The Assembly of DNA Amphiphiles at Liquid Crystal-Aqueous Interface
Abstract
:1. Introduction
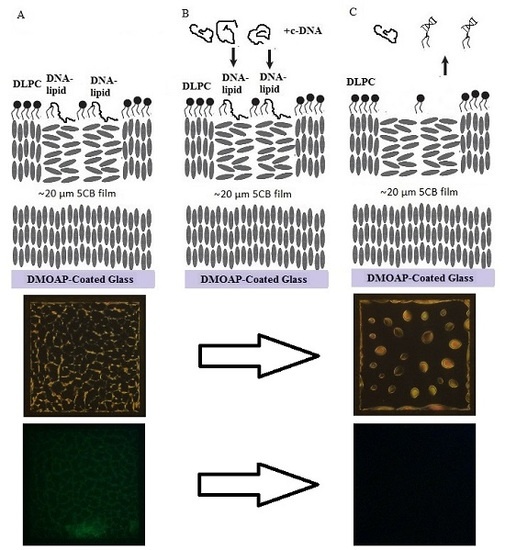
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Synthesis of DNA-Lipids
3.3. Preparation of DMOAP-Treated Glass
3.4. Preparation of Optical Cells
3.5. Using of SYBR Green to Label DNA Strands
3.6. The Preparation of l-DLPC and DNA-Lipids Mixture
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Brake, J.M.; Daschner, M.K.; Luk, Y.Y.; Abbott, N.L. Biomolecular interactions at phospholipid-decorated surfaces of liquid crystals. Science 2003, 302, 2094–2097. [Google Scholar] [CrossRef] [PubMed]
- Brake, J.M.; Abbott, N.L. An experimental system for imaging the reversible adsorption of amphiphiles at aqueous-liquid crystal interfaces. Langmuir 2002, 18, 6101–6109. [Google Scholar] [CrossRef]
- Lockwood, N.A.; Gupta, J.L.; Abbott, N.L. Self-assembly of amphiphiles, polymers and proteins at interfaces between thermotropic liquid crystals and aqueous phases. Surf. Sci. Rep. 2008, 63, 255–293. [Google Scholar] [CrossRef]
- Brake, J.M.; Mezera, A.D.; Abbott, N.L. Effect of surfactant structure on the orientation of liquid crystals at aqueous-liquid crystal interfaces. Langmuir 2003, 19, 6436–6442. [Google Scholar] [CrossRef]
- Brake, J.M.; Mezera, A.D.; Abbott, N.L. Active control of the anchoring of 4′-pentyl-4-cyanobiphenyl (5CB) at an aqueous-liquid crystal interface by using a redox-active ferrocenyl surfactant. Langmuir 2003, 19, 8629–8637. [Google Scholar] [CrossRef]
- Brake, J.M.; Abbott, N.L. Coupling of the orientations of thermotropic liquid crystals to protein binding events at lipid-decorated interfaces. Langmuir 2007, 23, 8497–8507. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Teren, S.; Tepp, W.H.; Beebe, D.J.; Johnson, E.A.; Abbott, N.L. Formation of oligopeptide-based polymeric membranes at interfaces between aqueous phases and thermotropic liquid crystals. Chem. Mater. 2006, 18, 6147–6151. [Google Scholar] [CrossRef]
- Kinsinger, M.I.; Lynn, D.M.; Abbott, N.L. Nematic ordering drives the phase separation of mixed monolayers containing phospholipids modified with poly(ethylene glycol) at aqueous–liquid crystal interfaces. Soft Matter 2010, 6, 4095–4104. [Google Scholar] [CrossRef]
- Jang, C.H.; Cheng, L.L.; Olsen, C.W.; Abbott, N.L. Anchoring of nematic liquid crystals on viruses with different envelope structures. Nano Lett. 2006, 6, 1053–1058. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Hartono, D.; Yang, K.L. Detecting and differentiating Escherichia coli strain TOP10 using optical textures of liquid crystals. Liq. Cryst. 2010, 37, 1269–1274. [Google Scholar] [CrossRef]
- Sivakumar, S.; Wark, K.L.; Gupta, J.K.; Abbott, N.L.; Caruso, F. Liquid crystal emulsions as the basis of biological sensors for the optical detection of bacteria and viruses. Adv. Funct. Mater. 2009, 19, 2260–2265. [Google Scholar] [CrossRef]
- Lin, I.H.; Miller, D.S.; Bertics, P.J.; Murphy, C.J.; de Pablo, J.J.; Abbott, N.L. Endotoxin-induced structural transformations in liquid crystalline droplets. Science 2011, 332, 1297–1300. [Google Scholar] [CrossRef] [PubMed]
- Bi, X.; Hartono, D.; Yang, K.L. Real-time liquid crystal pH sensor for monitoring enzymatic activities of penicillinase. Adv. Funct. Mater. 2009, 19, 3760–3765. [Google Scholar] [CrossRef]
- Hu, Q.Z.; Jang, C.H. Orientational behaviour of ultraviolet-tailored 4-cyano-4′-pentylbiphenyl at the aqueous/liquid crystal interface. Liq. Cryst. 2011, 38, 1209–1216. [Google Scholar] [CrossRef]
- Tan, L.N.; Orler, V.J.; Abbott, N.L. Ordering transitions triggered by specific binding of vesicles to protein-decorated interfaces of thermotropic liquid crystals. Langmuir 2012, 28, 6364–6376. [Google Scholar] [CrossRef] [PubMed]
- Lowe, A.M.; Abbott, N.L. Liquid crystalline materials for biological applications. Chem. Mater. 2011, 24, 746–758. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Park, S. Liquid crystal-based proton sensitive glucose biosensor. Anal. Chem. 2014, 86, 1493–1501. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yang, P.Y.; Mondiot, F.; Li, Y.; Miller, D.S.; Chen, Z.; Abbott, N.L. Interfacial ordering of thermotropic liquid crystals triggered by the secondary structures of oligopeptides. Chem. Commun. 2015, 51, 16844–16847. [Google Scholar] [CrossRef] [PubMed]
- Sadati, M.; Apik, A.I.; Armas-Perez, J.C.; Martinez-Gonzalez, J.A.; Hernandez-Ortiz, J.P.; Abbott, N.L.; de Pablo, J.J. Liquid crystal enabled early stage detection of beta amyloid formation on lipid monolayers. Adv. Funct. Mater. 2015, 25, 6050–6060. [Google Scholar] [CrossRef]
- Zhu, Q.; Yang, K.L. Microfluidic immunoassay with plug-in liquid crystal for optical detection of antibody. Anal. Chim. Acta 2015, 853, 696–701. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yang, K.L. Applications of metal ions and liquid crystals for multiplex detection of DNA. J. Colloid Interface Sci. 2015, 439, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Ong, L.H.; Yang, K.L. Surfactant-driven assembly of poly(ethylenimine)-coated microparticles at the liquid crystal/water interface. J. Phys. Chem. B 2016, 120, 825–833. [Google Scholar] [CrossRef] [PubMed]
- Kwiat, M.; Elnathan, R.; Kwak, M.; de Vries, J.B.; Pevzner, A.; Engel, Y.; Burstein, L.; Khatchtourints, A.; Lichtenstein, A.; Flaxer, E.; et al. Non-covalent monolayer-piercing anchoring of lipophilic nucleic acids: Preparation, characterization, and sensing applications. J. Am. Chem. Soc. 2012, 134, 280–292. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Yang, Z. Beyond displays: The recent progress of liquid crystals for bio/chemical detections. Chin. Sci. Bull. 2013, 58, 2557–2562. [Google Scholar] [CrossRef]
- Price, A.D.; Schwartz, D.K. DNA hybridization-induced reorientation of liquid crystal anchoring at the nematic liquid crystal/aqueous interface. J. Am. Chem. Soc. 2008, 130, 8188–8194. [Google Scholar] [CrossRef] [PubMed]
- Mcumber, A.C.; Noonan, P.S.; Schwartz, D.K. Surfactant–DNA interactions at the liquid crystal–aqueous interface. Soft Matter 2012, 8, 4335–4342. [Google Scholar] [CrossRef]
- Noonan, P.S.; Roberts, R.H.; Schwartz, D.K. Liquid crystal reorientation induced by aptamer conformational changes. J. Am. Chem. Soc. 2013, 135, 5183–5189. [Google Scholar] [CrossRef] [PubMed]
- Noonan, P.S.; Mohan, P.; Goodwin, A.P.; Schwartz, D.K. DNA hybridization-mediated liposome fusion at the aqueous liquid crystal interface. Adv. Funct. Mater. 2014, 24, 3206–3212. [Google Scholar] [CrossRef] [PubMed]
- Lai, S.L.; Hartono, D.; Yang, K.L. Self-assembly of cholesterol DNA at liquid crystal/aqueous interface and its application for DNA detection. Appl. Phys. Lett. 2009, 95, 153702. [Google Scholar] [CrossRef]
- Gupta, J.K.; Meli, M.V.; Teren, S.; Abbott, N.L. Elastic energy-driven phase separation of phospholipid monolayers at the nematic liquid-crystal–aqueous interface. Phys. Rev. Lett. 2008, 100, 048301. [Google Scholar] [CrossRef] [PubMed]
- Mackellar, C.; Graham, D.; Will, D.W.; Burgess, S.; Brown, T. Synthesis and physical properties of anti-HIV antisense oligonucleotides bearing terminal lipophilic groups. Nucleic Acids Res. 1992, 20, 3411–3417. [Google Scholar] [CrossRef] [PubMed]






© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, J.; Dong, Y.; Zhang, Y.; Liu, D.; Yang, Z. The Assembly of DNA Amphiphiles at Liquid Crystal-Aqueous Interface. Nanomaterials 2016, 6, 229. https://doi.org/10.3390/nano6120229
Zhou J, Dong Y, Zhang Y, Liu D, Yang Z. The Assembly of DNA Amphiphiles at Liquid Crystal-Aqueous Interface. Nanomaterials. 2016; 6(12):229. https://doi.org/10.3390/nano6120229
Chicago/Turabian StyleZhou, Jingsheng, Yuanchen Dong, Yiyang Zhang, Dongsheng Liu, and Zhongqiang Yang. 2016. "The Assembly of DNA Amphiphiles at Liquid Crystal-Aqueous Interface" Nanomaterials 6, no. 12: 229. https://doi.org/10.3390/nano6120229
APA StyleZhou, J., Dong, Y., Zhang, Y., Liu, D., & Yang, Z. (2016). The Assembly of DNA Amphiphiles at Liquid Crystal-Aqueous Interface. Nanomaterials, 6(12), 229. https://doi.org/10.3390/nano6120229