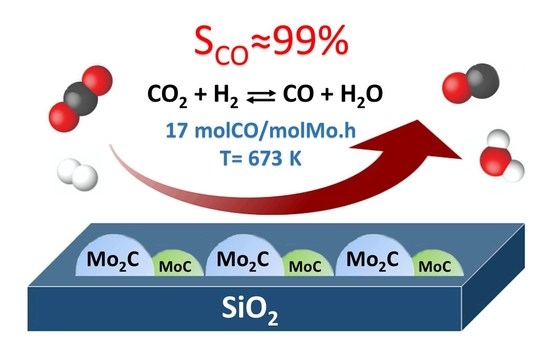
Supported Nanostructured MoxC Materials for the Catalytic Reduction of CO2 through the Reverse Water Gas Shift Reaction
Abstract
:1. Introduction
2. Experimental
2.1. Preparation of Catalysts
2.2. Characterization of Catalysts
2.3. RWGS Catalytic Tests
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Leung, D.Y.C.; Caramanna, G.; Maroto-Valer, M.M. An Overview of Current Status of Carbon Dioxide Capture and Storage Technologies. Renew. Sustain. Energy Rev. 2014, 39, 426–443. [Google Scholar] [CrossRef]
- Cuéllar-Franca, R.M.; Azapagic, A. Carbon Capture, Storage and Utilisation Technologies: A Critical Analysis and Comparison of Their Life Cycle Environmental Impacts. J. CO2 Util. 2015, 9, 82–102. [Google Scholar] [CrossRef]
- Choi, S.; Drese, J.H.; Jones, C.W. Adsorbent Materials for Carbon Dioxide Capture from Large Anthropogenic Point Sources. Chem. Sus. Chem. 2009, 2, 796–854. [Google Scholar] [CrossRef] [PubMed]
- D'Alessandro, D.M.; Smit, B.; Long, J.R. Carbon Dioxide Capture: Prospects for New Materials. Angew. Chem. Int. Ed. Engl. 2010, 49, 6058–6082. [Google Scholar] [CrossRef] [PubMed]
- Homs, N.; Toyir, J.; Ramirez de la Piscina, P. Catalytic Processes for Activation of CO2. In New and Future Developments in Catalysis; Elsevier: Amsterdam, The Netherlands, 2013; pp. 1–26. [Google Scholar]
- Din, I.U.; Shaharun, M.S.; Alotaibi, M.A.; Alharthi, A.I.; Naeem, A. Recent Developments on Heterogeneous Catalytic CO2 Reduction to Methanol. J. CO2 Util. 2019, 34, 20–33. [Google Scholar]
- Saeidi, S.; Najari, S.; Hessel, V.; Wilson, K.; Keil, F.J.; Concepción, P.; Suib, S.L.; Rodrigues, A.E. Recent Advances in CO2 Hydrogenation to Value-Added Products-Current Challenges and Future Directions. Prog. Energy Combust. 2021, 85, 100905. [Google Scholar]
- Bahmanpour, A.M.; Signorile, M.; Kröcher, O. Recent Progress in Syngas Production Via Catalytic CO2 Hydrogenation Reaction. Appl. Catal. B 2021, 295, 120319. [Google Scholar] [CrossRef]
- Boot-Handford, M.E.; Abanades, J.C.; Anthony, E.J.; Blunt, M.J.; Brandani, S.; Mac Dowell, N.; Fernández, J.R.; Ferrari, M.C.; Gross, R.; Hallett, J.P.; et al. Carbon Capture and Storage Update. Energy Environ. Sci. 2014, 7, 130–189. [Google Scholar]
- Daza, Y.A.; Kuhn, J.N. CO2 Conversion by Reverse Water Gas Shift Catalysis: Comparison of Catalysts, Mechanisms and Their Consequences for CO2 Conversion to Liquid Fuels. RSC Adv. 2016, 6, 49675–49691. [Google Scholar] [CrossRef]
- Liu, X.M.; Lu, G.Q.; Yan, Z.F.; Beltramini, J. Recent Advances in Catalysts for Methanol Synthesis Via Hydrogenation of CO and CO2. Ind. Eng. Chem. Res. 2003, 42, 6518–6530. [Google Scholar] [CrossRef]
- Jahangiri, H.; Bennett, J.; Mahjoubi, P.; Wilson, K.; Gu, S. A Review of Advanced Catalyst Development for Fischer–Tropsch Synthesis of Hydrocarbons from Biomass Derived Syn-Gas. Catal. Sci. Technol. 2014, 4, 2210–2229. [Google Scholar] [CrossRef]
- Saeidi, S.; Amin, N.A.S.; Rahimpour, M.R. Hydrogenation of CO2 to Value-Added Products—A Review and Potential Future Developments. J. CO2 Util. 2014, 5, 66–81. [Google Scholar] [CrossRef]
- Elsernagawy, O.Y.H.; Hoadley, A.; Patel, J.; Bhatelia, T.; Lim, S.; Haque, N.; Li, C. Thermo-Economic Analysis of Reverse Water-Gas Shift Process With Different Temperatures for Green Methanol Production as a Hydrogen Carrier. J. CO2 Util. 2020, 41, 101280. [Google Scholar] [CrossRef]
- Kirsch, H.; Sommer, U.; Pfeifer, P.; Dittmeyer, R. Power-To-Fuel Conversion Based on Reverse Water-Gas-Shift, Fischer-Tropsch Synthesis and Hydrocracking: Mathematical Modeling and Simulation in Matlab/Simulink. Chem. Eng. Sci. 2020, 227, 115930. [Google Scholar] [CrossRef]
- Liang, B.; Duan, H.; Su, X.; Chen, X.; Huang, Y.; Chen, X.; Delgado, J.J.; Zhang, T. Promoting Role of Potassium in the Reverse Water Gas Shift Reaction on Pt/Mullite Catalyst. Catal. Today 2017, 281, 319–326. [Google Scholar] [CrossRef]
- Levy, R.B.; Boudart, M. Platinum-Like Behavior of Tungsten Carbide in Surface Catalysis. Science 1973, 181, 547–549. [Google Scholar] [CrossRef] [PubMed]
- Oyama, S.T. The Chemistry of Transition Metal Carbides and Nitrides; Blackie Academic & Professional: Glasgow, Scotland, 1996. [Google Scholar]
- Porosoff, M.D.; Yang, X.; Boscoboinik, J.A.; Chen, J.G. Molybdenum Carbide as Alternative Catalysts to Precious Metals for Highly Selective Reduction of CO2 to CO. Angew. Chem. Int. Ed. Engl. 2014, 53, 6705–6709. [Google Scholar] [CrossRef]
- Porosoff, M.D.; Kattel, S.; Li, W.; Liu, P.; Chen, J.G. Identifying Trends and Descriptors for Selective CO2 Conversion to CO Over Transition Metal Carbides. Chem. Commun. 2015, 51, 6988–6991. [Google Scholar] [CrossRef]
- Liu, X.; Kunkel, C.; Ramírez de la Piscina, P.; Homs, N.; Viñes, F.; Illas, F. Effective and Highly Selective CO Generation from CO2 Using a Polycrystalline α-Mo2C Catalyst. ACS Catal. 2017, 7, 4323–4335. [Google Scholar] [CrossRef]
- Pajares, A.; Prats, H.; Romero, A.; Viñes, F.; de la Piscina, P.; Sayós, R.; Homs, N.; Illas, F. Critical Effect of Carbon Vacancies on the Reverse Water Gas Shift Reaction over Vanadium Carbide Catalysts. Appl. Catal. B 2020, 267, 118719. [Google Scholar] [CrossRef]
- Kunkel, C.; Viñes, F.; Illas, F. Transition metal carbides as novel materials for CO2 capture, storage, and activation. Energy Environ. Sci. 2016, 9, 141–144. [Google Scholar] [CrossRef]
- Posada-Pérez, S.; Viñes, F.; Ramirez, P.J.; Vidal, A.B.; Rodriguez, J.A.; Illas, F. The bending machine: CO2 activation and hydrogenation on δ-MoC(001) and β-Mo2C(001) surfaces. Phys. Chem. Chem. Phys. 2014, 16, 14912–14921. [Google Scholar] [CrossRef] [PubMed]
- Reddy, K.P.; Dama, S.; Mhamane, N.B.; Ghosalya, M.K.; Raja, T.; Satyanarayana, C.V.; Gopinath, C.S. Molybdenum carbide catalyst for the reduction of CO2 to CO: Surface science aspects by NAPPES and catalysis studies. Dalton Trans. 2019, 48, 12199–12209. [Google Scholar] [CrossRef]
- Claridge, J.B.; York, A.P.E.; Brungs, A.J.; Green, M.L.H. Study of the Temperature-Programmed Reaction Synthesis of Early Transition Metal Carbide and Nitride Catalyst Materials from Oxide Precursors. Chem. Mater. 2000, 12, 132–142. [Google Scholar] [CrossRef]
- Volpe, L.; Boudart, M. Compounds of Molybdenum and Tungsten with High Specific Surface Area. J. Solid State Chem. 1985, 59, 348–356. [Google Scholar] [CrossRef]
- Naito, S.; Tsuji, M.; Sakamoto, Y.; Miyao, T. Marked Difference of Catalytic Behavior by Preparation Methods in CH4 Reforming with CO2 Over Mo2C and WC Catalysts. Stud. Surf. Sci. Catal. 2000, 143, 415–423. [Google Scholar]
- Giordano, C.; Erpen, C.; Yao, W.; Antonietti, M. Synthesis of Mo and W Carbide and Nitride Nanoparticles Via a Simple “Urea Glass” Route. Nano Lett. 2008, 8, 4659–4663. [Google Scholar] [CrossRef]
- Gao, J.; Wu, Y.; Jia, C.; Zhong, Z.; Gao, F.; Yang, Y.; Liu, B. Controllable Synthesis of α-MoC1-X and β-Mo2C Nanowires for Highly Selective CO2 Reduction to CO. Catal. Commun. 2016, 84, 147–150. [Google Scholar] [CrossRef]
- Liu, X.; Pajares, A.; Matienzo, D.D.C.; Ramírez de la Piscina, P.; Homs, N. Preparation and Characterization of Bulk MoxC Catalysts and Their Use in the Reverse Water-Gas Shift Reaction. Catal. Today 2020, 356, 384–389. [Google Scholar] [CrossRef]
- Tsuji, M.; Miyao, T.; Naito, S. Remarkable Support Effect of ZrO2 Upon the CO2 Reforming of CH4 Over Supported Molybdenum Carbide Catalysts. Catal. Lett. 2000, 60, 195–198. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, T.; Ying, P.; Zheng, M.; Wu, W.; Xia, L.; Li, T.; Wang, X.; Li, C. A Novel Catalyst for Hydrazine Decomposition: Molybdenum Carbide Supported on γ-Al2O3. Chem. Commun. 2002, 3, 288–289. [Google Scholar] [CrossRef] [PubMed]
- Aegerter, P.A.; Quigley, W.W.C.; Simpson, G.J.; Ziegler, D.D.; Logan, J.W.; McCrea, K.R.; Glazier, S.; Bussell, M.E. Thiophene Hydrodesulfurization over Alumina-Supported Molybdenum Carbide and Nitride Catalysts: Adsorption Sites, Catalytic Activities, and Nature of the Active Surface. J. Catal. 1996, 164, 109–121. [Google Scholar] [CrossRef]
- Da Costa, P.; Potvin, C.; Manoli, J.M.; Breysse, M.; Djéga-Mariadassou, G. Novel Phosphorus-Doped Alumina-Supported Molybdenum and Tungsten Carbides: Synthesis, Characterization and Hydrogenation Properties. Catal. Lett. 2001, 72, 91–97. [Google Scholar] [CrossRef]
- Vo, D.N.; Adesina, A.A. Fischer–Tropsch Synthesis over Alumina-Supported Molybdenum Carbide Catalyst. Appl. Catal. A Gen. 2011, 399, 221–232. [Google Scholar] [CrossRef]
- Nagai, M.; Kurakami, T. Reverse Water Gas Shift Reaction Over Molybdenum Carbide. J. Chem. Eng. Jpn. 2005, 38, 807–812. [Google Scholar] [CrossRef]
- Porosoff, M.D.; Baldwin, J.W.; Peng, X.; Mpourmpakis, G.; Willauer, H.D. Potassium-Promoted Molybdenum Carbide as a Highly Active and Selective Catalyst for CO2 Conversion to CO. Chem. Sus. Chem. 2017, 10, 2408–2415. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Guo, Z.; Jiang, Q.; Wu, K.; Gong, H.; Liu, Y. Molybdenum Carbide Clusters for Thermal Conversion of CO2 to CO Via Reverse Water-Gas Shift Reaction. J. Energy Chem. 2020, 50, 37–43. [Google Scholar] [CrossRef]
- Marquart, W.; Raseale, S.; Prieto, G.; Zimina, A.; Sarma, B.B.; Grunwaldt, J.-D.; Claeys, M.; Fischer, N. CO2 Reduction over Mo2C-Based Catalysts. ACS Catal. 2021, 11, 1624–1639. [Google Scholar] [CrossRef]
- Juneau, M.; Pope, C.; Liu, R.; Porosoff, M.D. Support Acidity as a Descriptor for Reverse Water-Gas Shift over Mo2C-Based Catalysts. Appl. Catal. A Gen. 2021, 620, 118034. [Google Scholar] [CrossRef]
- Zhang, H.; Banfield, J.F. Understanding Polymorphic Phase Transformation Behavior During Growth of Nanocrystalline Aggregates: Insights from TiO2. J. Phys. Chem. B 2000, 104, 3481–3487. [Google Scholar] [CrossRef]
- Snyder, R.L. The use of reference intensity ratios in X-ray quantitative analysis. Powder Diffr. 1992, 7, 186–193. [Google Scholar] [CrossRef]
- Dieterle, M.; Weinberg, G.; Mestl, G. Raman Spectroscopy of Molybdenum Oxides. Part I. Structural Characterization of Oxygen Defects in MoO1-x by DR UV/VIS, Raman Spectroscopy and X-Ray Diffraction. Phys. Chem. Chem. Phys. 2002, 4, 812–821. [Google Scholar] [CrossRef]
- Silveira, J.V.; Batista, J.A.; Saraiva, G.D.; Mendes Filho, J.; Souza Filho, A.G.; Hu, S.; Wang, X. Temperature Dependent Behavior of Single Walled MoO3 Nanotubes: A Raman Spectroscopy Study. Vib. Spectrosc. 2010, 54, 179–183. [Google Scholar] [CrossRef]
- Camacho-López, M.; Escobar-Alarcón, L.; Picquart, M.; Arroyo, R.; Córdoba, G.; Haro-Poniatowski, E. Micro-Raman Study of the m-MoO2 to α-MoO3. Opt. Mater. 2011, 33, 480–484. [Google Scholar] [CrossRef]
- Zhang, W.; He, Y.; Zhang, M.; Yin, Z.; Chen, Q. Raman Scattering Study on Anatase TiO2 nanocrystals. J. Phys. D Appl. Phys. 2000, 33, 912–916. [Google Scholar] [CrossRef]
- Suo, Z.; Kou, Y.; Niu, J.Z.; Zhang, W.Z.; Wang, H.I. Characterization of TiO2-, ZrO2- and Al2O3-Supported Iron Catalysts as Used for CO2 Hydrogenation. Appl. Catal. A Gen. 1997, 148, 301–313. [Google Scholar] [CrossRef]
- Mazza, T.; Barborini, E.; Piseri, P.; Milani, P.; Cattaneo, D.; Li Bassi, A.; Bottani, C.E.; Ducati, C. Raman Spectroscopy Characterization of TiO2 Rutile Nanocrystals. Phys. Rev. B 2007, 75, 045416. [Google Scholar] [CrossRef]
- Shrestha, A.; Gao, X.; Hicks, J.C.; Paolucci, C. Nanoparticle Size Effects on Phase Stability for Molybdenum and Tungsten Carbides. Chem. Mater. 2021, 33, 4606–4620. [Google Scholar] [CrossRef]
- Gao, Q.; Zhao, X.; Xiao, Y.; Zhao, D.; Cao, M. A Mild Route to Mesoporous Mo2C–C Hybrid Nanospheres for High Performance Lithium-Ion Batteries. Nanoscale 2014, 6, 6151. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, S.; Zhong, Y.; Cai, R.; Li, L.; Shao, Z. Facile Synthesis of a MoO2–Mo2C–C Composite and Its Application as Favorable Anode Material for Lithium-Ion Batteries. J. Power Sources 2016, 307, 552–560. [Google Scholar] [CrossRef]
- Ojha, K.; Saha, S.; Kolev, H.; Kumar, B.; Ganguli, A.K. Composites of Graphene-Mo2C Rods: Highly Active and Stable Electrocatalyst for Hydrogen Evolution Reaction. Electrochim. Acta 2016, 193, 268–274. [Google Scholar]
- Li, R.; Wang, S.; Wang, W.; Cao, M. Ultrafine Mo2C Nanoparticles Encapsulated in N-Doped Carbon Nanofibers with Enhanced Lithium Storage Performance. Phys. Chem. Chem. Phys. 2015, 17, 24803–24809. [Google Scholar] [CrossRef] [PubMed]
- Bhaskar, A.; Deepa, M.; Narasinga Rao, T.N. MoO2/Multiwalled Carbon Nanotubes (MWCNT) Hybrid for Use as a Li-Ion Battery Anode. ACS Appl. Mater. Interfaces 2013, 5, 2555–2566. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Liu, J.; Zhou, W.; Lin, J.; Shen, Z. Nitrogen-Doped Graphene-Supported Transition-Metals Carbide Electrocatalysts for Oxygen Reduction Reaction. Sci. Rep. 2015, 5, 10389–10398. [Google Scholar]
- Ji, W.; Shen, R.; Yang, R.; Yu, G.; Guo, X.; Peng, L.; Ding, W. Partially Nitride Molybdenum Trioxide With Promoted Performance as an Anode Material for Lithium-Ion Batteries. J. Mater. Chem. A 2014, 2, 699–704. [Google Scholar] [CrossRef]
- Spevack, P.A.; McIntyre, N.S. A Raman and XPS Investigation of Supported Molybdenum Oxide Thin Films. 1. Calcination and Reduction Studies. J. Phys Chem. 1993, 97, 11020–11030. [Google Scholar] [CrossRef]
- Oshikawa, K.; Nagai, M.; Omi, S. Characterization of Molybdenum Carbides for Methane Reforming by TPR, XRD, and XPS. J. Phys. Chem. B 2001, 105, 9124–9131. [Google Scholar] [CrossRef]
- Guil-López, R.; Nieto, E.; Botas, J.A.; Fierro, J.L.G. On the Genesis of Molybdenum Carbide Phases During Reduction-Carburization Reactions. J. Solid State Chem. 2012, 190, 285–295. [Google Scholar] [CrossRef]
- Ma, Y.; Guan, G.; Hao, X.; Zuo, Z.; Huang, W.; Phanthong, P.; Kusakabe, K.; Abudula, A. Highly-Efficient Steam Reforming of Methanol over Copper Modified Molybdenum Carbide. RSC Adv. 2014, 4, 44175–44184. [Google Scholar] [CrossRef]
- Su, X.; Yang, X.; Zhao, B.; Huang, Y. Designing of highly selective and high-temperature endurable RWGS heterogeneous catalysts: Recent advances and the future directions. J. Energy Chem. 2017, 26, 854–867. [Google Scholar] [CrossRef]
- Zhu, M.; Ge, Q.; Zhu, X. Catalytic Reduction of CO2 to CO via Reverse Water Gas Shift Reaction: Recent Advances in the Design of Active and Selective Supported Metal Catalysts. Trans. Tianjin Univ. 2020, 26, 172–187. [Google Scholar] [CrossRef] [Green Version]











| Catalyst | Mo (%wt) | SBET (m2 g−1) | ||
|---|---|---|---|---|
| Fresh a | Post-Reaction b | Post-Reaction c | ||
| MoxC/Al2O3 | 25.1 | 119 (204) | 93 | 97 |
| MoxC/SiO2 | 25.5 | 129 (181) | 115 | 107 |
| MoxC/TiO2 | 27.5 | 39 (13) | 32 | 25 |
| Catalyst | Ea (kJ·mol−1) | (Mo2+,3+/Total Mon+)XPS | (Mo2+,3+,4+/Total Mon+)XPS |
|---|---|---|---|
| MoxC/Al2O3 | 77.7 ± 1.7 | 0.277 | 0.347 |
| MoxC/SiO2 | 64.9 ± 3.2 | 0.431 | 0.690 |
| MoxC/TiO2 | 77.9 ± 4.1 | 0.098 | 0.316 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pajares, A.; Liu, X.; Busacker, J.R.; Ramírez de la Piscina, P.; Homs, N. Supported Nanostructured MoxC Materials for the Catalytic Reduction of CO2 through the Reverse Water Gas Shift Reaction. Nanomaterials 2022, 12, 3165. https://doi.org/10.3390/nano12183165
Pajares A, Liu X, Busacker JR, Ramírez de la Piscina P, Homs N. Supported Nanostructured MoxC Materials for the Catalytic Reduction of CO2 through the Reverse Water Gas Shift Reaction. Nanomaterials. 2022; 12(18):3165. https://doi.org/10.3390/nano12183165
Chicago/Turabian StylePajares, Arturo, Xianyun Liu, Joan R. Busacker, Pilar Ramírez de la Piscina, and Narcís Homs. 2022. "Supported Nanostructured MoxC Materials for the Catalytic Reduction of CO2 through the Reverse Water Gas Shift Reaction" Nanomaterials 12, no. 18: 3165. https://doi.org/10.3390/nano12183165
APA StylePajares, A., Liu, X., Busacker, J. R., Ramírez de la Piscina, P., & Homs, N. (2022). Supported Nanostructured MoxC Materials for the Catalytic Reduction of CO2 through the Reverse Water Gas Shift Reaction. Nanomaterials, 12(18), 3165. https://doi.org/10.3390/nano12183165