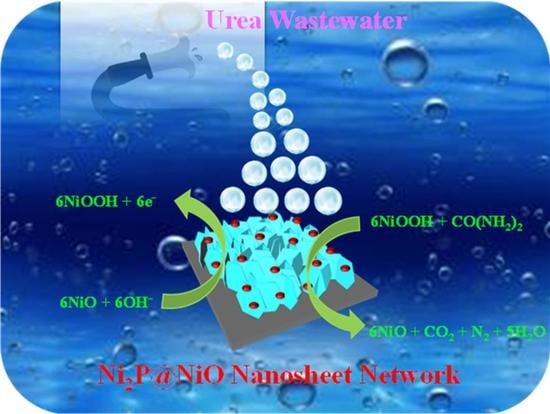
Ni2P Nanoparticle-Inserted Porous Layered NiO Hetero-Structured Nanosheets as a Durable Catalyst for the Electro-Oxidation of Urea
Abstract
:1. Introduction
2. Experimental Section
2.1. Chemicals and Reagents
2.2. Synthesis of the NiO/NiF Catalyst Sample
2.3. Synthesis of the Ni2P@NiO/NiF Catalyst Sample
2.4. Characterizations of the As-Synthesized Nanostructures
2.5. Electrochemical Measurements
3. Results and Discussion
3.1. Synthesis of the Ni2P@NiO Nanostructures
3.2. Electro-Oxidation of Urea (EOU) at the Ni Catalyst-Based Electrodes
3.3. EOU Stability and Wastewater Analyses at the Ni Catalyst-Based Electrodes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, X.; Wang, J.; Sun, X.; Wei, S.; Cui, L.; Yang, W.; Liu, J. Hierarchical coral-like NiMoS nanohybrids as highly efficient bifunctional electrocatalysts for overall urea electrolysis. Nano Res. 2018, 11, 988–996. [Google Scholar] [CrossRef]
- Wang, D.; Vijapur, S.H.; Wang, Y.; Botte, G.G. NiCo2O4 nanosheets grown on current collectors as binder-free electrodes for hydrogen production via urea electrolysis. Int. J. Hydrog. Energy 2017, 42, 3987–3993. [Google Scholar] [CrossRef] [Green Version]
- Theerthagiri, J.; Lee, S.J.; Karuppasamy, K.; Arulmani, S.; Veeralakshmi, S.; Ashokkumar, M.; Choi, M.Y. Application of advanced materials in sonophotocatalytic processes for the remediation of environmental pollutants. J. Hazard. Mater. 2021, 412, 125245. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.-Y.; Lang, C.-C.; Gao, M.-R.; Chen, Y.; Fu, Q.-Q.; Duan, Y.; Yu, S.-H. Ni–Mo–O nanorod-derived composite catalysts for efficient alkaline water-to-hydrogen conversion via urea electrolysis. Energy Environ. Sci. 2018, 11, 1890–1897. [Google Scholar] [CrossRef]
- Alajami, M.; Yassin, M.A.; Ghouri, Z.K.; Al-Meer, S.; Barakat, N.A.M. Influence of bimetallic nanoparticles composition and synthesis temperature on the electrocatalytic activity of NiMn-incorporated carbon nanofibers toward urea oxidation. Int. J. Hydrog. Energy 2018, 43, 5561–5575. [Google Scholar] [CrossRef]
- Sun, X.; Ding, R. Recent progress with electrocatalysts for urea electrolysis in alkaline media for energy-saving hydrogen production. Catal. Sci. Technol. 2020, 10, 1567–1581. [Google Scholar] [CrossRef]
- Xing, P.; Zhou, F.; Zhan, S. Catalytic conversion of seawater to fuels: Eliminating N vacancies in g-C3N4 to promote photocatalytic hydrogen production. Environ. Res. 2021, 197, 111167. [Google Scholar] [CrossRef]
- Wang, D.; Yan, W.; Botte, G.G. Exfoliated nickel hydroxide nanosheets for urea electrolysis. Electrochem. Commun. 2011, 13, 1135–1138. [Google Scholar] [CrossRef]
- Gopi, S.; Al-Mohaimeed, A.M.; Elshikh, M.S.; Yun, K. Facile fabrication of bifunctional SnO–NiO heteromixture for efficient electrocatalytic urea and water oxidation in urea-rich waste water. Environ. Res. 2021, 201, 111589. [Google Scholar] [CrossRef]
- Li, J.; Zhang, J.; Yang, J.-H. Research progress and applications of nickel-based catalysts for electrooxidation of urea. Int. J. Hydrog. Energy 2022, 47, 7693–7712. [Google Scholar] [CrossRef]
- Hu, L.; Jin, L.; Zhang, T.; Zhang, J.; He, J.; Chen, D.; Li, N.; Xu, Q.; Lu, J. Self-supported MoO2-MoO3/Ni2P hybrids as a bifunctional electrocatalyst for energy-saving hydrogen generation via urea–water electrolysis. J. Colloid Interface Sci. 2022, 614, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Shen, S.; Li, M.; Wang, Y.; Li, J.; Xing, Y.; Wang, C.; Pan, H. Rapid in situ synthesis of MgAl-LDH on η-Al2O3 for efficient hydrolysis of urea in wastewater. J. Catal. 2021, 395, 54–62. [Google Scholar] [CrossRef]
- Chouki, T.; Machreki, M.; Topić, J.; Butinar, L.; Stefanov, P.; Jez, E.; Summers, J.S.; Valant, M.; Fait, A.; Emin, S. Iron Phosphide Precatalyst for Electrocatalytic Degradation of Rhodamine B Dye and Removal of Escherichia coli from Simulated Wastewater. Catalysts 2022, 12, 269. [Google Scholar] [CrossRef]
- Li, S.-H.; Qi, M.-Y.; Tang, Z.-R.; Xu, Y.-J. Nanostructured metal phosphides: From controllable synthesis to sustainable catalysis. Chem. Soc. Rev. 2021, 50, 7539–7586. [Google Scholar] [CrossRef]
- Fu, X.; Lin, Y.; Yang, C.; Wu, S.; Wang, Y.; Li, X. Peroxymonosulfate activation via CoP nanoparticles confined in nitrogen-doped porous carbon for enhanced degradation of sulfamethoxazole in wastewater with high salinity. J. Environ. Chem. Eng. 2022, 10, 107734. [Google Scholar] [CrossRef]
- Yu, H.; Ji, J.; Yan, Q.; Xing, M. Transition metal phosphides for heterogeneous Fenton-like oxidation of contaminants in water. Chem. Eng. J. 2022, 449, 137856. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, S.; Ning, P.; Xin, P.; Chen, Z.; Wang, Q.; Uvdal, K.; Hu, Z. Nested hollow architectures of nitrogen-doped carbon-decorated Fe, Co, Ni-based phosphides for boosting water and urea electrolysis. Nano Res. 2022, 15, 1916–1925. [Google Scholar] [CrossRef]
- Feng, H.; Yu, J.; Tang, L.; Zeng, G.; Tang, W.; Wang, J.; Luo, T.; Peng, B.; Song, B.; Wang, L.; et al. Tuning Electron Density Endows Fe1–xCoxP with Exceptional Capability of Electrooxidation of Organic Pollutants. Environ. Sci. Technol. 2019, 53, 13878–13887. [Google Scholar] [CrossRef] [PubMed]
- Sayed, E.T.; Eisa, T.; Mohamed, H.O.; Abdelkareem, M.A.; Allagui, A.; Alawadhi, H.; Chae, K.-J. Direct urea fuel cells: Challenges and opportunities. J. Power Sources 2019, 417, 159–175. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, C. Yolk-shell nanostructural Ni2P/C composites as the high performance electrocatalysts toward urea oxidation. Chin. Chem. Lett. 2021, 32, 2222–2228. [Google Scholar] [CrossRef]
- Sun, W.; Li, J.; Gao, W.; Kang, L.; Lei, F.; Xie, J. Recent advances in the pre-oxidation process in electrocatalytic urea oxidation reactions. Chem. Commun. 2022, 58, 2430–2442. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, S.; Chang, J.; Feng, L. A review of Ni based powder catalyst for urea oxidation in assisting water splitting reaction. Adv. Powder Mater. 2022, 1, 100030. [Google Scholar] [CrossRef]
- Ye, K.; Wang, G.; Cao, D.; Wang, G. Recent Advances in the Electro-Oxidation of Urea for Direct Urea Fuel Cell and Urea Electrolysis. Top. Curr. Chem. 2018, 376, 42. [Google Scholar] [CrossRef]
- Singh, T.I.; Rajeshkhanna, G.; Singh, S.B.; Kshetri, T.; Kim, N.H.; Lee, J.H. Metal–Organic Framework-Derived Fe/Co-based Bifunctional Electrode for H2 Production through Water and Urea Electrolysis. ChemSusChem 2019, 12, 4810–4823. [Google Scholar] [CrossRef]
- Li, J.; Yao, C.; Kong, X.; Li, Z.; Jiang, M.; Zhang, F.; Lei, X. Boosting Hydrogen Production by Electrooxidation of Urea over 3D Hierarchical Ni4N/Cu3N Nanotube Arrays. ACS Sustain. Chem. Eng. 2019, 7, 13278–13285. [Google Scholar] [CrossRef]
- Wang, C.; Lu, H.; Mao, Z.; Yan, C.; Shen, G.; Wang, X. Bimetal Schottky Heterojunction Boosting Energy-Saving Hydrogen Production from Alkaline Water via Urea Electrocatalysis. Adv. Funct. Mater. 2020, 30, 2000556. [Google Scholar] [CrossRef]
- Zhu, X.; Dou, X.; Dai, J.; An, X.; Guo, Y.; Zhang, L.; Tao, S.; Zhao, J.; Chu, W.; Zeng, X.C.; et al. Metallic Nickel Hydroxide Nanosheets Give Superior Electrocatalytic Oxidation of Urea for Fuel Cells. Angew. Chem. Int. Ed. 2016, 55, 12465–12469. [Google Scholar] [CrossRef]
- Zhu, B.; Liang, Z.; Zou, R. Designing Advanced Catalysts for Energy Conversion Based on Urea Oxidation Reaction. Small 2020, 16, 1906133. [Google Scholar] [CrossRef]
- Pérez-Sosa, M.A.; Ramírez-Meneses, E.; Manzo-Robledo, A.; Mateos-Santiago, J.; Hernández-Pérez, M.A.; Garibay-Febles, V.; Lartundo-Rojas, L.; Zacahua-Tlacuatl, G. Enhanced performance of urea electro-oxidation in alkaline media on PtPdNi/C, PtNi/C, and Ni/C catalysts synthesized by one-pot reaction from organometallic precursors. Int. J. Hydrog. Energy 2021, 46, 21419–21432. [Google Scholar] [CrossRef]
- Abd-elnaby, A.E.; Shoueir, K.R.; Wazeer, W.; Kashyout, A.E.-H.B.; El-Kemary, M. Synthesis of binary nanohybrid-based polygonal Pd nanoparticles for proficient photoelectrochemical oxidation of methanol and urea. J. Mater. Sci. Mater. Electron. 2022, 33, 13255–13270. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, C.; Shang, H.; Yuan, M.; Wu, Z.; Li, J.; Du, Y. Self-driven Ru-modified NiFe MOF nanosheet as multifunctional electrocatalyst for boosting water and urea electrolysis. J. Colloid Interface Sci. 2022, 605, 779–789. [Google Scholar] [CrossRef]
- Lera, I.L.; Khasnabis, S.; Wangatia, L.M.; Femi, O.E.; Ramamurthy, P.C. An innovative catalyst of PdNiP nanosphere deposited PEDOT:PSS/rGO hybrid material as an efficient electrocatalyst for alkaline urea oxidation. Polym. Bull. 2022. [Google Scholar] [CrossRef]
- Zheng, S.; Zheng, Y.; Xue, H.; Pang, H. Ultrathin nickel terephthalate nanosheet three-dimensional aggregates with disordered layers for highly efficient overall urea electrolysis. Chem. Eng. J. 2020, 395, 125166. [Google Scholar] [CrossRef]
- Ji, X.; Zhang, Y.; Ma, Z.; Qiu, Y. Oxygen Vacancy-rich Ni/NiO@NC Nanosheets with Schottky Heterointerface for Efficient Urea Oxidation Reaction. ChemSusChem 2020, 13, 5004–5014. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Li, N.; An, J.; Pang, H. Controllable synthesis of a mesoporous NiO/Ni nanorod as an excellent catalyst for urea electro-oxidation. Inorg. Chem. Front. 2020, 7, 2089–2096. [Google Scholar] [CrossRef]
- Xie, J.; Gao, L.; Cao, S.; Liu, W.; Lei, F.; Hao, P.; Xia, X.; Tang, B. Copper-incorporated hierarchical wire-on-sheet α-Ni(OH)2 nanoarrays as robust trifunctional catalysts for synergistic hydrogen generation and urea oxidation. J. Mater. Chem. A 2019, 7, 13577–13584. [Google Scholar] [CrossRef]
- Xie, J.; Liu, W.; Lei, F.; Zhang, X.; Qu, H.; Gao, L.; Hao, P.; Tang, B.; Xie, Y. Iron-Incorporated α-Ni(OH)2 Hierarchical Nanosheet Arrays for Electrocatalytic Urea Oxidation. Chem. Eur. J. 2018, 24, 18408–18412. [Google Scholar] [CrossRef]
- He, G.-Y.; Wang, Y.-T.; Chen, X.-M.; Zhou, Y.; Meng, C.; Li, F.-T. Laser in situ synthesis of NiFe2O4 nanoparticle-anchored NiFe(OH)x nanosheets as advanced electrocatalysts for the oxygen evolution and urea oxidation reactions. Electrochim. Acta 2022, 411, 140074. [Google Scholar] [CrossRef]
- Liu, Z.; Xue, S.; Zhou, S.; Li, J.; Qu, K.; Cai, W. Mutual promotion effect of Ni and Mo2C encapsulated in N-doped porous carbon on bifunctional overall urea oxidation catalysis. J. Catal. 2022, 405, 606–613. [Google Scholar] [CrossRef]
- Liu, Y.; Guan, J.; Chen, W.; Wu, Y.; Li, S.; Du, X.; Zhang, M. Nickel-cobalt derived nanowires/nanosheets as electrocatalyst for efficient H2 generation via urea oxidation reaction. J. Alloys Compd. 2022, 891, 161790. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, H.; Ji, S.; Pollet, B.G.; Wang, X.; Wang, R. Engineered porous Ni2P-nanoparticle/Ni2P-nanosheet arrays via the Kirkendall effect and Ostwald ripening towards efficient overall water splitting. Nano Res. 2020, 13, 2098–2105. [Google Scholar] [CrossRef]
- Joo, J.; Kim, T.; Lee, J.; Choi, S.-I.; Lee, K. Morphology-Controlled Metal Sulfides and Phosphides for Electrochemical Water Splitting. Adv. Mater. 2019, 31, 1806682. [Google Scholar] [CrossRef]
- Tianou, H.; Wang, W.; Yang, X.; Cao, Z.; Kuang, Q.; Wang, Z.; Shan, Z.; Jin, M.; Yin, Y. Inflating hollow nanocrystals through a repeated Kirkendall cavitation process. Nat. Commun. 2017, 8, 1261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, Z.; Qiao, H.; Yang, H.; Zhang, C.; Yan, X. Characterization of NiO nanoparticles by anodic arc plasma method. J. Alloys Compd. 2009, 479, 855–858. [Google Scholar] [CrossRef]
- Yan, H.; Zhang, D.; Xu, J.; Lu, Y.; Liu, Y.; Qiu, K.; Zhang, Y.; Luo, Y. Solution growth of NiO nanosheets supported on Ni foam as high-performance electrodes for supercapacitors. Nanoscale Res. Lett. 2014, 9, 424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Zou, H.; Liu, Y.; Liu, Z.; Sun, W.; Lin, K.A.; Li, T.; Luo, S. Ni2P nanocrystals embedded Ni-MOF nanosheets supported on nickel foam as bifunctional electrocatalyst for urea electrolysis. Sci. Rep. 2021, 11, 21414. [Google Scholar] [CrossRef]
- Feng, L.; Vrubel, H.; Bensimon, M.; Hu, X. Easily-prepared dinickel phosphide (Ni2P) nanoparticles as an efficient and robust electrocatalyst for hydrogen evolution. Phys. Chem. Chem. Phys. 2014, 16, 5917–5921. [Google Scholar] [CrossRef] [Green Version]
- Hao, J.; Liu, J.; Wu, D.; Chen, M.; Liang, Y.; Wang, Q.; Wang, L.; Fu, X.-Z.; Luo, J.-L. In situ facile fabrication of Ni(OH)2 nanosheet arrays for electrocatalytic co-production of formate and hydrogen from methanol in alkaline solution. Appl. Catal. B Environ. 2021, 281, 119510. [Google Scholar] [CrossRef]
- Liu, G.; Qin, Y.; Lyu, Y.; Chen, M.; Qi, P.; Lu, Y.; Sheng, Z.; Tang, Y. Low-crystalline β-Ni(OH)2 nanosheets on nickel foam with enhanced areal capacitance for supercapacitor applications. Chem. Eng. J. 2021, 426, 131248. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, C.; Liu, H.; Feng, L. Efficient synergism of NiSe2 nanoparticle/NiO nanosheet for energy-relevant water and urea electrocatalysis. Appl. Catal. B Environ. 2020, 276, 119165. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, X.; Chen, C.; Lv, C.; Liu, H.; Lv, Y.; Guo, J.; Li, J.; Jia, D.; Tong, F. Spontaneous ruthenium doping in hierarchical flower-like Ni2P/NiO heterostructure nanosheets for superb alkaline hydrogen evolution. Chem. Eng. J. 2021, 417, 128069. [Google Scholar] [CrossRef]
- Yan, Y.; Ran, Z.; Zeng, T.; Wen, X.; Xu, H.; Li, R.; Zhao, C.; Shu, C. Interfacial Electron Redistribution of Hydrangea-like NiO@Ni2P Heterogeneous Microspheres with Dual-Phase Synergy for High-Performance Lithium–Oxygen Battery. Small 2022, 18, 2106707. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.-T.; Duan, J.-J.; Feng, J.-J.; Mei, L.-P.; Jiao, Y.; Zhang, L.; Wang, A.-J. Iron, rhodium-codoped Ni2P nanosheets arrays supported on nickel foam as an efficient bifunctional electrocatalyst for overall water splitting. J. Colloid Interface Sci. 2022, 605, 888–896. [Google Scholar] [CrossRef] [PubMed]
- Sha, L.; Ye, K.; Wang, G.; Shao, J.; Zhu, K.; Cheng, K.; Yan, J.; Wang, G.; Cao, D. Rational design of NiCo2S4 nanowire arrays on nickle foam as highly efficient and durable electrocatalysts toward urea electrooxidation. Chem. Eng. J. 2019, 359, 1652–1658. [Google Scholar] [CrossRef]
- Tammam, R.H.; Saleh, M.M. On the electrocatalytic urea oxidation on nickel oxide nanoparticles modified glassy carbon electrode. J. Electroanal. Chem. 2017, 794, 189–196. [Google Scholar] [CrossRef]
- Daramola, D.A.; Singh, D.; Botte, G.G. Dissociation Rates of Urea in the Presence of NiOOH Catalyst: A DFT Analysis. J. Phys. Chem. A 2010, 114, 11513–11521. [Google Scholar] [CrossRef]
- Vedharathinam, V.; Botte, G.G. Understanding the electro-catalytic oxidation mechanism of urea on nickel electrodes in alkaline medium. Electrochim. Acta 2012, 81, 292–300. [Google Scholar] [CrossRef]
- Ion-Ebrașu, D.; Andrei, R.D.; Enache, S.; Căprărescu, S.; Negrilă, C.C.; Jianu, C.; Enache, A.; Boerașu, I.; Carcadea, E.; Varlam, M.; et al. Nitrogen Functionalization of CVD Grown Three-Dimensional Graphene Foam for Hydrogen Evolution Reactions in Alkaline Media. Materials 2021, 14, 4952. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Hu, Q.-T.; Liu, J.; Zhang, W.-D.; Gu, Z.-G. Ultrafine-grained NiCo layered double hydroxide nanosheets with abundant active edge sites for highly enhanced electro-oxidation of urea. Electrochim. Acta 2021, 368, 137648. [Google Scholar] [CrossRef]
- Yan, W.; Wang, D.; Diaz, L.A.; Botte, G.G. Nickel nanowires as effective catalysts for urea electro-oxidation. Electrochim. Acta 2014, 134, 266–271. [Google Scholar] [CrossRef]
- Shen, F.; Jiang, W.; Qian, G.; Chen, W.; Zhang, H.; Luo, L.; Yin, S. Strongly coupled carbon encapsulated Ni-WO2 hybrids as efficient catalysts for water-to-hydrogen conversion via urea electro-oxidation. J. Power Sources 2020, 458, 228014. [Google Scholar] [CrossRef]
- Yu, T.; Xu, Q.; Qian, G.; Chen, J.; Zhang, H.; Luo, L.; Yin, S. Amorphous CoOx-Decorated Crystalline RuO2 Nanosheets as Bifunctional Catalysts for Boosting Overall Water Splitting at Large Current Density. ACS Sustain. Chem. Eng. 2020, 8, 17520–17526. [Google Scholar] [CrossRef]
- Yan, L.; Sun, Y.; Hu, E.; Ning, J.; Zhong, Y.; Zhang, Z.; Hu, Y. Facile in-situ growth of Ni2P/Fe2P nanohybrids on Ni foam for highly efficient urea electrolysis. J. Colloid Interface Sci. 2019, 541, 279–286. [Google Scholar] [CrossRef]
- Zhang, J.-Y.; He, T.; Wang, M.; Qi, R.; Yan, Y.; Dong, Z.; Liu, H.; Wang, H.; Xia, B.Y. Energy-saving hydrogen production coupling urea oxidation over a bifunctional nickel-molybdenum nanotube array. Nano Energy 2019, 60, 894–902. [Google Scholar] [CrossRef]
- Feng, Y.; Wang, X.; Huang, J.; Dong, P.; Ji, J.; Li, J.; Cao, L.; Feng, L.; Jin, P.; Wang, C. Decorating CoNi layered double hydroxides nanosheet arrays with fullerene quantum dot anchored on Ni foam for efficient electrocatalytic water splitting and urea electrolysis. Chem. Eng. J. 2020, 390, 124525. [Google Scholar] [CrossRef]
- Wang, T.; Wu, H.; Feng, C.; Zhang, L.; Zhang, J. MoP@NiCo-LDH on nickel foam as bifunctional electrocatalyst for high efficiency water and urea–water electrolysis. J. Mater. Chem. A 2020, 8, 18106–18116. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhao, J.; Wang, H.; Li, X.; Yang, L.; Zhao, Z.; Liu, X.; Liu, Y.; Liu, P.; Cai, Z. Porous flower-like nickel nitride as highly efficient bifunctional electrocatalysts for less energy-intensive hydrogen evolution and urea oxidation. Int. J. Hydrog. Energy 2020, 45, 14199–14207. [Google Scholar] [CrossRef]
- He, M.; Feng, C.; Liao, T.; Hu, S.; Wu, H.; Sun, Z. Low-Cost Ni2P/Ni0.96S Heterostructured Bifunctional Electrocatalyst toward Highly Efficient Overall Urea-Water Electrolysis. ACS Appl. Mater. Interfaces 2020, 12, 2225–2233. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Yang, D.; Liu, Z.; Wang, S.; Feng, L. Iron oxide promoted nickel/nickel oxide rough nanorods for efficient urea assisted water splitting. Electrochim. Acta 2020, 353, 136516. [Google Scholar] [CrossRef]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, K.; Wang, H.; Kannan, P.; Subramanian, P. Ni2P Nanoparticle-Inserted Porous Layered NiO Hetero-Structured Nanosheets as a Durable Catalyst for the Electro-Oxidation of Urea. Nanomaterials 2022, 12, 3633. https://doi.org/10.3390/nano12203633
Ma K, Wang H, Kannan P, Subramanian P. Ni2P Nanoparticle-Inserted Porous Layered NiO Hetero-Structured Nanosheets as a Durable Catalyst for the Electro-Oxidation of Urea. Nanomaterials. 2022; 12(20):3633. https://doi.org/10.3390/nano12203633
Chicago/Turabian StyleMa, Kun, Hui Wang, Palanisamy Kannan, and Palaniappan Subramanian. 2022. "Ni2P Nanoparticle-Inserted Porous Layered NiO Hetero-Structured Nanosheets as a Durable Catalyst for the Electro-Oxidation of Urea" Nanomaterials 12, no. 20: 3633. https://doi.org/10.3390/nano12203633
APA StyleMa, K., Wang, H., Kannan, P., & Subramanian, P. (2022). Ni2P Nanoparticle-Inserted Porous Layered NiO Hetero-Structured Nanosheets as a Durable Catalyst for the Electro-Oxidation of Urea. Nanomaterials, 12(20), 3633. https://doi.org/10.3390/nano12203633