D.; Fan, W.; Xiong, W.; Sun, H.; Cheng, Y.; Liu, X.; Meng, C.; Li, A.; Deng, W.Q. Study on the morphologies of covalent organic microporous polymers: The role of reaction solvents, Macromol. Chem. Phys. 2012, 213, 1435–1440. [Google Scholar] [CrossRef]
- Bunz, U.H.F. Poly(aryleneethynylene)s: Syntheses, Properties, Structures, and Applications. Chem. Rev. 2000, 100, 1605–1644. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Li, M.; Xiao, S.; Qu, Y.; Qiu, X.; Liu, T.; Tian, F.; Li, H.; Xiao, S. A graphyne-like porous carbon-rich network synthesized via alkyne metathesis. Nanoscale 2017, 9, 11939–11943. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.; Sun, M.; Gao, B.; Liu, W.; Ding, Z.; Anandan, S. A ball-milling synthesis of N-graphyne with controllable nitrogen doping sites for efficient electrocatalytic oxygen evolution and supercapacitors. Dalton Trans. 2020, 49, 10958–10969. [Google Scholar] [CrossRef]
- Chen, T.; Li, W.-Q.; Chen, X.-J.; Guo, Y.-Z.; Hu, W.-B.; Hu, W.-J.; Yahu, A.; Liu, Y.A.; Yang, H.; Wen, K. A Triazine-Based Analogue of Graphyne: Scalable Synthesis and Applications in Photocatalytic Dye Degradation and Bacterial Inactivation. Chem. Eur. J. 2020, 26, 2269–2275. [Google Scholar] [CrossRef]
- Zhou, W.; Shen, H.; Zeng, Y.; Yi, Y.; Zuo, Z.; Li, Y.; Li, Y. Controllable Synthesis of Graphdiyne Nanoribbons. Angew. Chem. Int. Ed. 2020, 59, 4908–4913. [Google Scholar] [CrossRef]
- Matsuoka, R.; Sakamoto, R.; Hoshiko, K.; Sasaki, S.; Masunaga, H.; Nagashio, K.; Nishihara, H. Crystalline graphdiyne nanosheets produced at a gas/liquid or liquid/liquid interface. J. Am. Chem. Soc. 2017, 139, 3145–3152. [Google Scholar] [CrossRef]
- Song, Y.; Li, X.; Yang, Z.; Wang, J.; Liu, C.; Xie, C.; Wang, H.; Huang, C. A facile liquid/liquid interface method to synthesize graphyne analogs. Chem. Commun. 2019, 55, 6571–6574. [Google Scholar] [CrossRef]
- Kan, X.; Ban, Y.; Wu, C.; Pan, Q.; Liu, H.; Song, J.; Zuo, Z.; Li, Z.; Zhao, Y. Interfacial Synthesis of Conjugated Two-Dimensional N-Graphdiyne. ACS Appl. Mater. Interfaces 2018, 10, 53–58. [Google Scholar] [CrossRef]
- Pan, Q.; Chen, S.; Wu, C.; Zhang, Z.; Li, Z.; Zhao, Y. Sulfur-Rich Graphdiyne-Containing Electrochemical Active Tetrathiafulvalene for Highly Efficient Lithium Storage Application. ACS Appl. Mater. Interfaces 2019, 11, 46070–46076. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Wang, N.; Zhang, M.; Sápi, A.; Yu, J.; Li, X.; Cui, W.; Yang, Z.; Huang, C. In situ growth of graphdiyne on arbitrary substrates with a controlled-release method. Chem. Commun. 2018, 54, 6004–6007. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Xie, Z.; Liu, R.; Gao, X.; Li, J.; Xiong, Y.; Tong, L.; Zhang, J.; Liu, Z. Synthesis of ultrathin graphdiyne film using a surface template. ACS Appl. Mater. Interfaces 2018, 11, 2632–2637. [Google Scholar] [CrossRef]
- Zhou, J.; Gao, X.; Liu, R.; Xie, Z.; Yang, J.; Zhang, S.; Zhang, G.; Liu, H.; Li, Y.; Zhang, J.; et al. Synthesis of Graphdiyne Nanowalls Using Acetylenic Coupling Reaction. J. Am. Chem. Soc. 2015, 137, 7596–7599. [Google Scholar] [CrossRef]
- He, J.; Bao, K.; Cui, W.; Yu, J.; Huang, C.; Shen, X.; Cui, Z.; Wang, N. Construction of Large-Area Uniform Graphdiyne Film for High-Performance Lithium-Ion Batteries. Chem. Eur. J. 2018, 24, 1187–1192. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Li, J.; Du, R.; Zhou, J.; Huang, M.-Y.; Liu, R.; Li, J.; Xie, Z.; Wu, L.-Z.; Liu, Z.; et al. Direct Synthesis of Graphdiyne Nanowalls on Arbitrary Substrates and Its Application for Photoelectrochemical Water Splitting Cell. Adv. Mater. 2017, 29, 1605308. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, C.; Hou, Z.; Wang, X.; He, J.; Li, X.; Song, Y.; Wang, N.; Wang, K.; Wang, H.; et al. Porous hydrogen substituted graphyne for high capacity and ultra-stable sodium ion storage. J. Mater. Chem. A 2019, 7, 11186–11194. [Google Scholar] [CrossRef]
- He, J.; Wang, N.; Yang, Z.; Shen, X.; Wang, K.; Huang, C.; Yi, Y.; Tu, Z.; Li, Y. Fluoride graphdiyne as a free-standing electrode displaying ultra-stable and extraordinary high Li storage performance. Energy Environ. Sci. 2018, 11, 2893–2903. [Google Scholar] [CrossRef]
- Wang, N.; He, J.; Tu, Z.; Yang, Z.; Zhao, F.; Li, X.; Huang, C.; Wang, K.; Jiu, T.; Yi, Y. Synthesis of Chlorine-Substituted Graphdiyne and Applications for Lithium-Ion Storage. Angew. Chem. Int. Ed. 2017, 129, 10880–10885. [Google Scholar] [CrossRef]
- Li, J.; Xie, Z.; Xiong, Y.; Li, Z.; Huang, Q.; Zhang, S.; Zhou, J.; Liu, R.; Gao, X.; Chen, C. Architecture of β-Graphdiyne-Containing Thin Film Using Modified Glaser–Hay Coupling Reaction for Enhanced Photocatalytic Property of TiO2. Adv. Mater. 2017, 29, 1700421. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, R.; Wang, N.; He, J.; Wang, K.; Li, X.; Shen, X.; Wang, X.; Lv, Q.; Zhang, M.; et al. Triazine-graphdiyne: A new nitrogen-carbonous material and its application as an advanced rechargeable battery anode. Carbon 2018, 137, 442–450. [Google Scholar] [CrossRef]
- Wu, L.M.; Dong, Y.Z.; Zhao, J.L.; Ma, D.T.; Huang, W.C.; Zhang, Y.; Wang, Y.Z.; Jiang, X.T.; Xiang, Y.J.; Li, J.Q.; et al. Kerr Nonlinearity in 2D Graphdiyne for Passive Photonic Diodes. Adv. Mater. 2019, 31, 1807981. [Google Scholar] [CrossRef]
- Wang, N.; Li, X.; Tu, Z.; Zhao, F.; He, J.; Guan, Z.; Huang, C.; Yi, Y.; Li, Y. Synthesis and Electronic Structure of Boron-Graphdiyne with an sp-Hybridized Carbon Skeleton and Its Application in Sodium Storage. Angew. Chem. Int. Ed. 2018, 130, 4032–4037. [Google Scholar] [CrossRef]
- Shang, H.; Zuo, Z.; Li, L.; Wang, F.; Liu, H.; Li, Y.; Li, Y. Ultrathin graphdiyne nanosheets grown in situ on copper nanowires and their performance as lithium-ion battery anodes. Angew. Chem. Int. Ed. 2018, 57, 774–778. [Google Scholar] [CrossRef]
- Li, G.; Li, Y.; Qian, X.; Liu, H.; Lin, H.; Chen, N.; Li, Y. Construction of tubular molecule aggregations of graphdiyne for highly efficient field emission. J. Phys. Chem. C 2011, 115, 2611–2615. [Google Scholar] [CrossRef]
- Huang, H.; Duan, W.; Liu, Z. The existence/absence of Dirac cones in graphynes. New J. Phys. 2013, 15, 023004. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Katsnelson, M.I.; Grigorieva, I.; Dubonos, S.; Firsov, A.A. Two-dimensional gas of massless Dirac fermions in graphene. Nature 2005, 438, 197–200. [Google Scholar] [CrossRef] [PubMed]
- Wallace, P.R. The band theory of graphite. Phys. Rev. 1947, 71, 622. [Google Scholar] [CrossRef]
- Malko, D.; Neiss, C.; Vines, F.; Görling, A. Competition for graphene: Graphynes with direction-dependent dirac cones. Phys. Rev. Lett. 2012, 108, 086804. [Google Scholar] [CrossRef] [Green Version]
- Xi, J.; Wang, D.; Shuai, Z. Electronic properties and charge carrier mobilities of graphynes and graphdiynes from first principles. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2015, 5, 215–227. [Google Scholar] [CrossRef]
- Narita, N.; Nagai, S.; Suzuki, S.; Nakao, K. Optimized geometries and electronic structures of graphyne and its family. Phys. Rev. B 1998, 58, 11009. [Google Scholar] [CrossRef]
- Ebadi, M.; Reisi-Vanani, A.; Houshmand, F.; Amani, P. Calcium-decorated graphdiyne as a high hydrogen storage medium: Evaluation of the structural and electronic properties. Int. J. Hydrogen Energy 2018, 43, 23346–23356. [Google Scholar] [CrossRef]
- Hou, X.; Xie, Z.; Li, C.; Li, G.; Chen, Z. Study of electronic structure, thermal conductivity, elastic and optical properties of α, β, γ-graphyne. Materials 2018, 11, 188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nulakani, N.V.R.; Subramanian, V. Cp-Graphyne: A Low-Energy Graphyne Polymorph with Double Distorted Dirac Points. ACS Omega 2017, 2, 6822–6830. [Google Scholar] [CrossRef]
- Srinivasu, K.; Ghosh, S.K. Graphyne and Graphdiyne: Promising Materials for Nanoelectronics and Energy Storage Applications. J. Phys. Chem. C 2012, 116, 5951–5956. [Google Scholar] [CrossRef]
- Kang, J.; Li, J.; Wu, F.; Li, S.-S.; Xia, J.-B. Elastic, Electronic, and Optical Properties of Two-Dimensional Graphyne Sheet. J. Phys. Chem. C 2011, 115, 20466–20470. [Google Scholar] [CrossRef]
- Yue, Q.; Chang, S.; Kang, J.; Qin, S.; Li, J. Mechanical and Electronic Properties of Graphyne and Its Family under Elastic Strain: Theoretical Predictions. J. Phys. Chem. C 2013, 117, 14804–14811. [Google Scholar] [CrossRef]
- Yun, J.; Zhang, Z.; Yan, J.; Zhao, W.; Xu, M. First-principles study of B or Al-doping effect on the structural, electronic structure and magnetic properties of γ-graphyne. Comput. Mater. Sci. 2015, 108, 147–152. [Google Scholar] [CrossRef]
- Singh, N.B.; Bhattacharya, B.; Sarkar, U. A first principle study of pristine and BN-doped graphyne family. J. Struct. Chem. 2014, 25, 1695–1710. [Google Scholar] [CrossRef]
- Koo, J.; Park, M.; Hwang, S.; Huang, B.; Jang, B.; Kwon, Y.; Lee, H. Widely tunable band gaps of graphdiyne: An ab initio study. Phys. Chem. Chem. Phys. 2014, 16, 8935–8939. [Google Scholar] [CrossRef] [Green Version]
- Luo, G.; Qian, X.; Liu, H.; Qin, R.; Zhou, J.; Li, L.; Gao, Z.; Wang, E.; Mei, W.-N.; Lu, J. Quasiparticle energies and excitonic effects of the two-dimensional carbon allotrope graphdiyne: Theory and experiment. Phys. Rev. B 2011, 84, 075439. [Google Scholar] [CrossRef] [Green Version]
- Jiao, Y.; Du, A.; Hankel, M.; Zhu, Z.; Rudolph, V.; Smith, S.C. Graphdiyne: A versatile nanomaterial for electronics and hydrogen purification. Chem. Commun. 2011, 47, 11843–11845. [Google Scholar] [CrossRef]
- Long, M.; Tang, L.; Wang, D.; Li, Y.; Shuai, Z. Electronic Structure and Carrier Mobility in Graphdiyne Sheet and Nanoribbons: Theoretical Predictions. ACS Nano 2011, 5, 2593–2600. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.-J.; Sheng, X.-L.; Yan, Q.-B.; Zheng, Q.-R.; Su, G. Strain-induced Dirac cone-like electronic structures and semiconductor–semimetal transition in graphdiyne. Phys. Chem. Chem. Phys. 2013, 15, 8179–8185. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Xi, J.; Wang, D.; Shuai, Z. Carrier Mobility in Graphyne Should Be Even Larger than That in Graphene: A Theoretical Prediction. J. Phys. Chem. Lett. 2013, 4, 1443–1448. [Google Scholar] [CrossRef] [PubMed]
- Narita, N.; Nagai, S.; Suzuki, S.; Nakao, K. Electronic structure of three-dimensional graphyne. Phys. Rev. B 2000, 62, 11146. [Google Scholar] [CrossRef]
- Luo, G.; Zheng, Q.; Mei, W.-N.; Lu, J.; Nagase, S. Structural, Electronic, and Optical Properties of Bulk Graphdiyne. J. Phys. Chem. C 2013, 117, 13072–13079. [Google Scholar] [CrossRef]
- Zheng, Q.; Luo, G.; Liu, Q.; Quhe, R.; Zheng, J.; Tang, K.; Gao, Z.; Nagase, S.; Lu, J. Structural and electronic properties of bilayer and trilayer graphdiyne. Nanoscale 2012, 4, 3990–3996. [Google Scholar] [CrossRef] [PubMed]
- Shohany, B.G.; Roknabadi, M.R.; Kompany, A. Computational study of edge configuration and the diameter effects on the electrical transport of graphdiyne nanotubes. Phys. E 2016, 84, 146–151. [Google Scholar] [CrossRef]
- Pan, L.D.; Zhang, L.Z.; Song, B.Q.; Du, S.X.; Gao, H.-J. Graphyne- and graphdiyne-based nanoribbons: Density functional theory calculations of electronic structures. Appl. Phys. Lett. 2011, 98, 173102. [Google Scholar] [CrossRef] [Green Version]
- Son, Y.-W.; Cohen, Y.-W.; Louie, S.G. Energy Gaps in Graphene Nanoribbons. Phys. Rev. Lett. 2006, 97, 216803. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Li, J.; Sheng, X.-L. Graphdiyne nanoribbons with open hexagonal rings: Existence of topological unprotected edge states. Phys. Lett. A 2017, 381, 3337–3341. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Liu, C.; Sun, Z.; Cao, T.; Zhang, Z.; Wang, E.; Liu, Z.; Liu, K. Interfacial engineering in graphene bandgap. Chem. Soc. Rev. 2018, 47, 3059–3099. [Google Scholar] [CrossRef] [PubMed]
- Makaremi, M.; Mortazavi, B.; Rabczuk, T.; Ozin, G.A.; Singh, C.V. Theoretical investigation: 2D N-graphdiyne nanosheets as promising anode materials for Li/Na rechargeable storage devices. ACS Appl. Nano Mater. 2018, 2, 127–135. [Google Scholar] [CrossRef] [Green Version]
- Qiu, H.; Xue, M.M.; Shen, C.; Zhang, Z.H.; Guo, W.L. Graphynes for Water Desalination and Gas Separation. Adv. Mater. 2019, 31, 1803772. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Magagula, S.; Zhao, J.; Chen, Z. Boosting ORR/OER activity of graphdiyne by simple heteroatom doping. Small Methods 2019, 3, 1800550. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, Y.; Guo, M.; Yun, J. Adsorption of hydrogen and oxygen on graphdiyne and its BN analog sheets: A density functional theory study. Comput. Mater. Sci. 2019, 160, 197–206. [Google Scholar] [CrossRef]
- Kang, J.; Wu, F.; Li, J. Modulating the bandgaps of graphdiyne nanoribbons by transverse electric fields. J. Phys. Condens. Matter. 2012, 24, 165301. [Google Scholar] [CrossRef] [Green Version]
- Leenaerts, O.; Partoens, B.; Peeters, F. Tunable double dirac cone spectrum in bilayer α-graphyne. Appl. Phys. Lett. 2013, 103, 013105. [Google Scholar] [CrossRef]
- Abdi, G.; Filip, A.; Krajewski, M.; Kazimierczuk, K.; Strawski, M.; Szarek, P.; Hamankiewicz, B.; Mazej, Z.; Cichowicz, G.; Leszczyński, P.J.; et al. Toward the synthesis, fluorination and application of N–graphyne. RSC Adv. 2020, 10, 40019–40029. [Google Scholar] [CrossRef]
- Haji-Nasiri, S.; Fotoohi, S. Doping induced diode behavior with large rectifying ratio in graphyne nanoribbons device. Phys. Lett. A 2018, 382, 2894–2899. [Google Scholar] [CrossRef]
- Jhon, Y.I.; Jhon, M.S. Electron Transport Properties of Graphene-Graphyne-Graphene Transistors: First Principles Study. arXiv 2013, arXiv:1307.4374. [Google Scholar]
- Li, Z.; Smeu, M.; Rives, A.; Maraval, V.; Chauvin, R.; Ratner, M.A.; Borguet, E. Towards graphyne molecular electronics. Nat. Commun. 2015, 6, 6321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhattacharya, B.; Sarkar, U. The Effect of Boron and Nitrogen Doping in Electronic, Magnetic, and Optical Properties of Graphyne. J. Phys.Chem. C 2016, 120, 26793–26806. [Google Scholar] [CrossRef]
- Yang, Z.; Ouyang, B.; Lan, G.; Xu, L.C.; Liu, R.; Liu, X. The tunneling magnetoresistance and spin-polarized optoelectronic properties of graphyne-based molecular magnetic tunnel junctions. J. Phys. D Appl. Phys. 2017, 50, 075103. [Google Scholar] [CrossRef]
- Jin, Z.; Zhou, Q.; Chen, Y.; Mao, P.; Li, H.; Liu, H.; Wang, J.; Li, Y. Graphdiyne:ZnO Nanocomposites for High-Performance UV Photodetectors. Adv. Mater. 2016, 28, 3697–3702. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Kuang, D.; Gao, Y.; Cheng, J.; Li, X.; Guo, J.; Yu, Z. Titania: Graphdiyne nanocomposites for high-performance deep ultraviolet photodetectors based on mixed-phase MgZnO. J. Alloys Compd. 2020, 825, 153882. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, P.; Guo, J.; Shi, R.; Huang, W.; Shi, Z.; Wu, L.; Zhang, F.; Gao, L.; Li, C.; et al. Graphdiyne-Based Flexible Photodetectors with High Responsivity and Detectivity. Adv.Mater. 2020, 32, 2001082. [Google Scholar] [CrossRef]
- Makarova, T. Magnetism of carbon-based materials. arXiv 2002, arXiv:cond-mat/0207368. [Google Scholar]
- Li, Z.; Sheng, W.; Ning, Z.; Zhang, Z.; Yang, Z.; Guo, H. Magnetism and spin-polarized transport in carbon atomic wires. Phys. Rev. B 2020, 80, 115429. [Google Scholar] [CrossRef]
- Cauchy, T.; Ruiz, E.; Jeannin, O.; Nomura, M.; Fourmigué, M. Strong magnetic interactions through weak bonding interactions in organometallic radicals: Combined experimental and theoretical study. Chem. Eur. J. 2007, 13, 8858–8866. [Google Scholar] [CrossRef]
- Ottaviani, M.F.; Cossu, E.; Turro, N.J.; Tomalia, D.A. Characterization of starburst dendrimers by electron paramagnetic resonance. 2. Positively charged nitroxide radicals of variable chain length used as spin probes. J. Am. Chem. Soc. 1995, 117, 4387–4398. [Google Scholar] [CrossRef]
- Chen, X.; Gao, P.; Guo, L.; Wen, Y.; Zhang, Y.; Zhang, S. Two-dimensional ferromagnetism and spin filtering in Cr and Mn-doped graphdiyne. J. Phys. Chem. Solids 2017, 105, 61–65. [Google Scholar] [CrossRef]
- He, J.; Ma, S.Y.; Zhou, P.; Zhang, C.; He, C.; Sun, L. Magnetic properties of single transition-metal atom absorbed graphdiyne and graphyne sheet from DFT+ U calculations. J. Phys. Chem. C 2012, 116, 26313–26321. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.; Puigdollers, A.R.; Gamallo, P.; Vines, F.; Lee, J.Y. Functionalization of γ-graphyne by transition metal adatoms. Carbon 2017, 120, 63–70. [Google Scholar] [CrossRef]
- Ren, J.; Zhang, S.-B.; Liu, P.-P. Magnetic and Electronic Properties of β-Graphyne Doped with Rare-Earth Atoms. Chin. Phys. Lett. 2019, 36, 076101. [Google Scholar] [CrossRef]
- Alaei, S.; Jalili, S.; Erkoc, S. Study of the influence of transition metal atoms on electronic and magnetic properties of graphyne nanotubes using density functional theory. Fuller. Nanotub. Carbon Nanostruct. 2015, 23, 494–499. [Google Scholar] [CrossRef]
- He, J.; Zhou, P.; Jiao, N.; Chen, X.; Lu, W.; Sun, L. Prediction of half-semiconductor antiferromagnets with vanishing net magnetization. RSC Adv. 2015, 5, 46640–46647. [Google Scholar] [CrossRef]
- Drogar, J.; Roknabadi, M.R.; Behdani, M.; Modarresi, M.; Kari, A. Hydrogen adsorption on the α-graphyne using ab initio calculations. Superlattices Microstruct. 2014, 75, 340–346. [Google Scholar] [CrossRef]
- Wang, Y.; Song, N.; Zhang, T.; Zheng, Y.; Gao, H.; Xu, K.; Wang, J. Tuning the electronic and magnetic properties of graphyne by hydrogenation. Appl. Surf. Sci. 2018, 452, 181–189. [Google Scholar] [CrossRef]
- Sofo, J.O.; Chaudhari, A.S.; Barber, G.D. Graphane: A two-dimensional hydrocarbon. Phys. Rev. B 2007, 75, 153401. [Google Scholar] [CrossRef] [Green Version]
- Feng, Z.; Ma, Y.; Li, Y.; Li, R.; Liu, J.; Li, H.; Tang, Y.; Dai, X. Importance of heteroatom doping site in tuning the electronic structure and magnetic properties of graphdiyne. Phys. E 2019, 114, 113590. [Google Scholar] [CrossRef]
- Ma, Y.; Foster, A.S.; Krasheninnikov, A.V.; Nieminen, R.M. Nitrogen in graphite and carbon nanotubes: Magnetism and mobility. Phys. Rev. B 2005, 72, 205416. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Wang, X.; Sun, H.; Wang, N.; Lv, Q.; Cui, W.; Long, Y.; Huang, C. Enhanced paramagnetism of mesoscopic graphdiyne by doping with nitrogen. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, Q.; Tang, N.; Liu, F.; Cao, Q.; Zheng, W.; Ren, W.; Wan, X.; Du, Y. Obtaining high localized spin magnetic moments by fluorination of reduced graphene oxide. ACS Nano 2013, 7, 6729–6734. [Google Scholar] [CrossRef]
- Zheng, Y.; Chen, Y.; Lin, L.; Sun, Y.; Liu, H.; Li, Y.; Du, Y.; Tang, N. Intrinsic magnetism of graphdiyne. Appl. Phys. Lett. 2017, 111, 033101. [Google Scholar] [CrossRef]
- Zhang, M.; Sun, H.; Wang, X.; Du, H.; He, J.; Long, Y.; Zhang, Y.; Huang, C. Room-Temperature Ferromagnetism in Sulfur-Doped Graphdiyne Semiconductors. J. Phys. Chem. C 2019, 123, 5010–5016. [Google Scholar] [CrossRef]
- Lee, C.; Wei, X.; Kysar, J.W.; Hone, J. Measurement of the Elastic Properties and Intrinsic Strength of Monolayer Graphene. Science 2008, 321, 385–388. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Y.; Pei, Q.X.; Wang, C.M. Mechanical properties of graphynes under tension: A molecular dynamics study. Appl. Phys. Lett. 2012, 101, 081909. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Si, Y.; Yuan, J.; Yang, B.; Chen, H. Tunable thermal transport and mechanical properties of graphyne heterojunctions. Phys. Chem. Chem. Phys. 2016, 18, 24210–24218. [Google Scholar] [CrossRef]
















| Synthetic Method | Year | Condition | Reaction | [Ref.] |
|---|---|---|---|---|
| Glaser coupling | 1869 |
| 2 R–C≡C–H → R–C≡C–C≡C–R | [43] |
| Eglinton coupling | 1959 |
| 2 R–C≡C–H → R–C≡C–C≡C–R | [44] |
| Hay coupling | 1962 |
| 2 R–C≡C–H → R–C≡C–C≡C–R | [45] |
| Negishi cross-coupling reaction | 1977 |
| RX + R′-ZnX′ + MLn → R-R′ X = Cl, Br, I, triflate and acetyloxy X′ = Cl, Br, I R = alkenyl, aryl, allyl, alkynyl or propargyl R′ = alkenyl, aryl, allyl, alkyl, benzyl, homoallyl, and homopropargyl. L = triphenylphosphine, DPPE, BINAP or chiraphos M = Ni, Pd | [46] |
| Hiyama coupling | 1988 |
| R-SiR′′3 + R′-X → R-R′ R: aryl, alkenyl or alkynyl R′: aryl, alkenyl, alkynyl or alkyl R′′: Cl, F or alkyl X: Cl, Br, I or OTf | [47] |
| Sonogashira cross-coupling reaction | 2002 |
| R1-X + H–C≡C–R2 → R1–C≡C–R2 R1: aryl R2: aryl or vinyl X: I, Br, Cl or OTf | [48] |
| Name/Percentage of Acetylenic Linkages % | Band Gap/Method | [Ref.] | Name | Band Gap/Method | [Ref.] |
|---|---|---|---|---|---|
| γ-GY | 0.447/PBE 0.448/PBE-D 0.45/PBE 1.2/MNDO 0.52/FP-LCAO 0.47/PBE 2.23/B3LYP 0.46/PBE 0.46/PBE 0.96/HSE06 0.94 HSE06 0.474 0.454/PBE | [122] [122] [124] [33] [121] [125] [125] [126] [127] [128] [127] [128] [129] | γ-GDY | 0.5/PBE 0.44/LDA 1.10/GW 0.53/FP-LCAO 1.22/HSE06 0.46/PBE 0.52/PBE 1.18/B3LYP 0.89/HSE06 0.47/PW91 0.9/HSE06 1.21/HSE06 0.485/PBE | [130] [131] [131] [121] [132] [133] [125] [125] [127] [134] [134] [106] [129] |
| β-GY | 0.028/PBE 0.04/PBE-D | [122] [122] | α-GY | 0/PBE 0.005/PBE-D | [122] [122] |
| 6,6,12-GY | 0/PBE | [135] a | Graphyne-3 | 0.6 at M/FP-LCAO 0.56/PBE 0.566/PBE | [121] b [127] [129] |
| Graphyne-4 | 0.59/FP-LCAO 0.54/PBE 0.542/PBE | [121] c [127] [129] | Bulk-GY | 0–0.5/FP-LSDA | [136] d |
| Bulk-GDY | 0.05–0.74/HSE06 | [137] d | Trilayer GDYs | 0.18–0.33/PW91 0.9/HSE06 | [138] d [106] |
| Bilayer GDYs | 0.14–0.35/PW91 0.99/HSE06 | [138] e [106] | (2,0)-AGDYNT (6.42 Å) | 0.95/PBE | [139] |
| (2,2)-ZGDYNT (10.25 Å) | 0.65/PBE | [139] | (3,0)-AGDYNT (9.08 Å) | 0.65/PBE | [139] |
| (3,3)-ZGDYNT (15.56 Å) | 0.55/PBE | [139] | (4,0)-AGDYNT (12.04 Å) | 0.55/PBE | [139] |
| (4,4)-ZGDYNT (20.81 Å) | 0.5/PBE | [139] | AGYNRs (10–45 Å) | 1.25–0.59/LDA | [140] |
| AGDYNRs (12–62 Å) | 0.97–0.54/LDA | [140] | ZGYNRs (14–38 Å) | 1.65–0.73/LDA | [140] |
| ZGYNRs (12–30 Å) | 1.32–0.75/LDA | [140] | AGNRs (20 Å) | 0.5/LDA | [141] |
| AGYNRs (20 Å) | 0.8/LDA | [140] | ZGDYNRs (19.2–28.6 Å) | 1.205–0.895/PBE | [133] |
| AGDYNRs (12.5–20.7 Å) | 0.954–0.817/PBE | [133] | GY (−2 to +10% A-strain) 0.87–1.47 | 0.4–0.17/PBE 0.87–0.56/HSE06 | [127] |
| GY (−2 to +10% H-strain) | 0.4–0.88/PBE 0.87–1.47/HSE06 | [127] [127] | GDY (−2 to +10% H-strain) | 0.41–0.94/PBE 0.8–1.53/HSE06 | [127] [127] |
| GY (−2 to +10% Z-strain) | 0.37–0.3/PBE 0.83–0.71/HSE06 | [127] [127] | GDY (−2 to +10% Z-strain) | 0.39–0.21/PBE 0.78–0.56/HSE06 | [127] [127] |
| GDY (−2 to +10% A-strain) | 0.39–0.31/PBE 0.78–0.69/HSE06 | [127] [127] | AGDYNRs (various n of nanoribbon) | 0.04–0.69/VASP | [142] |
| α-GYs (0–10% H-strain) | 0/PBE | [123] | AGDYNRs (various n of nanoribbon) | 0.01–0.69/OpenMX | [142] |
| β-GYs (0–10% H-strain) | 0.028–1.469/PBE | [123] | ZGDYNRs (28.6 Å) | 0.895/PBE | [133] |
| γ-GYs (0–10% H-strain) | 0.447–0.865/PBE | [123] | Graphene/0 | 0 | [143] |
| Lattic Constant (Å) | Interlayer Distance (Å) | Binding Energy (eV/atom) | Band Gap | [Ref.] | |
|---|---|---|---|---|---|
| α | 6.86 | 3.51 | 7.948 | 0 | [136] |
| β1 | 6.86 | 3.27 | 7.963 | 0.19 | [136] |
| β2 | 6.86 | 3.28 | 7.962 | 0.5 | [136] |
| β3 | 6.86 | 3.34 | 7.957 | 0 | [136] |
| 2D GY | 6.86 | - | 7.951 | 0.52 | [121] |
| graphite | 2.46 | 3.17 | 8.867 | 0 | [136] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdi, G.; Alizadeh, A.; Grochala, W.; Szczurek, A. Developments in Synthesis and Potential Electronic and Magnetic Applications of Pristine and Doped Graphynes. Nanomaterials 2021, 11, 2268. https://doi.org/10.3390/nano11092268
Abdi G, Alizadeh A, Grochala W, Szczurek A. Developments in Synthesis and Potential Electronic and Magnetic Applications of Pristine and Doped Graphynes. Nanomaterials. 2021; 11(9):2268. https://doi.org/10.3390/nano11092268
Chicago/Turabian StyleAbdi, Gisya, Abdolhamid Alizadeh, Wojciech Grochala, and Andrzej Szczurek. 2021. "Developments in Synthesis and Potential Electronic and Magnetic Applications of Pristine and Doped Graphynes" Nanomaterials 11, no. 9: 2268. https://doi.org/10.3390/nano11092268
APA StyleAbdi, G., Alizadeh, A., Grochala, W., & Szczurek, A. (2021). Developments in Synthesis and Potential Electronic and Magnetic Applications of Pristine and Doped Graphynes. Nanomaterials, 11(9), 2268. https://doi.org/10.3390/nano11092268