Facile Synthesis of Novel CaIn2S4/ZnIn2S4 Composites with Efficient Performance for Photocatalytic Reduction of Cr(VI) under Simulated Sunlight Irradiation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Composite Photocatalysts
2.3. Material Characterization
2.4. Photocatalytic Reduction of Cr(VI)
3. Results and Discussion
3.1. XRD Analysis and BET Surface Area
3.2. SEM and Elemental Mapping Analysis
3.3. XPS Analysis and Optical Properties
3.4. Photocatalytic Activity
3.5. Catalytic Stability
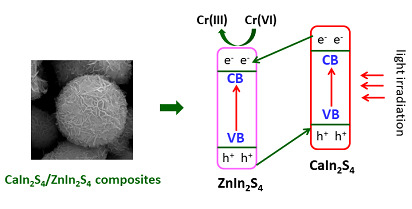
3.6. Enhancement Mechanism of Photocatalytic Activity and Stability
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Yang, L.X.; Luo, S.L.; Li, Y.; Xiao, Y.; Kang, Q.; Cai, Q.Y. High efficient photocatalytic degradation of p-nitrophenol on a unique Cu2O/TiO2 p-n heterojunction network catalyst. Environ. Sci. Technol. 2010, 44, 7641–7646. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.M.; Hou, C.L.; Jiao, T.F.; Song, J.W.; Zhang, X.; Xing, R.R.; Zhou, J.X.; Zhang, L.X.; Peng, Q.M. Self-assembled AgNP-containing nanocomposites constructed by electrospinning as efficient dye photocatalyst materials for wastewater treatment. Nanomaterials 2018, 8, 35. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.L.; Pehkonen, S.O.; Ray, A.K. Removal of aqueous Cr(VI) by a combination of photocatalytic reduction and coprecipitation. Ind. Eng. Chem. Res. 2004, 43, 1665–1672. [Google Scholar] [CrossRef]
- Yin, H.B.; Wada, Y.; Kitamura, T.; Yanagida, S. Photoreductive dehalogenation of halogenated benzene derivatives using ZnS or CdS nanocrystallites as photocatalysts. Environ. Sci. Technol. 2001, 35, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.X.; Guzman, M.I. CO2 reduction under periodic illumination of ZnS. J. Phys. Chem. C 2014, 118, 11649–11656. [Google Scholar] [CrossRef]
- Zhou, R.X.; Guzman, M.I. Photocatalytic reduction of fumarate to succinate on ZnS mineral surfaces. J. Phys. Chem. C 2016, 120, 7349–7357. [Google Scholar] [CrossRef]
- Baran, T.; Wojtyla, S.; Dibenedetto, A.; Aresta, M.; Macyk, W. Zinc sulfide functionalized with ruthenium nanoparticles for photocatalytic reduction of CO2. Appl. Catal. B Environ. 2015, 178, 170–176. [Google Scholar] [CrossRef]
- Wojtyla, S.; Baran, T. Insight on doped ZnS and its activity towards photocatalytic removing of Cr(VI) from wastewater in the presence of organic pollutants. Mater. Chem. Phys. 2018, 212, 103–112. [Google Scholar] [CrossRef]
- Wang, W.J.; Ng, T.W.; Ho, W.K.; Huang, J.H.; Liang, S.J.; An, T.C.; Li, G.Y.; Yu, J.C.; Wong, P.K. CdIn2S4 microsphere as an efficient visible-light-driven photocatalyst for bacterial inactivation: Synthesis, characterizations and photocatalytic inactivation mechanisms. Appl. Catal. B Environ. 2013, 129, 482–490. [Google Scholar] [CrossRef]
- Tu, X.L.; Lu, J.; Li, M.; Su, Y.J.; Yin, G.L.; He, D.N. Hierarchically ZnIn2S4 nanosheet-constructed microwire arrays: Template-free synthesis and excellent photocatalytic performances. Nanoscale 2018, 10, 4735–4744. [Google Scholar] [CrossRef] [PubMed]
- Gou, X.L.; Cheng, F.Y.; Shi, Y.H.; Zhang, L.; Peng, S.J.; Chen, J.; Shen, P.W. Shape-controlled synthesis of ternary chalcogenide ZnIn2S4 and CuIn(S,Se)(2) nano-/microstructures via facile solution route. J. Am. Chem. Soc. 2006, 128, 7222–7229. [Google Scholar] [CrossRef] [PubMed]
- Li, L.L.; Peng, S.J.; Wang, N.; Srinivasan, M.; Mhaisalkar, S.G.; Yan, Q.Y.; Ramakrishna, S. A general strategy toward carbon cloth-based hierarchical films constructed by porous nanosheets for superior photocatalytic activity. Small 2015, 11, 2429–2436. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Shavel, A.; An, X.Q.; Luo, Z.S.; Ibáñez, M.; Cabot, A. Cu2ZnSnS4-Pt and Cu2ZnSnS4-Au heterostructured nanoparticles for photocatalytic water splitting and pollutant degradation. J. Am. Chem. Soc. 2014, 136, 9236–9239. [Google Scholar] [CrossRef] [PubMed]
- Gao, B.; Liu, L.F.; Liu, J.D.; Yang, F.L. Photocatalytic degradation of 2,4,6-tribromophenol over Fe-doped ZnIn2S4: Stable activity and enhanced debromination. Appl. Catal. B Environ. 2013, 129, 89–97. [Google Scholar] [CrossRef]
- Guan, Z.J.; Xu, Z.Q.; Li, Q.Y.; Wang, P.; Li, G.Q.; Yang, J.J. AgIn5S8 nanoparticles anchored on 2D layered ZnIn2S4 to form OD/2D heterojunction for enhanced visible-light photocatalytic hydrogen evolution. Appl. Catal. B Environ. 2018, 227, 512–518. [Google Scholar] [CrossRef]
- Yuan, Y.J.; Chen, D.Q.; Zhong, J.S.; Yang, L.X.; Wang, J.J.; Liu, M.J.; Tu, W.G.; Yu, Z.T.; Zou, Z.G. Interface engineering of a noble-metal-free 2D-2D MoS2/Cu-ZnIn2S4 photocatalyst for enhanced photocatalytic H2 production. J. Mater. Chem. A 2017, 5, 15771–15779. [Google Scholar] [CrossRef]
- Tan, C.W.; Zhu, G.Q.; Hojamberdiev, M.; Lokesh, K.S.; Luo, X.C.; Jin, L.; Zhou, J.P.; Liu, P. Adsorption and enhanced photocatalytic activity of the {0001} faceted Sm-doped ZnIn2S4 microspheres. J. Hazard. Mater. 2014, 278, 572–583. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.Y.; Zhang, J.J.; Zhu, B.C.; Yu, J.G.; Xu, J.S. CuInS2 sensitized TiO2 hybrid nanofibers for improved photocatalytic CO2 reduction. Appl. Catal. B Environ. 2018, 230, 194–202. [Google Scholar] [CrossRef]
- Aguirre, M.E.; Zhou, R.X.; Eugene, A.J.; Guzman, M.I.; Grela, M.A. Cu2O/TiO2 heterostructures for CO2 reduction through a direct Z-scheme: Protecting Cu2O from photocorrosion. Appl. Catal. B Environ. 2017, 217, 485–493. [Google Scholar] [CrossRef]
- Wu, Z.; Gong, C.; Yu, J.; Sun, L.; Xiao, W.; Lin, C. Enhanced visible light photoelectrocatalytic activity over CuxZn1-xIn2S4@TiO2 nanotube array hetero-structures. J. Mater. Chem. A 2017, 5, 1292–1299. [Google Scholar] [CrossRef]
- Hou, J.; Yang, C.; Cheng, H.; Wang, Z.; Jiao, S.; Zhu, H. Ternary 3D architectures of CdS QDs/graphene/ZnIn2S4 heterostructures for efficient photocatalytic H2 production. Phys. Chem. Chem. Phys. 2013, 15, 15660–15668. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Liu, K.; Feng, Z.; Bao, Y.; Dong, B. Hierarchical sheet-on-sheet ZnIn2S4/g-C3N4 heterostructure with highly efficient photocatalytic H2 production based on photoinduced interfacial charge transfer. Sci. Rep. 2016, 6, 19221. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Lin, Y.; Yang, J.H.; Yu, Y. CdS-based semiconductor photocatalysts for hydrogen production from water splitting under solar light. ACS Symp. Ser. 2013, 1140, 219–241. [Google Scholar] [CrossRef]
- Chen, J.Y.; Zhang, H.M.; Liu, P.R.; Li, Y.B.; Liu, X.L.; Li, G.Y.; Wong, P.K.; An, T.C.; Zhao, H.J. Cross-linked ZnIn2S4/rGO composite photocatalyst for sunlight-driven photocatalytic degradation of 4-nitrophenol. Appl. Catal. B Environ. 2015, 168–169, 266–273. [Google Scholar] [CrossRef]
- Yuan, W.H.; Yang, S.; Li, L. Synthesis of g-C3N4/CaIn2S4 composites with enhanced photocatalytic activity under visible light irradiation. Dalton Trans. 2015, 44, 16091–16098. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.J.; Yan, W.H.; Sun, S.; Bao, J.; Gao, C. Hydrothermal synthesis of CaIn2S4-reduced graphene oxide nanocomposites with increased photocatalytic performance. ACS Appl. Mater. Interfance 2014, 6, 12877–12884. [Google Scholar] [CrossRef] [PubMed]
- Jo, W.K.; Natarajan, T.S. Facile synthesis of novel redox-mediator-free direct Z-Scheme Caln2S4 marigold-flower-like/TiO2 photocatalysts with superior photocatalytic efficiency. ACS Appl. Mater. Interfance 2015, 7, 17138–17154. [Google Scholar] [CrossRef] [PubMed]
- Testa, J.J.; Grela, M.A.; Litter, M.I. Heterogeneous photocatalytic reduction of chromium(VI) over TiO2 particles in the presence of oxalate: Involvement of Cr(V) species. Environ. Sci. Technol. 2004, 38, 1589–1594. [Google Scholar] [CrossRef] [PubMed]
- Fu, F.L.; Wang, Q. Removal of heavy metal ions from wastewaters: A review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.H.; Yu, J.; Tu, G.Y.; Qu, L.; Xiao, J.C.; Liu, Y.D.; Wang, L.Z.; Lei, J.Y.; Zhang, J.L. Carbon nitride coupled Ti-SBA15 catalyst for visible-light-drivenphotocatalytic reduction of Cr (VI) and the synergistic oxidation of phenol. Appl. Catal. B Environ. 2017, 201, 1–11. [Google Scholar] [CrossRef]
- Nayak, A.K.; Lee, S.; Choi, Y.I.; Yoon, H.J.; Sohn, Y.; Pradhan, D. Crystal phase and size-controlled synthesis of tungsten trioxide hydrate nanoplates at room temperature: Enhanced Cr(VI) photoreduction and methylene blue adsorption properties. ACS Sustain. Chem. Eng. 2017, 5, 2741–2750. [Google Scholar] [CrossRef]
- Yang, J.; You, J.; Dai, J.; Chen, Y.M.; Li, Y. In-situ synthesis of hydrogen-titanate nanotubes/graphene composites with chemically bonded interface and enhanced visible photocatalytic activity. Nanomaterials 2018, 8, 229. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, C.B.; Gao, F.; Mailhot, G.; Pan, G. Algae decorated TiO2/Ag hybrid nanofiber membrane with enhanced photocatalytic activity for Cr(VI) removal under visible light. Chem. Eng. J. 2017, 314, 622–630. [Google Scholar] [CrossRef]
- Ye, L.; Fu, J.L.; Xu, Z.; Yuan, R.S.; Li, Z.H. Facile one-pot solvothermal method to synthesize sheet-on-sheet reduced graphene oxide (RGO)/ZnIn2S4 nanocomposites with superior photocatalytic performance. ACS Appl. Mater. Interfaces 2014, 6, 3483–3490. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Sun, S.; Yan, W.; Bao, J.; Gao, C. Photocatalytic H2 evolution on a novel CaIn2S4 photocatalyst under visible light irradiation. Int. J. Hydrogen Energy 2013, 38, 13153–13158. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, J.; Yu, J.Q.; Zhang, Y.; Cui, Z.X.; Sun, Y.; Hou, B.R. Fabrication of InVO4/AgVO3 heterojunctions with enhanced photocatalytic antifouling efficiency under visible-light. Appl. Catal. B Environ. 2018, 220, 57–66. [Google Scholar] [CrossRef]
- Shi, L.; Yin, P.Q.; Dai, Y.M. Synthesis and photocatalytic performance of ZnIn2S4 nanotubes and nanowires. Langmuir 2013, 29, 12818–12822. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.C.; Tang, L.; Zeng, G.M.; Zhu, Z.J.; Yan, M.; Zhou, Y.Y.; Wang, J.J.; Liu, Y.N.; Wang, J.J. Insight into highly efficient simultaneous photocatalytic removal of Cr(VI) and 2,4-diclorophenol under visible light irradiation by phosphorus doped porous ultrathin g-C3N4 nanosheets from aqueous media: Performance and reaction mechanism. Appl. Catal. B Environ. 2017, 203, 343–354. [Google Scholar] [CrossRef]
- Liu, S.Q.; Yang, M.Q.; Tang, Z.R.; Xu, Y.J. A nanotree-like CdS/ZnO nanocomposite with spatially branched hierarchical structure for photocatalytic fine-chemical synthesis. Nanoscale 2014, 6, 7193–7198. [Google Scholar] [CrossRef] [PubMed]
- Gilja, V.; Novakovic, K.; Travas-Sejdic, J.; Hrnjak-Murgic, Z.; Rokovic, M.K.; Zic, M. Stability and synergistic effect of polyaniline/TiO2 photocatalysts in degradation of Azo dye in wastewater. Nanomaterials 2017, 7, 412. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.; Wang, J.; Tafen, D.N.; Wang, H.; Zheng, J.-G.; Lewis, J.P.; Liu, X.; Leonard, S.S.; Manivannan, A. Shape-enhanced photocatalytic activity of single-crystalline anatase TiO2 (101) nanobelts. J. Am. Chem. Soc. 2010, 132, 6679–6685. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Surendar, T.; Baruah, A.; Shanker, V. Synthesis of a novel and stable g-C3N4-Ag3PO4 hybrid nanocomposite photocatalyst and study of the photocatalytic activity under visible light irradiation. J. Mater. Chem. A 2013, 1, 5333–5340. [Google Scholar] [CrossRef]
- Xiong, P.; Zhu, J.W.; Wang, X. Cadmium sulfide-ferrite nanocomposite as a magnetically recyclable photocatalyst with enhanced visible-light-driven photocatalytic activity and photostability. Ind. Eng. Chem. Res. 2013, 52, 17126–17133. [Google Scholar] [CrossRef]
- Qiu, J.H.; Zhang, X.F.; Zhang, X.G.; Feng, Y.; Li, Y.X.; Yang, L.Y.; Lu, H.Q.; Yao, J.F. Constructing Cd0.5Zn0.5S@ZIF-8 nanocomposites through self-assembly strategy to enhance Cr(VI) photocatalytic reduction. J. Hazard. Mater. 2018, 349, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.J.; Chen, D.Q.; Shi, X.F.; Tu, J.R.; Hu, B.; Yang, L.X.; Yu, Z.T.; Zou, Z.G. Facile fabrication of “green” SnS2 quantum dots/reduced graphene oxide composites with enhanced photocatalytic performance. Chem. Eng. J. 2017, 313, 1438–1446. [Google Scholar] [CrossRef]












| Samples | SBET (m2/g) | Molar Ratios of Ca:Zn (%) | AQE (%) |
|---|---|---|---|
| pure ZnIn2S4 | 59.2 | 0 | 3.7 |
| 5% CaIn2S4/ZnIn2S4 | 56.3 | 5.05 | 4.1 |
| 10% CaIn2S4/ZnIn2S4 | 54.7 | 9.67 | 4.5 |
| 20% CaIn2S4/ZnIn2S4 | 53.4 | 18.30 | 5.2 |
| 30% CaIn2S4/ZnIn2S4 | 52.1 | 26.52 | 6.6 |
| 50% CaIn2S4/ZnIn2S4 | 50.5 | 43.29 | 5.5 |
| pure CaIn2S4 | 46.0 | – | 2.3 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, S.; Dai, J.; Yang, J.; You, J.; Hao, J. Facile Synthesis of Novel CaIn2S4/ZnIn2S4 Composites with Efficient Performance for Photocatalytic Reduction of Cr(VI) under Simulated Sunlight Irradiation. Nanomaterials 2018, 8, 472. https://doi.org/10.3390/nano8070472
Xu S, Dai J, Yang J, You J, Hao J. Facile Synthesis of Novel CaIn2S4/ZnIn2S4 Composites with Efficient Performance for Photocatalytic Reduction of Cr(VI) under Simulated Sunlight Irradiation. Nanomaterials. 2018; 8(7):472. https://doi.org/10.3390/nano8070472
Chicago/Turabian StyleXu, Siyu, Jun Dai, Juan Yang, Jun You, and Jingyi Hao. 2018. "Facile Synthesis of Novel CaIn2S4/ZnIn2S4 Composites with Efficient Performance for Photocatalytic Reduction of Cr(VI) under Simulated Sunlight Irradiation" Nanomaterials 8, no. 7: 472. https://doi.org/10.3390/nano8070472
APA StyleXu, S., Dai, J., Yang, J., You, J., & Hao, J. (2018). Facile Synthesis of Novel CaIn2S4/ZnIn2S4 Composites with Efficient Performance for Photocatalytic Reduction of Cr(VI) under Simulated Sunlight Irradiation. Nanomaterials, 8(7), 472. https://doi.org/10.3390/nano8070472