DNA Origami Reorganizes upon Interaction with Graphite: Implications for High-Resolution DNA Directed Protein Patterning
Abstract
:1. Introduction
2. Results
2.1. Contrasting DNA Origami Interactions with Mica and Graphite Substrates
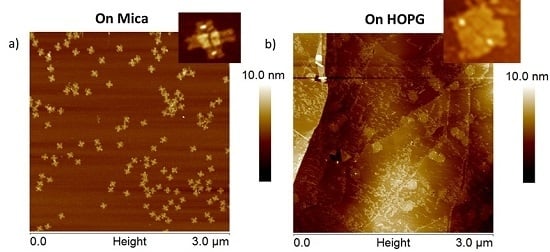
2.1.1. Test Object Deposition on Mica (Control)
2.1.2. Adsorption of DNA Origami from Solutions Containing Excess Staples onto HOPG
2.1.3. Adsorption of Purified DNA Origami onto HOPG
2.2. Protein Patterns on DNA Origami Are Perturbed during Adsorption onto Graphite Substrates
3. Discussion
4. Materials and Methods
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mody, C.C.M. Instrumental Community: Probe Microscopy and the Path to Nanotechnology; The MIT Press: Cambridge, MA, USA, 2011. [Google Scholar]
- Clemmer, C.; Beebe, T. Graphite: A mimic for DNA and other biomolecules in scanning tunneling microscope studies. Science 1991, 251, 640–642. [Google Scholar] [CrossRef] [PubMed]
- Heckl, W.M.; Binnig, G. Domain walls on graphite mimic DNA. Ultramicroscopy 1992, 42, 1073–1078. [Google Scholar] [CrossRef]
- Chen, Y.; Michael, Z.P.; Kotchey, G.P.; Zhao, Y.; Star, A. Electronic detection of bacteria using holey reduced graphene oxide. ACS Appl. Mater. Interfaces 2014, 6, 3805–3810. [Google Scholar] [CrossRef] [PubMed]
- Seeman, N.C. An overview of structural DNA nanotechnology. Mol. Biotechnol. 2007, 37, 246–257. [Google Scholar] [CrossRef] [PubMed]
- Rothemund, P.W.K. Folding DNA to create nanoscale shapes and patterns. Nature 2006, 440, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Pillers, M.; Goss, V.; Lieberman, M. Electron-beam lithography and molecular liftoff for directed attachment of DNA nanostructures on silicon: Top-down meets bottom-up. Accounts Chem. Res. 2014, 47, 1759–1767. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Wang, T.; Liu, W.; Xin, H.L.; Li, H.; Ke, Y.; Shih, W.M.; Gang, O. Prescribed nanoparticle cluster architectures and low-dimensional arrays built using octahedral DNA origami frames. Nat. Nanotechnol. 2015, 10, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Dietz, H.; Douglas, S.M.; Shih, W.M. Folding DNA into twisted and curved nanoscale shapes. Science 2009, 325, 725–730. [Google Scholar] [CrossRef] [PubMed]
- Seeman, N.C.; Kallenbach, N.R. Design of immobile nucleic acid junctions. Biophys. J. 1983, 44, 201–209. [Google Scholar] [CrossRef]
- Seeman, N.C. DNA in a material world. Nature 2003, 421, 427–431. [Google Scholar] [CrossRef] [PubMed]
- Winfree, E.; Liu, F.; Wenzler, L.A.; Seeman, N.C. Design and self-assembly of two-dimensional DNA crystals. Nature 1998, 394, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Park, S.H.; Finkelstein, G.; Reif, J.H.; LaBean, T.H. DNA-templated self-assembly of protein arrays and highly conductive nanowires. Science 2003, 301, 1882–1884. [Google Scholar] [CrossRef] [PubMed]
- Shih, W.M.; Quispe, J.D.; Joyce, G.F. A 1.7-kilobase single-stranded DNA that folds into a nanoscale octahedron. Nature 2004, 427, 618–621. [Google Scholar] [CrossRef] [PubMed]
- Douglas, S.M.; Dietz, H.; Liedl, T.; Hogberg, B.; Graf, F.; Shih, W.M. Self-assembly of DNA into nanoscale three-dimensional shapes. Nature 2009, 459, 414–418. [Google Scholar] [CrossRef] [PubMed]
- Wei, B.; Dai, M.; Myhrvold, C.; Ke, Y.; Jungmann, R.; Yin, P. Design space for complex DNA structures. J. Am. Chem. Soc. 2013, 135, 18080–18088. [Google Scholar] [CrossRef] [PubMed]
- Roller, E.M.; Khorashad, L.K.; Fedoruk, M.; Schreiber, R.; Govorov, A.O.; Liedl, T. DNA-assembled nanoparticle rings exhibit electric and magnetic resonances at visible frequencies. Nano Lett. 2015, 15, 1368–1373. [Google Scholar] [CrossRef] [PubMed]
- Urban, M.J.; Dutta, P.K.; Wang, P.; Duan, X.; Shen, X.; Ding, B.; Ke, Y.; Liu, N. Plasmonic toroidal metamolecules assembled by DNA origami. J. Am. Chem. Soc. 2016, 138, 5495–5498. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Gaitanaros, S.; Lee, S.; Bathe, M.; Shih, W.M.; Ke, Y. Programming self-assembly of DNA origami honeycomb two-dimensional lattices and plasmonic metamaterials. J. Am. Chem. Soc. 2016, 138, 7733–7740. [Google Scholar] [CrossRef] [PubMed]
- Young, K.L.; Ross, M.B.; Blaber, M.G.; Rycenga, M.; Jones, M.R.; Zhang, C.; Senesi, A.J.; Lee, B.; Schatz, G.C.; Mirkin, C.A. Using DNA to design plasmonic metamaterials with tunable optical properties. Adv. Mater. 2014, 26, 653–659. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Jagota, A.; Semke, E.D.; Diner, B.A.; McLean, R.S.; Lustig, S.R.; Richardson, R.E.; Tassi, N.G. DNA-assisted dispersion and separation of carbon nanotubes. Nat. Mater. 2003, 2, 338–342. [Google Scholar] [CrossRef] [PubMed]
- Tu, X.; Manohar, S.; Jagota, A.; Zheng, M. DNA sequence motifs for structure-specific recognition and separation of carbon nanotubes. Nature 2009, 460, 250–253. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; McLean, R.S.; Zheng, M. High-resolution length sorting and purification of DNA-wrapped carbon nanotubes by size-exclusion chromatography. Anal. Chem. 2005, 77, 6225–6228. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Jagota, A.; Strano, M.S.; Santos, A.P.; Barone, P.; Chou, S.G.; Diner, B.A.; Dresselhaus, M.S.; McLean, R.S.; Onoa, G.B.; et al. Structure-based carbon nanotube sorting by sequence-dependent DNA assembly. Science 2003, 302, 1545–1548. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y. Theoretical evidence for the stronger ability of thymine to disperse swcnt than cytosine and adenine: Self-stacking of DNA bases vs. their cross-stacking with swcnt. J. Phys. Chem. C 2008, 112, 14297–14305. [Google Scholar] [CrossRef]
- Husale, B.S.; Sahoo, S.; Radenovic, A.; Traversi, F.; Annibale, P.; Kis, A. Ssdna binding reveals the atomic structure of graphene. Langmuir 2010, 26, 18078–18082. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Choi, Y.-K.; Kim, H.-J.; Scheicher, R.H.; Cho, J.-H. Physisorption of DNA nucleobases on h-bn and graphene: Vdw-corrected dft calculations. J. Phys. Chem. C 2013, 117, 13435–13441. [Google Scholar] [CrossRef]
- Lu, Y.; Goldsmith, B.R.; Kybert, N.J.; Johnson, A.T.C. DNA-decorated graphene chemical sensors. Appl. Phys. Lett. 2010, 97, 083107. [Google Scholar] [CrossRef]
- Yun, J.M.; Kim, K.N.; Kim, J.Y.; Shin, D.O.; Lee, W.J.; Lee, S.H.; Lieberman, M.; Kim, S.O. DNA origami nanopatterning on chemically modified graphene. Angew. Chem. Int. Ed. Engl. 2012, 51, 912–915. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Mao, C. Molecular lithography with DNA nanostructures. Angew. Chem. Int. Ed. Engl. 2004, 43, 4068–4070. [Google Scholar] [CrossRef] [PubMed]
- Surwade, S.P.; Zhao, S.; Liu, H. Molecular lithography through DNA-mediated etching and masking of sio2. J. Am. Chem. Soc. 2011, 133, 11868–11871. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Sun, W.; Ke, Y.; Shih, C.J.; Paulus, G.L.; Hua Wang, Q.; Mu, B.; Yin, P.; Strano, M.S. Metallized DNA nanolithography for encoding and transferring spatial information for graphene patterning. Nat. Commun. 2013, 4, 1663. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.; Zhong, H.; Neff, D.; Norton, M.L. Nta directed protein nanopatterning on DNA origami nanoconstructs. J. Am. Chem. Soc. 2009, 131, 6660–6661. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhong, H.; Wang, R.; Seeman, N.C. Crystalline two-dimensional DNA-origami arrays. Angew. Chem. Int. Ed. Engl. 2011, 50, 264–267. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.C.; Rahman, M.; Norton, M.L. From nonfinite to finite 1d arrays of origami tiles. Acc. Chem. Res. 2014, 47, 1750–1758. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.; Norton, M.L. Enhancing DNA origami binding to graphene via π-π interactions. In Proceedings of the 9th Annual Conference Foundations of Nanoscience (FNANO12), Snowbird Cliff Lodge, Snowbird, UT, USA, 16–19 April 2012; pp. 214–215.
- Chiorcea-Paquim, A.-M.; Santos, P.V.; Oliveira-Brett, A.M. Atomic force microscopy and voltammetric characterisation of synthetic homo-oligodeoxynucleotides. Electrochim. Acta 2013, 110, 599–607. [Google Scholar] [CrossRef]
- Khripin, C.Y.; Arnold-Medabalimi, N.; Zheng, M. Molecular-crowding-induced clustering of DNA-wrapped carbon nanotubes for facile length fractionation. ACS Nano 2011, 5, 8258–8266. [Google Scholar] [CrossRef] [PubMed]
- Martin, W.; Zhu, W.; Krilov, G. Simulation study of noncovalent hybridization of carbon nanotubes by single-stranded DNA in water. J. Phys. Chem. B 2008, 112, 16076–16089. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.R.; Kohlmeyer, A.; Johnson, A.T.; Klein, M.L. Free energy landscape of a DNA-carbon nanotube hybrid using replica exchange molecular dynamics. Nano Lett. 2009, 9, 537–541. [Google Scholar] [CrossRef] [PubMed]








© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahman, M.; Neff, D.; Green, N.; Norton, M.L. DNA Origami Reorganizes upon Interaction with Graphite: Implications for High-Resolution DNA Directed Protein Patterning. Nanomaterials 2016, 6, 196. https://doi.org/10.3390/nano6110196
Rahman M, Neff D, Green N, Norton ML. DNA Origami Reorganizes upon Interaction with Graphite: Implications for High-Resolution DNA Directed Protein Patterning. Nanomaterials. 2016; 6(11):196. https://doi.org/10.3390/nano6110196
Chicago/Turabian StyleRahman, Masudur, David Neff, Nathaniel Green, and Michael L. Norton. 2016. "DNA Origami Reorganizes upon Interaction with Graphite: Implications for High-Resolution DNA Directed Protein Patterning" Nanomaterials 6, no. 11: 196. https://doi.org/10.3390/nano6110196
APA StyleRahman, M., Neff, D., Green, N., & Norton, M. L. (2016). DNA Origami Reorganizes upon Interaction with Graphite: Implications for High-Resolution DNA Directed Protein Patterning. Nanomaterials, 6(11), 196. https://doi.org/10.3390/nano6110196