The Novel Z-Scheme Ternary-Component Ag/AgI/α-MoO3 Catalyst with Excellent Visible-Light Photocatalytic Oxidative Desulfurization Performance for Model Fuel
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.1.1. Preparation of α-MoO3 Nanobelt
2.1.2. Synthesis of A Series of Ag/AgI/α-MoO3 Heterojunctions
2.2. Characterization
2.3. Photocatalytic Activity
3. Results
3.1. Formation and Characterization of Ag/AgI/α-MoO3 Heterojunctions
3.2. Photocatalytic Oxidative Desulfurization Activity
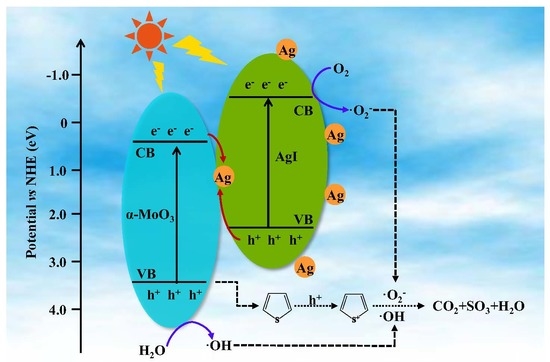
3.3. Photocatalytic Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Dai, C.N.; Zhang, J.; Huang, C.P.; Lei, Z.G. Ionic liquids in selective oxidation: Catalysts and solvents. Chem. Rev. 2017, 117, 6929–6983. [Google Scholar] [CrossRef] [PubMed]
- Stanislaus, A.; Marafi, A.; Rana, M.S. Recent advances in the science and technology of ultra low sulfur diesel (ULSD) production. Catal. Today 2010, 153, 1–68. [Google Scholar] [CrossRef]
- Samokhvalov, A. Desulfurization of real and model liquid fuels using light: Photocatalysis and photochemistry. Catal. Rev. 2012, 54, 281–343. [Google Scholar] [CrossRef]
- Wei, S.N.; He, H.J.; Cheng, Y.; Yang, C.P.; Zeng, G.M.; Qiu, L. Performances, kinetics and mechanisms of catalytic oxidative desulfurization from oils. RSC Adv. 2016, 6, 103253–103269. [Google Scholar] [CrossRef]
- Abdelaal, M.Y.; Mohamed, R.M. Environmental remediation from thiophene solution by photocatalytic oxidation using a Pd/ZrO2-chitosan nanocomposite. Ceram. Int. 2014, 40, 7693–7699. [Google Scholar] [CrossRef]
- Miao, G.; Huang, D.; Ren, X.; Li, X.; Li, Z.; Xiao, J. Visible-light induced photocatalytic oxidative desulfurization using BiVO4/C3N4@SiO2 with air/cumene hydroperoxide under ambient conditions. Appl. Catal. B Environ. 2016, 192, 72–79. [Google Scholar] [CrossRef]
- Lin, F.; Li, C.; Wang, D.G.; Jiang, Z.X.; Ma, Y.; Li, J.; Li, R.G. Photocatalytic oxidation of thiophene on BiVO4 with dual co-catalysts Pt and RuO2 under visible light irradiation using molecular oxygen as oxidant. Energy Environ. Sci. 2012, 5, 6400–6406. [Google Scholar] [CrossRef]
- Gao, X.M.; Fu, F.; Zhang, L.P.; Li, W.H. The preparation of Ag-BiVO4 metal compositeoxides and its application in efficient photocatalytic oxidative thiophene. Phys. B Condens. Matter 2013, 419, 80–85. [Google Scholar] [CrossRef]
- Zhang, B.; Li, J.; Zhang, B.Q.; Chong, R.F.; Li, R.G.; Yuan, B.S.; Lu, M.; Li, C. Selective oxidation of sulfides on Pt/BiVO4 photocatalyst under visible light irradiation using water as the oxygen source and dioxygen as the electron acceptor. J. Catal. 2015, 332, 95–100. [Google Scholar] [CrossRef]
- Lu, X.; Li, X.; Qian, J.; Miao, N.; Yao, C.; Chen, Z. Synthesis and characterization of CeO2/TiO2 nanotube arrays and enhanced photocatalytic oxidative desulfurization performance. J. Alloys Compd. 2016, 661, 363–371. [Google Scholar] [CrossRef]
- Zarrabi, M.; Entezari, M.H.; Goharshadi, E.K. Photocatalytic oxidative desulfurization of dibenzothiophene by C/TiO2@MCM-41 nanoparticles under visible light and mild conditions. RSC Adv. 2015, 5, 34652–34662. [Google Scholar] [CrossRef]
- Mahd Zaid, H.F.; Chong, F.K.; Abdul Mutalib, M.I. Photooxidative–extractive deep desulfurization of diesel using Cu-Fe/TiO2 and eutectic ionic liquid. Fuel 2015, 156, 54–62. [Google Scholar] [CrossRef]
- Miao, G.; Ye, F.Y.; Wu, L.M.; Ren, X.L.; Xiao, J.; Li, Z.; Wang, H.H. Selective adsorption of thiophenic compounds from fuel over TiO2/SiO2 under UV-irradiation. J. Hazard. Mater. 2015, 300, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhu, W.S.; Xu, Y.H.; Xu, H.; Zhang, M.; Chao, Y.H.; Yin, S.; Li, H.M.; Wang, J.G. Preparation of TiO2/g-C3N4 composites and their application in photocatalytic oxidative desulfurization. Ceram. Int. 2014, 40, 11627–11635. [Google Scholar] [CrossRef]
- Dedual, G.; MacDonald, M.J.; Alshareef, A.; Wu, Z.; Tsang, D.C.W.; Yip, A.C.K. Requirements for effective photocatalytic oxidative desulfurization of a thiophene-containing solution using TiO2. J. Environ. Chem. Eng. 2014, 2, 1947–1955. [Google Scholar] [CrossRef]
- Lin, F.; Zhang, Y.; Wang, L.; Zhang, Y.; Wang, D.; Yang, M.; Yang, J.; Zhang, B.; Jiang, Z.; Li, C. Highly efficient photocatalytic oxidation of sulfur-containing organic compounds and dyes on TiO2 with dual cocatalysts Pt and RuO2. Appl. Catal. B Environ. 2012, 127, 363–370. [Google Scholar] [CrossRef]
- Wang, X.J.; Li, F.T.; Liu, J.X.; Kou, C.G.; Zhao, Y.; Hao, Y.J.; Zhao, D.S. Preparation of TiO2 in ionic liquid via microwave radiation and in situ photocatalytic oxidative desulfurization of diesel oil. Energy Fuel. 2012, 26, 6777–6782. [Google Scholar] [CrossRef]
- Zeng, X.Y.; Xiao, X.Y.; Li, Y.; Chen, J.Y.; Wang, H.L. Deep desulfurization of liquid fuels with molecular oxygen through graphene photocatalytic oxidation. Appl. Catal. B Environ. 2017, 209, 98–109. [Google Scholar] [CrossRef]
- Li, S.; Mominou, N.; Wang, Z.; Liu, L.; Wang, L. Ultra-deep desulfurization of gasoline with CuW/TiO2-GO through photocatalytic oxidation. Energy Fuel. 2016, 30, 962–967. [Google Scholar] [CrossRef]
- Aazam, E.S. Visible light photocatalytic degradation of thiophene using Ag-TiO2/multi-walled carbon nanotubes nanocomposite. Ceram. Int. 2014, 40, 6705–6711. [Google Scholar] [CrossRef]
- Yang, C.; Ji, H.; Chen, C.; Ma, W.; Zhao, J. Desulfurization of thiophenes in oils into H2SO4 using molecular oxygen. Appl. Catal. B Environ. 2018, 235, 207–213. [Google Scholar] [CrossRef]
- Zeng, X.; Xiao, X.; Chen, J.; Wang, H. Electron-hole interactions in choline-phosphotungstic acid boosting molecular oxygen activation for fuel desulfurization. Appl. Catal. B Environ. 2018, 248, 573–586. [Google Scholar] [CrossRef]
- Cheng, L.; Shao, M.H.; Hu, H.B. Single-crystalline molybdenum trioxide nanoribbons: Photocatalytic, photoconductive, and electrochemical properties. Chem. Eur. J. 2009, 15, 2310–2316. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.P.; Lu, C.L.; Xu, L.; Ma, Y.; Hou, W.H.; Zhu, J.J. Single-crystalline orthorhombic molybdenum oxide nanobelts: Synthesis and photocatalytic properties. CrystEngComm 2010, 12, 3740–3747. [Google Scholar] [CrossRef]
- Shakir, I.; Shahid, M.; Kang, D.J. MoO3 and Cu0.33MoO3 nanorods for unprecedented UV/Visible light photocatalysis. Chem. Commun. 2010, 46, 4324–4326. [Google Scholar] [CrossRef] [PubMed]
- Feng, B.; Wu, Z.Y.; Liu, J.S.; Zhu, K.J.; Li, Z.Q.; Jin, X.; Hou, Y.D.; Xi, Q.X.; Cong, M.Q.; Liu, P.C.; et al. Combination of ultrafast dye-sensitized-assisted electron transfer process and novel Z-scheme system: AgBr nanoparticles interspersed MoO3 nanobelts for enhancing photocatalytic performance of RhB. Appl. Catal. B Environ. 2017, 206, 242–251. [Google Scholar] [CrossRef]
- De Castro, I.A.; Datta, R.S.; Ou, J.Z.; Castellanos-Gomez, A.; Sriram, S.; Daeneke, T.; Kalantar-Zadeh, K. Molybdenum oxides—from fundamentals to functionality. Adv. Mater. 2017, 29, 1701619–1701650. [Google Scholar] [CrossRef]
- Lin, F.; Cai, J.B.; Li, Y.H.; Yu, H.W.; Li, S.X. Constituting fully integrated colorimetric analysis system for Fe(III) on multifunctional nitrogen-doped MoO3/cellulose paper. Talanta 2018, 180, 352–357. [Google Scholar] [CrossRef]
- Liu, Z.L.; Jin, Y.J.; Teng, F.; Hua, X.; Chen, M.D. An efficient Ce-doped MoO3 catalyst and its photo-thermal catalytic synergetic degradation performance for dye pollutant. Catal. Commun. 2015, 66, 42–45. [Google Scholar] [CrossRef]
- Liu, T.X.; Li, B.X.; Hao, Y.G.; Yao, Z.Y. MoO3-nanowire membrane and Bi2Mo3O12/MoO3 nano-heterostructural photocatalyst for wastewater treatment. Chem. Eng. J. 2014, 244, 382–390. [Google Scholar] [CrossRef]
- Li, H.; Yu, K.; Tang, Z.; Fu, H.; Zhu, Z. High photocatalytic performance of a type-II α-MoO3@MoS2 heterojunction: From theory to experiment. Chem. Chem. Phys. 2016, 18, 14074–14085. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.N.; Yi, J.J.; Chen, H.X.; Mao, M.; Liu, L.; She, X.J.; Ji, H.Y.; Wu, X.Y.; Yuan, S.Q.; Xu, H.; et al. Construction of a few-layer g-C3N4/α-MoO3 nanoneedles all-solid-state Z-scheme photocatalytic system for photocatalytic degradation. J. Energy Chem. 2019, 29, 65–71. [Google Scholar] [CrossRef]
- Huang, L.Y.; Xu, H.; Zhang, R.X.; Cheng, X.N.; Xia, J.X.; Xu, Y.G.; Li, H.M. Synthesis and characterization of g-C3N4/MoO3 photocatalyst with improved visible-light photoactivity. Appl. Surf. Sci. 2013, 283, 25–32. [Google Scholar] [CrossRef]
- Liu, H.; Lv, T.; Zhu, C.K.; Zhu, Z.F. Direct band gap narrowing of TiO2/MoO3 heterostructure composites for enhanced solar-driven photocatalytic activity. Sol. Energy Mater. Sol. Cells 2016, 153, 1–8. [Google Scholar] [CrossRef]
- Qu, Y.Q.; Duan, X.F. Progress, challenge and perspective of heterogeneous photocatalysts. Chem. Soc. Rev. 2013, 42, 2568–2580. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.J.; Feng, Y.P.; Wang, F.L.; Chen, D.N.; Zhang, Q.X.; Zeng, Y.Q.; Lv, W.Y.; Liu, G.G. Construction of carbon dots modified MoO3/g-C3N4 Z-scheme photocatalyst with enhanced visible-light photocatalytic activity for the degradation of tetracycline. Appl. Catal. B Environ. 2018, 229, 96–104. [Google Scholar] [CrossRef]
- Wang, P.; Huang, B.; Qin, X.; Zhang, X.; Dai, Y.; Wei, J.; Whangbo, M.H. Ag@AgCl: A highly efficient and stable photocatalyst active under visible light. Angew. Chem. Int. Ed. Engl. 2008, 47, 7931–7933. [Google Scholar] [CrossRef]
- Ji, H.; Zhang, L.; Hu, C. Chemical-bond conjugated BiO(OH)x I1-x -AgI heterojunction with high visible light activity and stability in degradation of pollutants. Appl. Catal. B Environ. 2017, 218, 443–451. [Google Scholar] [CrossRef]
- Wan, Z.; Zhang, G.K. Synthesis and facet-dependent enhanced photocatalytic activity of Bi2SiO5/AgI nanoplate photocatalysts. J. Mater. Chem. A 2015, 3, 16737–16745. [Google Scholar] [CrossRef]
- Gao, X.M.; Shang, Y.Y.; Gao, K.L.; Fu, F. Plasmon sensitized heterojunction 2D ultrathin Ag/AgI-δ-Bi2O3 for enhanced photocatalytic nitrogen fixation. Nanomaterials 2019, 9, 781. [Google Scholar] [CrossRef]
- Sun, L.; Wu, W.; Tian, Q.; Lei, M.; Liu, J.; Xiao, X.; Zheng, X.; Ren, F.; Jiang, C. In situ oxidation and self-assembly synthesis of dumbbell-like α-Fe2O3/Ag/AgX (X = Cl, Br, I) heterostructures with enhanced photocatalytic properties. ACS Sustain. Chem. Engin. 2015, 4, 1521–1530. [Google Scholar] [CrossRef]
- Zeng, C.; Hu, Y.M.; Guo, Y.; Zhang, T.R.; Dong, F.; Zhang, Y.H.; Huang, H.W. Facile in situ self-sacrifice approach to ternary hierarchical architecture Ag/AgX (X = Cl, Br, I)/AgIO3 distinctively promoting visible-light photocatalysis with composition-dependent mechanism. ACS Sustain. Chem. Eng. 2016, 4, 3305–3315. [Google Scholar] [CrossRef]
- Chen, F.; Yang, Q.; Wang, Y.; Zhao, J.; Wang, D.; Li, X.; Guo, Z.; Wang, H.; Deng, Y.; Niu, C.; et al. Novel ternary heterojunction photcocatalyst of Ag nanoparticles and g-C3N4 nanosheets co-modified BiVO4 for wider spectrum visible-light photocatalytic degradation of refractory pollutant. Appl. Catal. B Environ. 2017, 205, 133–147. [Google Scholar] [CrossRef]
- Lin, F.; Jiang, Z.X.; Tang, N.F.; Zhang, C.; Chen, Z.P.; Liu, T.F.; Dong, B. Photocatalytic oxidation of thiophene on RuO2/SO42−-TiO2: Insights for cocatalyst and solid-acid. Appl. Catal. B Environ. 2016, 188, 253–258. [Google Scholar] [CrossRef]
- Zhen, Y.Z.; Li, J.; Wang, D.J.; Fu, F.; Xue, G.L. Synthesis of α-MoO3 nanobelts and its photocatalytic oxidative desulfurization (Photo-ODS) activity of simulation fuel. J. Inorg. Mater. 2015, 30, 408–412. [Google Scholar]
- Zhen, Y.Z.; Wang, J.; Li, J.; Fu, M.X.; Fu, F.; Zhang, Y.Z.; Feng, J.H. Enhanced photocatalytic degradation for thiophene by Ag/α-MoO3 heterojunction under visible-light irradiation. J. Mater. Sci. Mater. Electron. 2018, 29, 3672–3681. [Google Scholar] [CrossRef]
- Shi, H.; Chen, J.; Li, G.; Nie, X.; Zhao, H.; Wong, P.K.; An, T. Synthesis and characterization of novel plasmonic Ag/AgX-CNTs (X= Cl, Br, I) nanocomposite photocatalysts and synergetic degradation of organic pollutant under visible light. ACS Appl. Mater. Interfaces 2013, 5, 6959–6967. [Google Scholar] [CrossRef]
- Zhou, F.Q.; Fan, J.C.; Xu, Q.J.; Min, Y.L. BiVO4 nanowires decorated with CdS nanoparticles as Z-scheme photocatalyst with enhanced H2 generation. Appl. Catal. B Environ. 2017, 201, 77–83. [Google Scholar] [CrossRef]
- Wang, D.J.; Shen, H.D.; Guo, L.; Wang, C.; Fu, F.; Liang, Y.C. Ag/Bi2MoO6-x with enhanced visible-light-responsive photocatalytic activities via the synergistic effect of surface oxygen vacancies and surface plasmon. Appl. Surf. Sci. 2018, 436, 536–547. [Google Scholar] [CrossRef]
- Nishio, J.; Tokumura, M.; Znad, H.T.; Kawase, Y. Photocatalytic decolorization of azo-dye with zinc oxide powder in an external UV light irradiation slurry photoreactor. J. Hazard. Mater. 2006, 138, 106–115. [Google Scholar] [CrossRef]
- Chen, F.; Yang, Q.; Sun, J.; Yao, F.; Wang, S.; Wang, Y.; Wang, X.; Li, X.; Niu, C.; Wang, D.; et al. Enhanced photocatalytic degradation of tetracycline by AgI/BiVO4 heterojunction under visible-light irradiation: Mineralization efficiency and mechanism. ACS Appl. Mater. Interfaces 2016, 8, 32887–32900. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Wang, W.; Zhang, Z.; Fang, X. High-efficiency visible-light-driven Ag3PO4/AgI photocatalysts: Z-scheme photocatalytic mechanism for their enhanced photocatalytic activity. J. Phys. Chem. C 2013, 117, 19346–19352. [Google Scholar] [CrossRef]











© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhen, Y.; Wang, J.; Fu, F.; Fu, W.; Liang, Y. The Novel Z-Scheme Ternary-Component Ag/AgI/α-MoO3 Catalyst with Excellent Visible-Light Photocatalytic Oxidative Desulfurization Performance for Model Fuel. Nanomaterials 2019, 9, 1054. https://doi.org/10.3390/nano9071054
Zhen Y, Wang J, Fu F, Fu W, Liang Y. The Novel Z-Scheme Ternary-Component Ag/AgI/α-MoO3 Catalyst with Excellent Visible-Light Photocatalytic Oxidative Desulfurization Performance for Model Fuel. Nanomaterials. 2019; 9(7):1054. https://doi.org/10.3390/nano9071054
Chicago/Turabian StyleZhen, Yanzhong, Jie Wang, Feng Fu, Wenhao Fu, and Yucang Liang. 2019. "The Novel Z-Scheme Ternary-Component Ag/AgI/α-MoO3 Catalyst with Excellent Visible-Light Photocatalytic Oxidative Desulfurization Performance for Model Fuel" Nanomaterials 9, no. 7: 1054. https://doi.org/10.3390/nano9071054
APA StyleZhen, Y., Wang, J., Fu, F., Fu, W., & Liang, Y. (2019). The Novel Z-Scheme Ternary-Component Ag/AgI/α-MoO3 Catalyst with Excellent Visible-Light Photocatalytic Oxidative Desulfurization Performance for Model Fuel. Nanomaterials, 9(7), 1054. https://doi.org/10.3390/nano9071054