Preparation of N-Doped Carbon Nanosheets from Sewage Sludge for Adsorption Studies of Cr(VI) from Aqueous Solution
Abstract
:1. Introduction
2. Materials and Methods
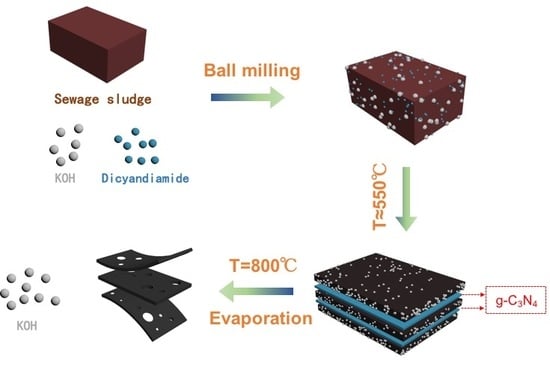
2.1. Materials Synthesis
2.2. Adsorption Characterization
2.3. Density Functional Theory (DFT)
3. Results and Discussion
3.1. Proximate Analysis and Yield of Activated Carbon (SAC, N-SAC)
3.2. Characterization
3.2.1. Textural Properties
3.2.2. Morphology Analysis
3.2.3. FT-IR and XPS Analysis
3.3. Adsorption Studies of Cr(VI) on N-SAC
3.3.1. Effect of PH
3.3.2. Comparison of Adsorption Performance
3.3.3. Adsorption Kinetics
3.3.4. Adsorption Isotherm
3.3.5. Intraparticle Diffusion Model (IPD)
3.3.6. Adsorption Thermodynamics
3.4. Cr (VI) Adsorption Mechanism
3.5. DFT Calculation
3.6. Regeneration Investigation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Smith, K.; Fowler, G.; Pullket, S.; Graham, N. Sewage sludge-based adsorbents: A review of their production, properties and use in water treatment applications. Water Res. 2009, 43, 2569–2594. [Google Scholar] [CrossRef] [PubMed]
- Caicedo, C.; Rosenwinkel, K.-H.; Exner, M.; Verstraete, W.; Suchenwirth, R.; Hartemann, P.; Nogueira, R. Legionella occurrence in municipal and industrial wastewater treatment plants and risks of reclaimed wastewater reuse: Review. Water Res. 2019, 149, 21–34. [Google Scholar] [CrossRef] [PubMed]
- Tong, X.-J.; Li, J.-Y.; Yuan, J.-H.; Xu, R.-K. Adsorption of Cu(II) by biochars generated from three crop straws. Chem. Eng. J. 2011, 172, 828–834. [Google Scholar] [CrossRef]
- Lu, H.; Zhang, W.; Yang, Y.; Huang, X.; Wang, S.; Qiu, R. Relative distribution of Pb2+ sorption mechanisms by sludge-derived biochar. Water Res. 2012, 46, 854–862. [Google Scholar] [CrossRef]
- Liu, S.; Chen, X.; Liu, Z.; Wang, H. Activated carbon with excellent chromium(VI) adsorption performance prepared by acid–base surface modification. J. Hazardous Mat. 2007, 141, 315–319. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, Q.; Liu, B.; Hu, T.; Liu, W.; Yao, J. Green synthesis of tannin-hexamethylendiamine based adsorbents for efficient removal of Cr(VI). J. Hazardous Mat. 2018, 352, 27–35. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, S.; Liu, Q.; Chen, Z.; Gu, J.; Zhu, C.; Lu, T.; Zhang, D.; Ma, J. N-doped porous carbon with magnetic particles formed in situ for enhanced Cr(VI) removal. Water Res. 2013, 47, 4188–4197. [Google Scholar] [CrossRef]
- Li, D.; Chen, L.; Zhao, J.; Zhang, X.; Wang, Q.; Wang, H.; Ye, N. Evaluation of the pyrolytic and kinetic characteristics of Enteromorpha prolifera as a source of renewable bio-fuel from the Yellow Sea of China. Chem. Eng. Res. Design 2010, 88, 647–652. [Google Scholar] [CrossRef]
- Jeyaseelan, S.; Lu, G. Development of adsorbent/catalyst from municipal wastewater sludge. Water Sci. Tech. 1996, 34, 499–505. [Google Scholar] [CrossRef]
- Li, W.-H.; Yue, Q.-Y.; Gao, B.-Y.; Wang, X.-J.; Qi, Y.-F.; Zhao, Y.-Q.; Li, Y.-J. Preparation of sludge-based activated carbon made from paper mill sewage sludge by steam activation for dye wastewater treatment. Desalination 2011, 278, 179–185. [Google Scholar] [CrossRef]
- Wang, H.; Xu, Z.; Kohandehghan, A.; Li, Z.; Cui, K.; Tan, X.; Stephenson, T.J.; King’Ondu, C.K.; Holt, C.M.B.; Olsen, B.C.; et al. Interconnected carbon nanosheets derived from hemp for ultrafast supercapacitors with high energy. ACS Nano 2013, 7, 5131–5141. [Google Scholar] [CrossRef] [PubMed]
- Du, W.; Wang, X.; Sun, X.; Zhan, J.; Zhang, H.; Zhao, X. Nitrogen-doped hierarchical porous carbon using biomass-derived activated carbon/carbonized polyaniline composites for supercapacitor electrodes. J. Electro. Chem. 2018, 827, 213–220. [Google Scholar] [CrossRef]
- Ye, X.-N.; Lu, Q.; Wang, X.; Guo, H.-Q.; Cui, M.-S.; Dong, C.-Q.; Yang, Y.-P. Catalytic Fast Pyrolysis of Cellulose and Biomass to Selectively Produce Levoglucosenone Using Activated Carbon Catalyst. ACS Sustainable Chem. Eng. 2017, 5, 10815–10825. [Google Scholar] [CrossRef]
- Li, X.; Li, H.; Liu, T.; Hei, Y.; Hassan, M.; Zhang, S.; Lin, J.; Wang, T.; Bo, X.; Wang, H.-L.; et al. The biomass of ground cherry husks derived carbon nanoplates for electroChem. sensing. Sensors Actuators B Chem. 2018, 255, 3248–3256. [Google Scholar] [CrossRef]
- Weng, C.-H.; Lin, Y.-T.; Tzeng, T.-W. Removal of methylene blue from aqueous solution by adsorption onto pineapple leaf powder. J. Hazardous Mat. 2009, 170, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Luo, T.; Tian, X.; Yang, C.; Luo, W.; Nie, Y.; Wang, Y. Polyethylenimine-Functionalized Corn Bract, an Agricultural Waste Material, for Efficient Removal and Recovery of Cr(VI) from Aqueous Solution. J. Agric. Food Chem. 2017, 65, 7153–7158. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Jiang, W.-J.; Zhang, Y.; Niu, S.; Tang, T.; Huang, L.-B.; Chen, Y.-Y.; Wei, Z.-D.; Hu, J.-S. Self-terminated activation for high-yield production of N,P-codoped nanoporous carbon as an efficient metal-free electrocatalyst for Zn-air battery. Carbon 2018, 128, 97–105. [Google Scholar] [CrossRef]
- Bedia, J.; Monsalvo, V.; Rodriguez, J.; Mohedano, A.; Garcia-Matamoros, J.B. Iron catalysts by Chem. activation of sewage sludge with FeCl 3 for CWPO. Chem. Eng. J. 2017, 318, 224–230. [Google Scholar] [CrossRef]
- Sud, D.; Mahajan, G.; Kaur, M. Agricultural waste material as potential adsorbent for sequestering heavy metal ions from aqueous solutions – A review. Bioresource Tech. 2008, 99, 6017–6027. [Google Scholar] [CrossRef]
- Pezoti, O.; Cazetta, A.L.; Bedin, K.C.; Souza, L.S.; Martins, A.C.; Silva, T.L.; Júnior, O.O.S.; Visentainer, J.V.; Almeida, V.C. NaOH-activated carbon of high surface area produced from guava seeds as a high-efficiency adsorbent for amoxicillin removal: Kinetic, isotherm and thermodynamic studies. Chem. Eng. J. 2016, 288, 778–788. [Google Scholar] [CrossRef]
- Jiang, T.-Y.; Jiang, J.; Xu, R.-K.; Li, Z. Adsorption of Pb(II) on variable charge soils amended with rice-straw derived biochar. Chemosphere 2012, 89, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Guo, S.; Ma, N.; Wang, G.; Ma, C.; Hao, J.; Xue, M.; Zhang, X. Sewage sludge pretreatment by microwave irradiation combined with activated carbon fibre at alkaline pH for anaerobic digestion. Water Sci. Tech. 2016, 73, 2882–2887. [Google Scholar] [CrossRef] [PubMed]
- Silva, T.L.; Ronix, A.; Pezoti, O.; Souza, L.S.; Leandro, P.K.; Bedin, K.C.; Beltrame, K.K.; Cazetta, A.L.; Almeida, V.C. Mesoporous activated carbon from industrial laundry sewage sludge: Adsorption studies of reactive dye Remazol Brilliant Blue R. Chem. Eng. J. 2016, 303, 467–476. [Google Scholar] [CrossRef]
- Jiang, J.; Xu, R.-K. Application of crop straw derived biochars to Cu(II) contaminated Ultisol: Evaluating role of alkali and organic functional groups in Cu(II) immobilization. Bioresource Tech. 2013, 133, 537–545. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Cheng, H.; Chen, G. Preparation and characterization of carbonaceous adsorbents from sewage sludge using a pilot-scale microwave heating equipment. J. Anal. Appl. Pyrolysis 2012, 93, 113–119. [Google Scholar] [CrossRef]
- Wen, X.; Liu, H.; Zhang, L.; Zhang, J.; Fu, C.; Shi, X.; Chen, X.; Mijowska, E.; Chen, M.-J.; Wang, D.-Y.; et al. Large-scale converting waste coffee grounds into functional carbon materials as high-efficient adsorbent for organic dyes. Bioresource Tech. 2019, 272, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zhang, G.; Dai, Z.; Zhou, L.; Bian, P.; Zheng, K.; Wu, Z.; Cai, D. Sandwich-like Nanosystem for Simultaneous Removal of Cr(VI) and Cd(II) from Water and Soil. ACS Appl. Mater. Interfaces 2018, 10, 18316–18326. [Google Scholar] [CrossRef] [PubMed]
- Jia, S.; Wang, Y.; Tian, P.; Zhou, S.; Cai, H.; Gao, H.; Zang, J. A simple synthetic route of N-doped mesoporous carbon derived from casein extracted with cobalt ions for high rate performance supercapacitors. Electrochim. Acta 2017, 250, 16–24. [Google Scholar] [CrossRef]
- Hameed, B.; Ahmad, A. Batch adsorption of methylene blue from aqueous solution by garlic peel, an agricultural waste biomass. J. Hazardous Mat. 2009, 164, 870–875. [Google Scholar] [CrossRef]
- Choi, H.-D.; Cho, J.-M.; Baek, K.; Yang, J.-S.; Lee, J.-Y. Influence of cationic surfactant on adsorption of Cr(VI) onto activated carbon. J. Hazardous Mat. 2009, 161, 1565–1568. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, M.; Li, H.; Ge, H.; Bian, Z. The enhanced photoreduction of Cr(VI) to Cr(III) using carbon dots coupled TiO 2 mesocrystals. Appl. Catal. B Environ. 2018, 226, 213–219. [Google Scholar] [CrossRef]
- Li, H.; Li, Y.; Chen, Y.; Yin, M.; Jia, T.; He, S.; Deng, Q.; Wang, S. Carbon Tube Clusters with Nanometer Walls Thickness, Micrometer Diameter from Biomass, and Its Adsorption Property as Bioadsorbent. ACS Sustain. Chem. Eng. 2018, 7, 858–866. [Google Scholar] [CrossRef]
- El-Nagar, G.A.; Hassan, M.A.; Fetyan, A.; Kayarkatte, M.K.; Lauermann, I.; Roth, C. A promising N-doped carbon-metal oxide hybrid electrocatalyst derived from crustacean’s shells: Oxygen reduction and oxygen evolution. Appl. Catal. B Environ. 2017, 214, 137–147. [Google Scholar] [CrossRef]
- Sun, J.T.; Zhang, Z.P.; Ji, J.; Dou, M.L.; Wang, F. Removal of Cr(VI) from wastewater via adsorption with high-specific-surface-area nitrogen-doped hierarchical porous carbon derived from silkworm cocoon. Appl. Surf. Sci. 2017, 405, 372–379. [Google Scholar] [CrossRef]
- Covelo, E.; Andrade, M.L.; Vega, F. Heavy metal adsorption by humic umbrisols: selectivity sequences and competitive sorption kinetics. J. Colloid Interface Sci. 2004, 280, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Mohan, D.; Rajput, S.; Singh, V.K.; Steele, P.H.; Pittman, C.U., Jr. Modeling and evaluation of chromium remediation from water using low cost bio-char, a green adsorbent. J. Hazardous Mat. 2011, 188, 319–333. [Google Scholar] [CrossRef]
- Chu, Y.; Gu, L.; Qu, K.G.; Zhang, Y.; Zhao, J.S.; Xie, Y. The synthesis of phenanthroline and bipyridine based ligand for the preparation of Fe-Nx/C type electrocatalyst for oxygen reduction. Int. J. Hydrogen. Energ. 2018, 43, 21810–21823. [Google Scholar] [CrossRef]
- Sen, T.K.; Afroze, S.; Ang, H.M. Equilibrium, Kinetics and Mechanism of Removal of Methylene Blue from Aqueous Solution by Adsorption onto Pine Cone Biomass of Pinus radiata. Water Air Soil Pollut. 2010, 218, 499–515. [Google Scholar] [CrossRef]
- Li, L.-L.; Feng, X.-Q.; Han, R.-P.; Zang, S.-Q.; Yang, G. Cr(VI) removal via anion exchange on a silver-triazolate MOF. J. Hazardous Mat. 2017, 321, 622–628. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Li, J.; Jin, Q.; Ren, Z.; Sun, Y.; Zhang, R.; Zhai, Y.; Liu, Y. Photocatalytic reduction of Cr (VI) on nano-sized red phosphorus under visible light irradiation. J. Colloid Interface Sci. 2019, 537, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.-N.; Lu, Y.-Z.; Shen, N.; Lau, T.-C.; Zeng, R.J. Investigation of Cr(VI) reduction potential and mechanism by Caldicellulosiruptor saccharolyticus under glucose fermentation condition. J. Hazardous Mat. 2018, 344, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Li, X.; Sun, B.; Ji, H.; Wang, C. Diethylenetriamine-assisted synthesis of amino-rich hydrothermal carbon-coated electrospun polyacrylonitrile fiber adsorbents for the removal of Cr(VI) and 2,4-dichlorophenoxyacetic acid. J Colloid Interface Sci. 2017, 487, 297–309. [Google Scholar] [CrossRef]
- Zhu, K.; Chen, C.; Xu, H.; Gao, Y.; Tan, X.; Alsaedi, A.; Hayat, T. Cr(VI) Reduction and Immobilization by Core-Double-Shell Structured Magnetic Polydopamine@Zeolitic Idazolate Frameworks-8 Microspheres. ACS Sustain. Chem. Eng. 2017, 5, 6795–6802. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, J.; Ma, C.; Li, Y. Cr 2 O 3 ultrasmall nanoparticles filled carbon nanocapsules deriving from Cr(VI) for enhanced lithium storage. Chem. Phys. Lett. 2018, 704, 31–36. [Google Scholar] [CrossRef]
- Gong, K.; Hu, Q.; Yao, L.; Li, M.; Sun, D.; Shao, Q.; Qiu, B.; Guo, Z. Ultrasonic Pretreated Sludge Derived Stable Magnetic Active Carbon for Cr(VI) Removal from Wastewater. ACS Sustain. Chem. Eng. 2018, 6, 7283–7291. [Google Scholar] [CrossRef]
- Song, B.; Wang, T.; Wang, L.; Liu, H.; Mai, X.; Wang, X.; Wang, N.; Huang, Y.; Ma, Y.; Lu, Y. Interfacially reinforced carbon fiber/epoxy composite laminates via in-situ synthesized graphitic carbon nitride (g-C3N4). Compos. Part B Eng. 2019, 158, 259–268. [Google Scholar] [CrossRef]
- Gong, K.; Hu, Q.; Xiao, Y.; Cheng, X.; Liu, H.; Wang, N.; Qiu, B.; Guo, Z. Triple layered core–shell ZVI@carbon@polyaniline composite enhanced electron utilization in Cr(vi) reduction. J. Mat. Chem. A 2018, 6, 11119–11128. [Google Scholar] [CrossRef]







| Sample | Dap (nm) | SBET (m2g−1) | Smic (m2g−1) | Vt (cm3g−1) | Vmic (cm3g−1) | Vmic/Vt (%) |
|---|---|---|---|---|---|---|
| N-SAC | 5.50 | 2327.8 | 1736.1 | 1.35 | 0.97 | 71.8 |
| SAC | 1.62 | 1235.2 | 1126.2 | 0.62 | 0.59 | 95.1 |
| C0 (mg·L−1) | qe,exp (mg·g−1) | Pseudo-First Order | Pseudo-Second Order | ||||
|---|---|---|---|---|---|---|---|
| qe,cal1 (mg·g−1) | k1 (min−1) | R2 | qe,cal1 (mg·g−1) | k2 (mg·min−1) | R2 | ||
| 10 | 6.03 | 3.068 | 0.0263 | 0.9184 | 6.271 | 0.0264 | 0.9984 |
| 15 | 7.84 | 3.092 | 0.0167 | 0.08775 | 7.831 | 0.0186 | 0.9993 |
| Langmuir | Freundlich |
|---|---|
| Qm = 7.74 (mg·g−1) | kF = 4.57 |
| ka = 0.23 (L·mg−1) | nF = 9.81 |
| R2 = 0.8773 | R2 = 0.9710 |
| C0, mg·L−1 | C, mg·g−1 | Step I | Step II | ||
|---|---|---|---|---|---|
| Kid1 | R2 | Kid2 | R2 | ||
| 10 | 0.42 | 2.69 | 0.94 | 0.62 | 0.92 |
| 15 | 0.59 | 3.23 | 0.99 | 0.73 | 0.94 |
| ΔG0/kJ·mol−1 | ΔH0 (kJ·mol−1) | ΔS0 (kJ·mol−1·K−1) | ||||
|---|---|---|---|---|---|---|
| 288K | 298K | 308K | 318K | 328K | ||
| −9.75 | −12.85 | −15.95 | −19.05 | −22.14 | 79.46 | 0.3098 |
| Adsorbents | HOMO Energy (eV) | Adsorption Energy (eV) |
|---|---|---|
| Gra | −5.57492 | −0.1865 |
| Gra-N | −3.39052 | −0.5782 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Zhao, W.; Zheng, W.; Chen, S.; Zhao, J. Preparation of N-Doped Carbon Nanosheets from Sewage Sludge for Adsorption Studies of Cr(VI) from Aqueous Solution. Nanomaterials 2019, 9, 265. https://doi.org/10.3390/nano9020265
Wang Y, Zhao W, Zheng W, Chen S, Zhao J. Preparation of N-Doped Carbon Nanosheets from Sewage Sludge for Adsorption Studies of Cr(VI) from Aqueous Solution. Nanomaterials. 2019; 9(2):265. https://doi.org/10.3390/nano9020265
Chicago/Turabian StyleWang, Yi, Weinan Zhao, Wanlan Zheng, Shuang Chen, and Jinsheng Zhao. 2019. "Preparation of N-Doped Carbon Nanosheets from Sewage Sludge for Adsorption Studies of Cr(VI) from Aqueous Solution" Nanomaterials 9, no. 2: 265. https://doi.org/10.3390/nano9020265
APA StyleWang, Y., Zhao, W., Zheng, W., Chen, S., & Zhao, J. (2019). Preparation of N-Doped Carbon Nanosheets from Sewage Sludge for Adsorption Studies of Cr(VI) from Aqueous Solution. Nanomaterials, 9(2), 265. https://doi.org/10.3390/nano9020265