Realizing Broadband NIR Photodetection and Ultrahigh Responsivity with Ternary Blend Organic Photodetector
Abstract
:1. Introduction
2. Materials and Methods
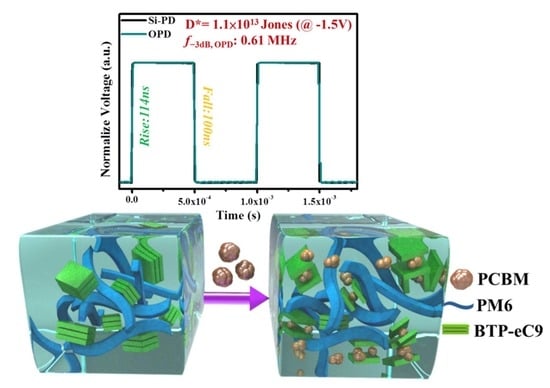
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dong, S.; Zhang, K.; Xie, B.; Xiao, J.; Yip, H.-L.; Yan, H.; Huang, F.; Cao, Y. High-Performance Large-Area Organic Solar Cells Enabled by Sequential Bilayer Processing via Nonhalogenated Solvents. Adv. Energy Mater. 2019, 9, 1802832. [Google Scholar] [CrossRef]
- Jang, W.; Rasool, S.; Kim, B.G.; Kim, J.; Yoon, J.; Manzhos, S.; Lee, H.K.; Jeon, I.; Wang, D.H. Superior Noise Suppression, Response Time, and Device Stability of Non-Fullerene System over Fullerene Counterpart in Organic Photodiode. Adv. Funct. Mater. 2020, 30, 2001402. [Google Scholar] [CrossRef]
- Strobel, N.; Seiberlich, M.; Eckstein, R.; Lemmer, U.; Hernandez-Sosa, G. Organic photodiodes: Printing, coating, benchmarks, and applications. Flex. Print. Electron. 2019, 4, 043001. [Google Scholar] [CrossRef] [Green Version]
- Li, N.; Lan, Z.; Cai, L.; Zhu, F. Advances in solution-processable near-infrared phototransistors. J. Mater. Chem. C 2019, 7, 3711–3729. [Google Scholar] [CrossRef]
- Lan, Z.J.; Lei, Y.L.; Chan, W.K.E.; Chen, S.M.; Luo, D.; Zhu, F.R. Near-infrared and visible light dual-mode organic photodetectors. Sci. Adv. 2020, 6, eaaw8065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simone, G.; Dyson, M.J.; Meskers, S.C.J.; Janssen, R.A.J.; Gelinck, G.H. Organic Photodetectors and their Application in Large Area and Flexible Image Sensors: The Role of Dark Current. Adv. Funct. Mater. 2020, 30, 1904205. [Google Scholar] [CrossRef] [Green Version]
- Wu, Z.; Yao, W.; London, A.E.; Azoulay, J.D.; Ng, T.N. Temperature-Dependent Detectivity of Near-Infrared Organic Bulk Heterojunction Photodiodes. ACS Appl. Mater. Interfaces 2017, 9, 1654–1660. [Google Scholar] [CrossRef] [Green Version]
- Li, N.; Lau, Y.S.; Xiao, Z.; Ding, L.; Zhu, F. NIR to Visible Light Upconversion Devices Comprising an NIR Charge Generation Layer and a Perovskite Emitter. Adv. Opt. Mater. 2018, 6, 1801084. [Google Scholar] [CrossRef]
- Tang, H.; Zhong, J.; Chen, W.; Shi, K.; Mei, G.; Zhang, Y.; Wen, Z.; Müller-Buschbaum, P.; Wu, D.; Wang, K.; et al. Lead Sulfide Quantum Dot Photodetector with Enhanced Responsivity through a Two-Step Ligand-Exchange Method. ACS Appl. Nano Mater. 2019, 2, 6135–6143. [Google Scholar] [CrossRef]
- Gasparini, N.; Gregori, A.; Salvador, M.; Biele, M.; Wadsworth, A.; Tedde, S.; Baran, D.; McCulloch, I.; Brabec, C.J. Visible and Near-Infrared Imaging with Nonfullerene-Based Photodetectors. Adv. Mater. Technol. 2018, 3, 1800104. [Google Scholar] [CrossRef]
- Zafar, Q.; Ahmad, Z. Dual donor bulk-heterojunction to realize a quick and more sensitive organic visible photodector. J. Mater. Sci. Mater. Electron. 2018, 29, 11144–11150. [Google Scholar] [CrossRef]
- Büchele, P.; Richter, M.; Tedde, S.F.; Matt, G.J.; Ankah, G.N.; Fischer, R.; Biele, M.; Metzger, W.; Lilliu, S.; Bikondoa, O.; et al. X-ray imaging with scintillator-sensitized hybrid organic photodetectors. Nat. Photon. 2015, 9, 843–848. [Google Scholar] [CrossRef]
- Lee, C.C.; Estrada, R.; Li, Y.Z.; Biring, S.; Al Amin, N.R.A.; Li, M.Z.; Liu, S.W.; Wong, K.T. Vacuum-Processed Small Molecule Organic Photodetectors with Low Dark Current Density and Strong Response to Near-Infrared Wavelength. Adv. Opt. Mater. 2020, 8, 2000519. [Google Scholar] [CrossRef]
- Grimoldi, A.; Colella, L.; La Monaca, L.; Azzellino, G.; Caironi, M.; Bertarelli, C.; Natali, D.; Sampietro, M. Inkjet printed polymeric electron blocking and surface energy modifying layer for low dark current organic photodetectors. Org. Electron. 2016, 36, 29–34. [Google Scholar] [CrossRef]
- Biele, M.; Montenegro Benavides, C.; Hürdler, J.; Tedde, S.F.; Brabec, C.J.; Schmidt, O. Spray-Coated Organic Photodetectors and Image Sensors with Silicon-Like Performance. Adv. Mater. Technol. 2019, 4, 1800158. [Google Scholar] [CrossRef] [Green Version]
- Xie, B.; Xie, R.; Zhang, K.; Yin, Q.; Hu, Z.; Yu, G.; Huang, F.; Cao, Y. Self-filtering narrowband high performance organic photodetectors enabled by manipulating localized Frenkel exciton dissociation. Nat. Commun. 2020, 11, 2871. [Google Scholar] [CrossRef]
- Cui, Y.; Yao, H.; Zhang, J.; Xian, K.; Zhang, T.; Hong, L.; Wang, Y.; Xu, Y.; Ma, K.; An, C.; et al. Single-Junction Organic Photovoltaic Cells with Approaching 18% Efficiency. Adv. Mater. 2020, 32, 1908205. [Google Scholar] [CrossRef]
- Kim, I.K.; Li, X.; Ullah, M.; Shaw, P.E.; Wawrzinek, R.; Namdas, E.B.; Lo, S.C. High-Performance, Fullerene-Free Organic Photodiodes Based on a Solution-Processable Indigo. Adv. Mater. 2015, 27, 6390–6395. [Google Scholar] [CrossRef]
- Bristow, H.; Jacoutot, P.; Scaccabarozzi, A.D.; Babics, M.; Moser, M.; Wadsworth, A.; Anthopoulos, T.D.; Bakulin, A.; McCulloch, I.; Gasparini, N. Nonfullerene-Based Organic Photodetectors for Ultrahigh Sensitivity Visible Light Detection. ACS Appl. Mater. Interfaces 2020, 12, 48836–48844. [Google Scholar] [CrossRef]
- Zhao, Z.; Wang, J.; Xu, C.; Yang, K.; Zhao, F.; Wang, K.; Zhang, X.; Zhang, F. Photomultiplication Type Broad Response Organic Photodetectors with One Absorber Layer and One Multiplication Layer. J. Phys. Chem. Lett. 2020, 11, 366–373. [Google Scholar] [CrossRef]
- Jiang, B.H.; Wang, Y.P.; Liao, C.Y.; Chang, Y.M.; Su, Y.W.; Jeng, R.J.; Chen, C.P. Improved Blend Film Morphology and Free Carrier Generation Provide a High-Performance Ternary Polymer Solar Cell. ACS Appl. Mater. Interfaces 2021, 13, 1076–1085. [Google Scholar] [CrossRef]
- Li, W.; Xu, Y.L.; Meng, X.Y.; Xiao, Z.; Li, R.M.; Jiang, L.; Cui, L.H.; Zheng, M.J.; Liu, C.; Ding, L.M.; et al. Visible to Near-Infrared Photodetection Based on Ternary Organic Heterojunctions. Adv. Funct. Mater. 2019, 29, 1808948. [Google Scholar] [CrossRef]
- Jiang, B.-H.; Chen, C.-P.; Liang, H.-T.; Jeng, R.-J.; Chien, W.-C.; Yu, Y.-Y. The role of Y6 as the third component in fullerene-free ternary organic photovoltaics. Dyes Pigm. 2020, 181, 108613. [Google Scholar] [CrossRef]
- Wang, F.; Yang, X.-Y.; Niu, M.-S.; Feng, L.; Hao, X.-T. Förster resonance energy transfer and morphology optimization for high-performance ternary organic photodetectors. Org. Electron. 2019, 67, 146–152. [Google Scholar] [CrossRef]
- Liang, W.Q.; Li, Y.; Ma, J.L.; Wang, Y.; Yan, J.J.; Chen, X.; Wu, D.; Tian, Y.T.; Li, X.J.; Shi, Z.F. A solution-processed ternary copper halide thin films for air-stable and deep-ultraviolet-sensitive photodetector. Nanoscale 2020, 12, 17213–17221. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, C.; Shen, L. Recent advances on organic-inorganic hybrid perovskite photodetectors with fast response. InfoMat 2019, 1, 164–182. [Google Scholar] [CrossRef] [Green Version]
- Liu, G.; Li, T.; Zhan, X.; Wu, H.; Cao, Y. High-Sensitivity Visible-Near Infrared Organic Photodetectors Based on Non-Fullerene Acceptors. ACS Appl. Mater. Interfaces 2020, 12, 17769–17775. [Google Scholar] [CrossRef]
- Strobel, N.; Seiberlich, M.; Rodlmeier, T.; Lemmer, U.; Hernandez-Sosa, G. Non-Fullerene-Based Printed Organic Photodiodes with High Responsivity and Megahertz Detection Speed. ACS Appl. Mater. Interfaces 2018, 10, 42733–42739. [Google Scholar] [CrossRef]
- Huang, J.; Lee, J.; Vollbrecht, J.; Brus, V.V.; Dixon, A.L.; Cao, D.X.; Zhu, Z.; Du, Z.; Wang, H.; Cho, K.; et al. A High-Performance Solution-Processed Organic Photodetector for Near-Infrared Sensing. Adv. Mater. 2020, 32, 1906027. [Google Scholar] [CrossRef]
- Wu, Y.L.; Fukuda, K.; Yokota, T.; Someya, T. A Highly Responsive Organic Image Sensor Based on a Two-Terminal Organic Photodetector with Photomultiplication. Adv. Mater. 2019, 31, 1903687. [Google Scholar] [CrossRef]
- Yoon, S.; Lee, G.S.; Sim, K.M.; Kim, M.J.; Kim, Y.H.; Chung, D.S. End-Group Functionalization of Non-Fullerene Acceptors for High External Quantum Efficiency over 150000% in Photomultiplication Type Organic Photodetectors. Adv. Funct. Mater. 2021, 31, 2006448. [Google Scholar] [CrossRef]
- Saggar, S.; Sanderson, S.; Gedefaw, D.; Pan, X.; Philippa, B.; Andersson, M.R.; Lo, S.C.; Namdas, E.B. Toward Faster Organic Photodiodes: Tuning of Blend Composition Ratio. Adv. Funct. Mater. 2021, 31, 2010661. [Google Scholar] [CrossRef]
- Jiang, B.-H.; Chan, P.-H.; Su, Y.-W.; Hsu, H.-L.; Jeng, R.-J.; Chen, C.-P. Surface properties of buffer layers affect the performance of PM6:Y6–based organic photovoltaics. Org. Electron. 2020, 87, 105944. [Google Scholar] [CrossRef]
- Strobel, N.; Droseros, N.; Kontges, W.; Seiberlich, M.; Pietsch, M.; Schlisske, S.; Lindheimer, F.; Schroder, R.R.; Lemmer, U.; Pfannmoller, M.; et al. Color-Selective Printed Organic Photodiodes for Filterless Multichannel Visible Light Communication. Adv. Mater. 2020, 32, 1908258. [Google Scholar] [CrossRef]
- Lu, J.H.; Cheng, M.T.; Hsu, H.L.; Liu, S.W.; Chen, C.P. Perovskite Photosensors Integrated with Silver Resonant-Cavity Color Filters Display Color Perception Beyond That of the Human Eye. Adv. Funct. Mater. 2020, 30, 2002503. [Google Scholar] [CrossRef]
- Kushnir, K.; Qin, Y.; Shen, Y.; Li, G.; Fregoso, B.M.; Tongay, S.; Titova, L.V. Ultrafast Zero-Bias Surface Photocurrent in Germanium Selenide: Promise for Terahertz Devices and Photovoltaics. ACS Appl. Mater. Interfaces 2019, 11, 5492–5498. [Google Scholar] [CrossRef]
- Nath, D.; Dey, P.; Joseph, A.M.; Rakshit, J.K.; Roy, J.N. CuPc/C60 heterojunction for high responsivity zero bias organic red light photodetector. Appl. Phys. A 2020, 126, 627. [Google Scholar] [CrossRef]
- Lin, Y.; Adilbekova, B.; Firdaus, Y.; Yengel, E.; Faber, H.; Sajjad, M.; Zheng, X.; Yarali, E.; Seitkhan, A.; Bakr, O.M.; et al. 17% Efficient Organic Solar Cells Based on Liquid Exfoliated WS2 as a Replacement for PEDOT:PSS. Adv. Mater. 2019, 31, 1902965. [Google Scholar] [CrossRef]
- Chen, C.-P.; Li, Y.-C.; Tsai, Y.-Y.; Lu, Y.-W. Efficient ternary polymer solar cells with a shelf-life stability for longer than 410 days. Sol. Energy Mater Sol. Cells 2018, 183, 120–128. [Google Scholar] [CrossRef]
- Hu, R.; Zhang, L.; Peng, J.; Zhang, W. Comparative study of charge characteristics in PCPDTBT:fullerenes solar cells. Chem. Phys. 2021, 540, 111004. [Google Scholar] [CrossRef]
- Chuppina, S.V. Anti-Icing gradient organosilicate coatings. Glass Phys. Chem. 2007, 33, 502–509. [Google Scholar] [CrossRef]
- An, Q.; Wang, J.; Gao, W.; Ma, X.; Hu, Z.; Gao, J.; Xu, C.; Hao, M.; Zhang, X.; Yang, C.; et al. Alloy-like ternary polymer solar cells with over 17.2% efficiency. Sci. Bull. 2020, 65, 538–545. [Google Scholar] [CrossRef] [Green Version]
- Ma, R.; Liu, T.; Luo, Z.; Gao, K.; Chen, K.; Zhang, G.; Gao, W.; Xiao, Y.; Lau, T.-K.; Fan, Q.; et al. Adding a Third Component with Reduced Miscibility and Higher LUMO Level Enables Efficient Ternary Organic Solar Cells. ACS Energy Lett. 2020, 5, 2711–2720. [Google Scholar] [CrossRef]
- Jiao, C.; Pang, C.; An, Q. Nonfullerene organic photovoltaic cells exhibiting 13.76% efficiency by employing upside-down solvent vapor annealing. Int. J. Energy Res. 2019, 43, 8716–8724. [Google Scholar] [CrossRef]
- Pan, M.-A.; Lau, T.-K.; Tang, Y.; Wu, Y.-C.; Liu, T.; Li, K.; Chen, M.-C.; Lu, X.; Ma, W.; Zhan, C. 16.7%-efficiency ternary blended organic photovoltaic cells with PCBM as the acceptor additive to increase the open-circuit voltage and phase purity. J. Mater. Chem. A 2019, 7, 20713–20722. [Google Scholar] [CrossRef]
- Zhang, D.; Zhao, D.; Wang, Z.; Yu, J. Processes Controlling the Distribution of Vertical Organic Composition in Organic Photodetectors by Ultrasonic-Assisted Solvent Vapor Annealing. Appl. Electron. Mater. 2020, 2, 2188–2195. [Google Scholar] [CrossRef]
- Hosono, N.; Terashima, A.; Kusaka, S.; Matsuda, R.; Kitagawa, S. Highly responsive nature of porous coordination polymer surfaces imaged by in situ atomic force microscopy. Nat. Chem. 2019, 11, 109–116. [Google Scholar] [CrossRef]
- Bangsund, J.S.; Fielitz, T.R.; Steiner, T.J.; Shi, K.; Van Sambeek, J.R.; Clark, C.P.; Holmes, R.J. Formation of aligned periodic patterns during the crystallization of organic semiconductor thin films. Nature Mater. 2019, 18, 725–731. [Google Scholar] [CrossRef]
- Park, H.G.; Jeong, H.C.; Jung, Y.H.; Seo, D.S. Control of the wrinkle structure on surface-reformed poly(dimethylsiloxane) via ion-beam bombardment. Sci. Rep. 2015, 5, 12356. [Google Scholar] [CrossRef] [Green Version]
- Smolyakov, G.; Cauquil, M.; Severac, C.; Lachaize, V.; Guilbeau-Frugier, C.; Senard, J.M.; Gales, C.; Dague, E. Biophysical properties of cardiomyocyte surface explored by multiparametric AFM. J. Struct. Biol. 2017, 198, 28–37. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Meng, Y.; Guo, X.; Zhu, L.; Liu, F.; Zhang, M. All-polymer solar cells based on a novel narrow-bandgap polymer acceptor with power conversion efficiency over 10%. J. Mater. Chem. A 2019, 7, 16190–16196. [Google Scholar] [CrossRef]
- Lin, Q.; Armin, A.; Lyons, D.M.; Burn, P.L.; Meredith, P. Low noise, IR-blind organohalide perovskite photodiodes for visible light detection and imaging. Adv. Mater. 2015, 27, 2060–2064. [Google Scholar] [CrossRef]
- Jansen-van Vuuren, R.D.; Armin, A.; Pandey, A.K.; Burn, P.L.; Meredith, P. Organic Photodiodes: The Future of Full Color Detection and Image Sensing. Adv. Mater. 2016, 28, 4766–4802. [Google Scholar] [CrossRef]
- Park, J.B.; Ha, J.W.; Yoon, S.C.; Lee, C.; Jung, I.H.; Hwang, D.H. Visible-Light-Responsive High-Detectivity Organic Photodetectors with a 1 mum Thick Active Layer. ACS Appl. Mater. Interfaces 2018, 10, 38294–38301. [Google Scholar] [CrossRef]
- Lee, J.; Ko, S.-J.; Lee, H.; Huang, J.; Zhu, Z.; Seifrid, M.; Vollbrecht, J.; Brus, V.V.; Karki, A.; Wang, H.; et al. Side-Chain Engineering of Nonfullerene Acceptors for Near-Infrared Organic Photodetectors and Photovoltaics. ACS Energy Lett. 2019, 4, 1401–1409. [Google Scholar] [CrossRef]
- Liu, Z.-X.; Lau, T.-K.; Zhou, G.; Li, S.; Ren, J.; Das, S.K.; Xia, R.; Wu, G.; Zhu, H.; Lu, X.; et al. Achieving efficient organic solar cells and broadband photodetectors via simple compositional tuning of ternary blends. Nano Energy 2019, 63, 103807. [Google Scholar] [CrossRef]
- Ko, H.; Park, S.; Son, H.J.; Chung, D.S. Wide-Linear-Dynamic-Range Polymer Photodiode with a New Benzo [1,2-b:4,5-b′] dithiophene-Copolymer: The Role of Crystalline Orientation. Chem. Mater. 2020, 32, 3219–3228. [Google Scholar] [CrossRef]
- Zhong, Z.; Peng, F.; Huang, Z.; Ying, L.; Yu, G.; Huang, F.; Cao, Y. High-Detectivity Non-Fullerene Organic Photodetectors Enabled by a Cross-Linkable Electron Blocking Layer. ACS Appl. Mater. Interfaces 2020, 12, 45092–45100. [Google Scholar] [CrossRef] [PubMed]
- Sim, H.R.; Kang, M.; Yu, S.H.; Nam, G.H.; Lim, B.; Chung, D.S. Design and Synthesis of a New Non-Fullerene Acceptor for High-Performance Photomultiplication-Type—Organic Photodiodes. Adv. Opt. Mater. 2021, 9, 2001836. [Google Scholar] [CrossRef]
- Fuentes-Hernandez, C.; Chou, W.F.; Khan, T.M.; Diniz, L.; Lukens, J.; Larrain, F.A.; Rodriguez-Toro, V.A.; Kippelen, B. Large-area low-noise flexible organic photodiodes for detecting faint visible light. Science 2020, 370, 698–701. [Google Scholar] [CrossRef] [PubMed]
- Seiberlich, M.; Strobel, N.; Ruiz-Preciado, L.A.; Ruscello, M.; Lemmer, U.; Hernandez-Sosa, G. Aerosol-Jet-Printed Donor-Blocking Layer for Organic Photodiodes. Adv. Electron. Mater. 2021, 7, 2000811. [Google Scholar] [CrossRef]
- Yang, W.; Qiu, W.; Georgitzikis, E.; Simoen, E.; Serron, J.; Lee, J.; Lieberman, I.; Cheyns, D.; Malinowski, P.; Genoe, J.; et al. Mitigating Dark Current for High-Performance Near-Infrared Organic Photodiodes via Charge Blocking and Defect Passivation. ACS Appl. Mater. Interfaces 2021, 13, 16766–16774. [Google Scholar] [CrossRef] [PubMed]
- Eun, H.J.; Kye, H.; Kim, D.; Jin, I.S.; Jung, J.W.; Ko, S.J.; Heo, J.; Kim, B.G.; Kim, J.H. Effective Dark Current Suppression for High-Detectivity Organic Near-Infrared Photodetectors Using a Non-Fullerene Acceptor. ACS Appl. Mater. Interfaces 2021, 13, 11144–11150. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Lee, J.; Nakayama, H.; Schrock, M.; Cao, D.X.; Cho, K.; Bazan, G.C.; Nguyen, T.Q. Understanding and Countering Illumination-Sensitive Dark Current: Toward Organic Photodetectors with Reliable High Detectivity. ACS Nano 2021, 15, 1753–1763. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, Y.-Y.; Peng, Y.-C.; Chiu, Y.-C.; Liu, S.-J.; Chen, C.-P. Realizing Broadband NIR Photodetection and Ultrahigh Responsivity with Ternary Blend Organic Photodetector. Nanomaterials 2022, 12, 1378. https://doi.org/10.3390/nano12081378
Yu Y-Y, Peng Y-C, Chiu Y-C, Liu S-J, Chen C-P. Realizing Broadband NIR Photodetection and Ultrahigh Responsivity with Ternary Blend Organic Photodetector. Nanomaterials. 2022; 12(8):1378. https://doi.org/10.3390/nano12081378
Chicago/Turabian StyleYu, Yang-Yen, Yan-Cheng Peng, Yu-Cheng Chiu, Song-Jhe Liu, and Chih-Ping Chen. 2022. "Realizing Broadband NIR Photodetection and Ultrahigh Responsivity with Ternary Blend Organic Photodetector" Nanomaterials 12, no. 8: 1378. https://doi.org/10.3390/nano12081378
APA StyleYu, Y. -Y., Peng, Y. -C., Chiu, Y. -C., Liu, S. -J., & Chen, C. -P. (2022). Realizing Broadband NIR Photodetection and Ultrahigh Responsivity with Ternary Blend Organic Photodetector. Nanomaterials, 12(8), 1378. https://doi.org/10.3390/nano12081378