Supported MOCVD TiO2 Thin Films Grown on Modified Stainless Steel Mesh for Sensing Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Acid-Based Chemical Etching of Stainless Steel Mesh
2.2. Deposition of Nanostructured TiO2 Thin Films via MOCVD
2.3. Microstructural Investigations
2.4. Photocatalytic Measurements
3. Results
3.1. Increasing the SS Surface Area: Acid-Based Etchings
3.1.1. HCl, H2SO4, and HCl/H2SO4 Etchings: Morphological Characterization
3.1.2. HCl, H2SO4, and HCl/H2SO4 Etchings: EDX Characterization
3.1.3. HCl, H2SO4, and HCl/H2SO4 Etchings: XPS Characterization
3.2. TiO2 Deposition via MOCVD on SS and SS-AT Meshes
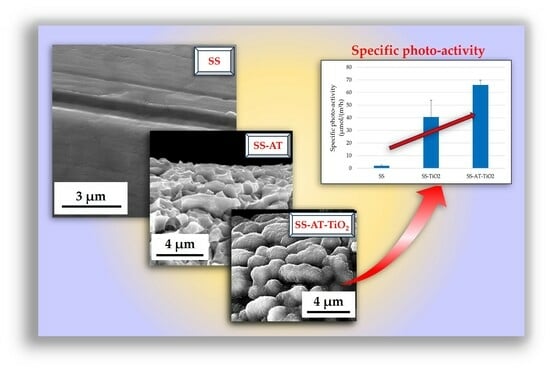
3.3. Photocatalytic Activity
4. Conclusions
- (i)
- Considering the scalability of the CVD technology for the growth of the TiO2 films, we will investigate the scalability of the etching uniformity and the possibility of enlarging the size of the AISI 316 mesh to target industrial-scale sensing application;
- (ii)
- Considering that the efficiency of the etching process could also depend on the mesh composition and impurity, it is worth optimizing and developing protocols for other low-cost meshes;
- (iii)
- As many performance parameters depend on the structure of the TiO2, specifically sensitivity, lifetime, and the working range, a further direction of our study will be unveiling the effect of the mesh microstructure on the time response of the TiO2 COD sensor;
- (iv)
- In order to move towards wastewater applications, the selectivity and sensitivity to specific organic pollutants, such as repeatable detection of blue-methylene as a probe of main organic contaminants of water, will be investigated.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mele, G.; Del Sole, R.; Lü, X. Applications of TiO2 in Sensor Devices. In Titanium Dioxide (TiO2) and Its Applications; Parrino, F., Palmisano, L., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 527–581. ISBN 9780128199602. [Google Scholar]
- Bai, J.; Zhou, B. Titanium Dioxide Nanomaterials for Sensor Applications. Chem. Rev. 2014, 114, 10131–10176. [Google Scholar] [CrossRef]
- El Mekkawi, D.M.; Galal, H.R.; Abd EL Wahab, R.M.; Mohamed, W.A.A. Photocatalytic Activity Evaluation of TiO2 Nanoparticles Based on COD Analyses for Water Treatment Applications: A Standardization Attempt. Int. J. Environ. Sci. Technol. 2016, 13, 1077–1088. [Google Scholar] [CrossRef]
- Kim, Y.; Lee, K.; Sasaki, S.; Hashimoto, K.; Ikebukuro, K.; Karube, I. Photocatalytic Sensor for Chemical Oxygen Demand Determination Based on Oxygen Electrode. Anal. Chem. 2000, 72, 3379–3382. [Google Scholar] [CrossRef] [PubMed]
- Bastug Azer, B.; Gulsaran, A.; Pennings, J.R.; Saritas, R.; Kocer, S.; Bennett, J.L.; Devdas Abhang, Y.; Pope, M.A.; Abdel-Rahman, E.; Yavuz, M. A Review: TiO2 Based Photoelectrocatalytic Chemical Oxygen Demand Sensors and Their Usage in Industrial Applications. J. Electroanal. Chem. 2022, 918, 116466. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, J.; Xian, Y.; Ying, X.; Liu, M.; Jin, L. Preparation and Application of TiO2 Photocatalytic Sensor for Chemical Oxygen Demand Determination in Water Research. Water Res. 2005, 39, 1340–1346. [Google Scholar] [CrossRef]
- Diebold, U. The Surface Science of Titanium Dioxide. Surf. Sci. Rep. 2003, 48, 53. [Google Scholar] [CrossRef]
- Murgolo, S.; Yargeau, V.; Gerbasi, R.; Visentin, F.; El Habra, N.; Ricco, G.; Lacchetti, I.; Carere, M.; Curri, M.L.; Mascolo, G. A New Supported TiO2 Film Deposited on Stainless Steel for the Photocatalytic Degradation of Contaminants of Emerging Concern. Chem. Eng. J. 2017, 318, 103–111. [Google Scholar] [CrossRef]
- Galenda, A.; Visentin, F.; Gerbasi, R.; Favaro, M.; Bernardi, A.; El Habra, N. Evaluation of Self-Cleaning Photocatalytic Paints: Are They Effective under Actual Indoor Lighting Systems? Appl. Catal. B Environ. 2018, 232, 194–204. [Google Scholar] [CrossRef]
- Galenda, A.; Natile, M.M.; El Habra, N. Large-Scale MOCVD Deposition of Nanostructured TiO2 on Stainless Steel Woven: A Systematic Investigation of Photoactivity as a Function of Film Thickness. Nanomaterials 2022, 12, 992. [Google Scholar] [CrossRef]
- Wang, N.; Xiong, D.; Li, M.; Deng, Y.; Shi, Y.; Wang, K. Superhydrophobic Surface on Steel Substrate and Its Anti-Icing Property in Condensing Conditions. Appl. Surf. Sci. 2015, 355, 226–232. [Google Scholar] [CrossRef]
- Nageswara Rao, P.; Kunzru, D. Fabrication of Microchannels on Stainless Steel by Wet Chemical Etching. J. Micromech. Microeng. 2007, 17, N99–N106. [Google Scholar] [CrossRef]
- Cremaldi, J.; Bhushan, B. Journal of Colloid and Interface Science Fabrication of Bioinspired, Self-Cleaning Superliquiphilic/Phobic Stainless Steel Using Different Pathways. J. Colloid Interface Sci. 2018, 518, 284–297. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, Z.; Yang, J.; Yue, Y.; Zhang, H. Fabrication of Superhydrophobic Surface on Stainless Steel by Two-Step Chemical Etching. Chem. Phys. Lett. 2022, 797, 139567. [Google Scholar] [CrossRef]
- Datta, M.; Harris, D. Electrochemical Micromachining: An Environmentally Friendly, High Speed Processing Technology. Electrochim. Acta 1997, 42, 3007–3013. [Google Scholar] [CrossRef]
- Cakir, O. Study of Etch Rate and Surface Roughness in Chemical Etching of Stainless Study of Etch Rate and Surface Roughness in Chemical Etching of Stainless Steel Orhan Çakır. Key Eng. Mater. 2008, 364, 837. [Google Scholar] [CrossRef]
- Sarvghad, M.; Del Aguila, D.; Will, G. Optimized Corrosion Performance of a Carbon Steel in Dilute Sulfuric Acid through Heat Treatment. Appl. Surf. Sci. 2019, 491, 460–468. [Google Scholar] [CrossRef]
- Rassu, P.; Malfatti, L.; Carboni, D.; Casula, M.F.; Garroni, S.; Zampetti, E.; Macagnano, A.; Bearzotti, A.; Innocenzi, P. Mesoscale Organization of Titania Thin Films Enables Oxygen Sensing at Room Temperature. J. Mater. Chem. C 2017, 5, 11815–11823. [Google Scholar] [CrossRef]
- Matthews, I.C.; Hanneman, G.B. Etching Solution. US Patent 2,266,430, 1941. [Google Scholar]
- Snyder, H.B.; Rosenberg, L.M. Chemical Milling. US Patent 2,940,838, 1960. [Google Scholar]
- Comert, B.; Akin, N.; Donmez, M.; Saglam, S.; Ozcelik, S. Titanium Dioxide Thin Films as Methane Gas Sensors. IEEE Sens. J. 2016, 16, 8890–8896. [Google Scholar] [CrossRef]
- Patil, M.; Shaikh, S.; Ganesh, I. Recent Advances on TiO2 Thin Film Based Photocatalytic Applications (A Review). Curr. Nanosci. 2015, 11, 271–285. [Google Scholar] [CrossRef]
- Visentin, F.; Galenda, A.; Fabrizio, M.; Battiston, S.; Brianese, N.; Gerbasi, R.; Zin, V.; El, N. Assessment of Synergistic Effects of LP-MOCVD TiO2 and Ti Surface Fi Nish for Dental Implant Purposes. Appl. Surf. Sci. 2019, 490, 568–579. [Google Scholar] [CrossRef]
- Battiston, G.A.; Gerbasi, R.; Porchia, M.; Marigo, A. Influence of Substrate on Structural Properties of TiO2 Thin Films Obtained via MOCVD. Thin Solid Film. 1994, 239, 186–191. [Google Scholar] [CrossRef]
- Choy, K.L. Chemical Vapour Deposition of Coatings. Prog. Mater. Sci. 2003, 48, 57–170. [Google Scholar] [CrossRef]
- Dell’Amico, D.B.; Bertagnolli, H.; Calderazzo, F.; D’Arienzo, M.; Gross, S.; Labella, L.; Rancan, M.; Scotti, R.; Bernd, M.S.; Supplit, R.; et al. Nanostructured Copper Oxide on Silica-Zirconia Mixed Oxides by Chemical Implantation. Chem. A Eur. J. 2009, 15, 4931–4943. [Google Scholar] [CrossRef]
- Pelizzo, M.G.; Corso, A.J.; Tessarolo, E.; Böttger, R.; Hübner, R.; Napolitani, E.; Bazzan, M.; Rancan, M.; Armelao, L.; Jark, W.; et al. Morphological and Functional Modifications of Optical Thin Films for Space Applications Irradiated with Low-Energy Helium Ions. ACS Appl. Mater. Interfaces 2018, 10, 34781–34791. [Google Scholar] [CrossRef]
- ISO 10678:2010; Fine Ceramics, Advanced Technical Ceramics—Determination of Photocatalytic Activity of Surfaces in an Aqueous Medium by Degradation of Methylene Blue. ISO: Geneve, Switzerland, 2010.
- Rokosz, K.; Hryniewicz, T.; Raaen, S.; Valiček, J. SEM/EDX, XPS, Korozija i Karakterizacija Hrapavosti Površine AISI 316L SS Nakon Elektrokemijske Obrade u Koncentriranoj HNO3. Teh. Vjesn. 2015, 22, 125–131. [Google Scholar] [CrossRef]
- Rouzmeh, S.S.; Naderi, R.; Mahdavian, M. A Sulfuric Acid Surface Treatment of Mild Steel for Enhancing the Protective Properties of an Organosilane Coating. Prog. Org. Coat. 2017, 103, 156–164. [Google Scholar] [CrossRef]
- Rouzmeh, S.S.; Naderi, R.; Mahdavian, M. Steel Surface Treatment with Three Different Acid Solutions and Its Effect on the Protective Properties of the Subsequent Silane Coating. Prog. Org. Coat. 2017, 112, 133–140. [Google Scholar] [CrossRef]
- Stypula, B.; Banaś, J. Passivity of Chromium in Sulphuric Acid Solutions. Electrochim. Acta 1993, 38, 2309–2314. [Google Scholar] [CrossRef]
- Panossian, Z.; de Almeida, N.L.; de Sousa, R.M.F.; de Pimenta, G.S.; Marques, L.B.S. Corrosion of Carbon Steel Pipes and Tanks by Concentrated Sulfuric Acid: A Review. Corros. Sci. 2012, 58, 1–11. [Google Scholar] [CrossRef]
- Welsh, I.; Sherwood, P. Photoemission and Electronic Structure of FeOOH: Distinguishing between Oxide and Oxyhydroxide. Phys. Rev. B 1989, 40, 1898. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Payne, B.P.; Grosvenor, A.P.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Applied Surface Science Resolving Surface Chemical States in XPS Analysis of First Row Transition Metals, Oxides and Hydroxides: Cr, Mn, Fe, Co and Ni. Appl. Surf. Sci. 2011, 257, 2717–2730. [Google Scholar] [CrossRef]
- Comisso, N.; Armelao, L.; Cattarin, S.; Fasolin, S.; Mattarozzi, L.; Musiani, M.; Rancan, M.; Vázquez-gómez, L.; Verlato, E. Deposition of FeOOH Layers onto Porous PbO2 by Galvanic Displacement and Their Use as Electrocatalysts for Oxygen Evolution Reaction. J. Electroanal. Chem. 2021, 880, 114844. [Google Scholar] [CrossRef]
- Maller, R.R. Passivation of Stainless Steel. Trends Food Sci. Technol. 1998, 9, 28–32. [Google Scholar] [CrossRef]
- Velten, D.; Biehl, V.; Aubertin, F.; Valeske, B.; Possart, W.; Breme, J. Preparation of TiO2 Layers on Cp-Ti and Ti6Al4V by Thermal and Anodic Oxidation and by Sol-Gel Coating Techniques and Their Characterization. J. Biomed. Mater. Res. 2001, 59, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.; Hu, X.; Yue, P.L.; Lu, G.Q.; Greenfield, P.F. Synthesis of Anatase TiO2 Supported on Porous Solids by Chemical Vapor Deposition. Catal. Today 2001, 68, 173–182. [Google Scholar] [CrossRef]
- Jung, S.C.; Kim, S.J.; Imaishi, N.; Cho, Y.I. Effect of TiO2 Thin Film Thickness and Specific Surface Area by Low-Pressure Metal-Organic Chemical Vapor Deposition on Photocatalytic Activities. Appl. Catal. B Environ. 2005, 55, 253–257. [Google Scholar] [CrossRef]
- Carp, O.; Huisman, C.L.; Reller, A. Photoinduced Reactivity of Titanium Dioxide. Prog. Solid State Chem. 2004, 32, 33–177. [Google Scholar] [CrossRef]
- Tschirch, J.; Dillert, R.; Bahnemann, D.; Proft, B.; Biedermann, A.; Goer, B. Photodegradation of Methylene Blue in Water, a Standard Method to Determine the Activity of Photocatalytic Coatings? Res. Chem. Intermed. 2008, 34, 381–392. [Google Scholar] [CrossRef]
- Mills, A. An Overview of the Methylene Blue ISO Test for Assessing the Activities of Photocatalytic Films. Appl. Catal. B Environ. 2012, 128, 144–149. [Google Scholar] [CrossRef]











| Acid Solution (10M) | Temperature (°C) | Treatment Duration (Min) |
|---|---|---|
| HCl | 25–40–50–55–60 | 10–15–20 |
| H2SO4 | 25–40–60 | 10–15–20 |
| HCl/H2SO4 | 25–40–50–55–60 | 10–15–20 |
| Oxide % | Hydroxide % | Oxide/Hydroxide Ratio | |
|---|---|---|---|
| SS | 42.2 | 57.8 | 0.7 |
| SS-AT-HCl | 41.9 | 58.1 | 0.7 |
| SS-AT-H2SO4 | 34.0 | 66.0 | 0.5 |
| SS-AT-HCl/H2SO4 | 33.7 | 66.3 | 0.5 |
| Deposition Time (Min) | Thickness (nm) | Growth Rate (nm/Min) |
| 20 | 330 ± 5 | 16.5 ± 0.2 |
| 25 | 390 ± 11 | 15.6 ± 0.4 |
| 30 | 481 ± 17 | 16.0 ± 0.6 |
| 33 | 545 ± 19 | 16.5 ± 0.6 |
| 35 | 631 ± 18 | 18.0 ± 0.5 |
| 40 | 713 ± 21 | 17.8 ± 0.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Habra, N.; Visentin, F.; Russo, F.; Galenda, A.; Famengo, A.; Rancan, M.; Losurdo, M.; Armelao, L. Supported MOCVD TiO2 Thin Films Grown on Modified Stainless Steel Mesh for Sensing Applications. Nanomaterials 2023, 13, 2678. https://doi.org/10.3390/nano13192678
El Habra N, Visentin F, Russo F, Galenda A, Famengo A, Rancan M, Losurdo M, Armelao L. Supported MOCVD TiO2 Thin Films Grown on Modified Stainless Steel Mesh for Sensing Applications. Nanomaterials. 2023; 13(19):2678. https://doi.org/10.3390/nano13192678
Chicago/Turabian StyleEl Habra, Naida, Francesca Visentin, Francesca Russo, Alessandro Galenda, Alessia Famengo, Marzio Rancan, Maria Losurdo, and Lidia Armelao. 2023. "Supported MOCVD TiO2 Thin Films Grown on Modified Stainless Steel Mesh for Sensing Applications" Nanomaterials 13, no. 19: 2678. https://doi.org/10.3390/nano13192678
APA StyleEl Habra, N., Visentin, F., Russo, F., Galenda, A., Famengo, A., Rancan, M., Losurdo, M., & Armelao, L. (2023). Supported MOCVD TiO2 Thin Films Grown on Modified Stainless Steel Mesh for Sensing Applications. Nanomaterials, 13(19), 2678. https://doi.org/10.3390/nano13192678