An Optimization Framework for Silicon Photonic Evanescent-Field Biosensors Using Sub-Wavelength Gratings
Abstract
:1. Introduction
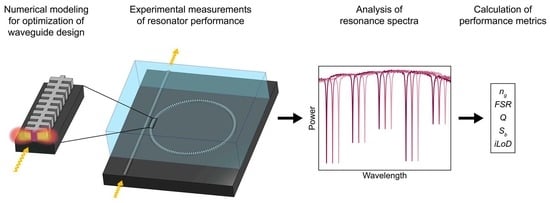
2. Materials and Methods
2.1. Numerical Models
2.1.1. Index and Bulk Sensitivity Simulations
2.1.2. Propagation Loss Simulations
2.2. Design and Optimization of Fishbone SWG Waveguides
2.3. Sensor Chip Design and Fabrication
2.4. Sensor Characterization
2.5. Microfluidic Design and Fabrication
2.6. Bulk Sensitivity Testing
2.7. SEM Imaging
3. Results
3.1. Simulation Overestimates In-Water Group Indices of SWG Waveguides
3.2. Empirical Characterization of Extinction Ratio vs. Coupling Gap Reveals Insights for Further Optimization and Highlights Performance Degradation Due to Peak Splitting
3.3. Fishbone SWG MRRs Achieve Comparable Performance to Previously Reported SWG-Based Sensors
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chrostowski, L.; Leanne, D.; Matthew, M.; Connor, M.; Luan, E.; Al-Qadasi, M.; Avineet, R.; Mojaver, H.R.; Lyall, E.; Gervais, A.; et al. A silicon photonic evanescent-field sensor architecture using a fixed-wavelength laser. In Optical Interconnects XXI; Schröder, H., Chen, R.T., Eds.; SPIE: Bellingham, WA, USA, 2021; Volume 11692. [Google Scholar]
- Ruiz-Vega, G.; Soler, M.; Lechuga, L.M. Nanophotonic biosensors for point-of-care COVID-19 diagnostics and coronavirus surveillance. J. Phys. Photonics 2021, 3, 011002. [Google Scholar] [CrossRef]
- Luan, E.; Yun, H.; Laplatine, L.; Dattner, Y.; Ratner, D.M.; Cheung, K.C.; Chrostowski, L. Enhanced Sensitivity of Subwavelength Multibox Waveguide Microring Resonator Label-Free Biosensors. IEEE J. Sel. Top. Quantum Electron. 2019, 25, 1–11. [Google Scholar] [CrossRef]
- Nishat, S.; Jafry, A.T.; Martinez, A.W.; Awan, F.R. Paper-based microfluidics: Simplified fabrication and assay methods. Sens. Actuators B Chem. 2021, 336, 129681. [Google Scholar] [CrossRef]
- Gonzalo Wangüemert-Pérez, J.; Cheben, P.; Ortega-Moñux, A.; Alonso-Ramos, C.; Pérez-Galacho, D.; Halir, R.; Molina-Fernández, I.; Xu, D.-X.; Schmid, J.H. Evanescent field waveguide sensing with subwavelength grating structures in silicon-on-insulator. Opt. Lett. 2014, 39, 4442–4445. [Google Scholar] [CrossRef] [PubMed]
- Luan, E.; Awan, K.M.; Cheung, K.C.; Chrostowski, L. High-performance sub-wavelength grating-based resonator sensors with substrate overetch. Opt. Lett. 2019, 44, 5981–5984. [Google Scholar] [CrossRef]
- Rahim, A.; Spuesens, T.; Baets, R.; Bogaerts, W. Open-Access Silicon Photonics: Current Status and Emerging Initiatives. Proc. IEEE 2018, 106, 2313–2330. [Google Scholar] [CrossRef] [Green Version]
- Shekhar, S. Silicon Photonics: A brief tutorial. IEEE Solid-State Circuits Mag. 2021, 13, 22–32. [Google Scholar] [CrossRef]
- Dincer, C.; Bruch, R.; Kling, A.; Dittrich, P.S.; Urban, G.A. Multiplexed Point-of-Care Testing—xPOCT. Trends Biotechnol. 2017, 35, 728–742. [Google Scholar] [CrossRef] [Green Version]
- Kaushik, A.; Mujawar, M.A. Point of care sensing devices: Better care for everyone. Sensors 2018, 18, 4303. [Google Scholar] [CrossRef] [Green Version]
- Soler, M.; Huertas, C.S.; Lechuga, L.M. Label-free plasmonic biosensors for point-of-care diagnostics: A review. Expert Rev. Mol. Diagn. 2019, 19, 71–81. [Google Scholar] [CrossRef]
- Bailey, R.C.; Washburn, A.L.; Qavi, A.J.; Iqbal, M.; Gleeson, M.; Tybor, F.; Gunn, L.C. A robust silicon photonic platform for multiparameter biological analysis. In Silicon Photonics IV; Kubby, J.A., Reed, G.T., Eds.; SPIE: Bellingham, WA, USA, 2009; Volume 7220, p. 72200N. [Google Scholar]
- Jarockyte, G.; Karabanovas, V.; Rotomskis, R.; Mobasheri, A. Multiplexed nanobiosensors: Current trends in early diagnostics. Sensors 2020, 20, 6890. [Google Scholar] [CrossRef] [PubMed]
- Valera, E.; Shia, W.W.; Bailey, R.C. Development and validation of an immunosensor for monocyte chemotactic protein 1 using a silicon photonic microring resonator biosensing platform. Clin. Biochem. 2016, 49, 121–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Washburn, A.L.; Shia, W.W.; Lenkeit, K.A.; Lee, S.-H.; Bailey, R.C. Multiplexed cancer biomarker detection using chip-integrated silicon photonic sensor arrays. Analyst 2016, 141, 5358–5365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luchansky, M.S.; Bailey, R.C. Silicon photonic microring resonators for quantitative cytokine detection and T-cell secretion analysis. Anal. Chem. 2010, 82, 1975–1981. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Washburn, A.L.; Gunn, L.C.; Bailey, R.C. Label-free quantitation of a cancer biomarker in complex media using silicon photonic microring resonators. Anal. Chem. 2009, 81, 9499–9506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McClellan, M.S.; Domier, L.L.; Bailey, R.C. Label-free virus detection using silicon photonic microring resonators. Biosens. Bioelectron. 2012, 31, 388–392. [Google Scholar] [CrossRef] [Green Version]
- Flueckiger, J.; Schmidt, S.; Donzella, V.; Sherwali, A.; Ratner, D.M.; Chrostowski, L.; Cheung, K.C. Sub-wavelength grating for enhanced ring resonator biosensor. Opt. Express 2016, 24, 15672–15686. [Google Scholar] [CrossRef]
- Bogaerts, W.; De Heyn, P.; Van Vaerenbergh, T.; De Vos, K.; Kumar Selvaraja, S.; Claes, T.; Dumon, P.; Bienstman, P.; Van Thourhout, D.; Baets, R. Silicon microring resonators. Laser Photonics Rev. 2012, 6, 47–73. [Google Scholar] [CrossRef]
- Schmidt, S.; Flueckiger, J.; Wu, W.; Grist, S.M.; Talebi Fard, S.; Donzella, V.; Khumwan, P.; Thompson, E.R.; Wang, Q.; Kulik, P.; et al. Improving the performance of silicon photonic rings, disks, and Bragg gratings for use in label-free biosensing. In Biosensing and Nanomedicine VII; Mohseni, H., Agahi, M.H., Razeghi, M., Eds.; SPIE: Bellingham, WA, USA, 2014; Volume 9166, p. 91660M. [Google Scholar]
- Luan, E.; Shoman, H.; Ratner, D.M.; Cheung, K.C.; Chrostowski, L. Silicon Photonic Biosensors Using Label-Free Detection. Sensors 2018, 18, 3519. [Google Scholar] [CrossRef] [Green Version]
- Steglich, P.; Hülsemann, M.; Dietzel, B.; Mai, A. Optical Biosensors Based on Silicon-On-Insulator Ring Resonators: A Review. Molecules 2019, 24, 519. [Google Scholar] [CrossRef]
- Iqbal, M.; Gleeson, M.A.; Spaugh, B.; Tybor, F.; Gunn, W.G.; Hochberg, M.; Baehr-Jones, T.; Bailey, R.C.; Gunn, L.C. Label-Free Biosensor Arrays Based on Silicon Ring Resonators and High-Speed Optical Scanning Instrumentation. IEEE J. Select. Top. Quantum Electron. 2010, 16, 654–661. [Google Scholar] [CrossRef]
- Steglich, P.; Lecci, G.; Mai, A. Surface plasmon resonance (SPR) spectroscopy and photonic integrated circuit (PIC) biosensors: A comparative review. Sensors 2022, 22, 2901. [Google Scholar] [CrossRef]
- Jayatilleka, H.; Boeck, R.; Murray, K.; Flueckiger, J.; Chrostowski, L.; Jaeger, N.A.; Shekhar, S. Automatic Wavelength Tuning of Series-Coupled Vernier Racetrack Resonators on SOI. In Optical Fiber Communication Conference; OSA: Washington, DC, USA, 2016. [Google Scholar]
- Chrostowski, L.; Grist, S.; Flueckiger, J.; Shi, W.; Wang, X.; Ouellet, E.; Yun, H.; Webb, M.; Nie, B.; Liang, Z.; et al. Silicon photonic resonator sensors and devices. In Laser Resonators, Microresonators, and Beam Control XIV; Kudryashov, A.V., Paxton, A.H., Ilchenko, V.S., Eds.; SPIE: Bellingham, WA, USA, 2012; Volume 8236, p. 823620. [Google Scholar]
- Chang, C.-W.; Xu, X.; Chakravarty, S.; Huang, H.-C.; Tu, L.-W.; Chen, Q.Y.; Dalir, H.; Krainak, M.A.; Chen, R.T. Pedestal subwavelength grating metamaterial waveguide ring resonator for ultra-sensitive label-free biosensing. Biosens. Bioelectron. 2019, 141, 111396. [Google Scholar] [CrossRef] [PubMed]
- Soni, V.; Chang, C.-W.; Xu, X.; Wang, C.; Yan, H.; D Agati, M.; Tu, L.-W.; Chen, Q.Y.; Tian, H.; Chen, R.T. Portable Automatic Microring Resonator System Using a Subwavelength Grating Metamaterial Waveguide for High-Sensitivity Real-Time Optical-Biosensing Applications. IEEE Trans. Biomed. Eng. 2021, 68, 1894–1902. [Google Scholar] [CrossRef] [PubMed]
- Sader, J.E.; Hughes, B.D.; Sanelli, J.A.; Bieske, E.J. Effect of multiplicative noise on least-squares parameter estimation with applications to the atomic force microscope. Rev. Sci. Instrum. 2012, 83, 055106. [Google Scholar] [CrossRef]
- White, I.M.; Fan, X. On the performance quantification of resonant refractive index sensors. Opt. Express 2008, 16, 1020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshie, T.; Tang, L.; Su, S.-Y. Optical microcavity: Sensing down to single molecules and atoms. Sensors 2011, 11, 1972–1991. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kazanskiy, N.L.; Butt, M.A.; Khonina, S.N. Silicon photonic devices realized on refractive index engineered subwavelength grating waveguides—A review. Opt. Laser Technol. 2021, 138, 106863. [Google Scholar] [CrossRef]
- Fard, S.T.; Donzella, V.; Schmidt, S.A.; Flueckiger, J.; Grist, S.M.; Talebi Fard, P.; Wu, Y.; Bojko, R.J.; Kwok, E.; Jaeger, N.A.F.; et al. Performance of ultra-thin SOI-based resonators for sensing applications. Opt. Express 2014, 22, 14166–14179. [Google Scholar] [CrossRef]
- Hoste, J.W.; Werquin, S.; Claes, T.; Bienstman, P. Conformational analysis of proteins with a dual polarisation silicon microring. Opt. Express 2014, 22, 2807–2820. [Google Scholar] [CrossRef]
- Barrios, C.A.; Bañuls, M.J.; González-Pedro, V.; Gylfason, K.B.; Sánchez, B.; Griol, A.; Maquieira, A.; Sohlström, H.; Holgado, M.; Casquel, R. Label-free optical biosensing with slot-waveguides. Opt. Lett. 2008, 33, 708–710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Claes, T.; Molera, J.G.; De Vos, K.; Schacht, E.; Baets, R.; Bienstman, P. Label-Free Biosensing With a Slot-Waveguide-Based Ring Resonator in Silicon on Insulator. IEEE Photonics J. 2009, 1, 197–204. [Google Scholar] [CrossRef]
- Halir, R.; Ortega-Monux, A.; Benedikovic, D.; Mashanovich, G.Z.; Wanguemert-Perez, J.G.; Schmid, J.H.; Molina-Fernandez, I.; Cheben, P. Subwavelength-Grating Metamaterial Structures for Silicon Photonic Devices. Proc. IEEE 2018, 106, 2144–2157. [Google Scholar] [CrossRef] [Green Version]
- Donzella, V.; Sherwali, A.; Flueckiger, J.; Grist, S.M.; Fard, S.T.; Chrostowski, L. Design and fabrication of SOI micro-ring resonators based on sub-wavelength grating waveguides. Opt. Express 2015, 23, 4791–4803. [Google Scholar] [CrossRef]
- Luan, E. Improving the Performance of Silicon Photonic Optical Resonator-Based Sensors for Biomedical Applications. Doctoral Dissertation, University of British Columbia: Vancouver, BC, Canada, 2020. [Google Scholar]
- Bock, P.J.; Cheben, P.; Schmid, J.H.; Lapointe, J.; Delâge, A.; Janz, S.; Aers, G.C.; Xu, D.-X.; Densmore, A.; Hall, T.J. Subwavelength grating periodic structures in silicon-on-insulator: A new type of microphotonic waveguide. Opt. Express 2010, 18, 20251–20262. [Google Scholar] [CrossRef] [Green Version]
- Luque-González, J.M.; Sánchez-Postigo, A.; Hadij-ElHouati, A.; Ortega-Moñux, A.; Wangüemert-Pérez, J.G.; Schmid, J.H.; Cheben, P.; Molina-Fernández, Í.; Halir, R. A review of silicon subwavelength gratings: Building break-through devices with anisotropic metamaterials. Nanophotonics 2021, 10, 2765–2797. [Google Scholar] [CrossRef]
- Brunetti, G.; Marocco, G.; Benedetto, A.D.; Giorgio, A.; Armenise, M.N.; Ciminelli, C. Design of a large bandwidth 2 × 2 interferometric switching cell based on a sub-wavelength grating. J. Opt. 2021, 23, 085801. [Google Scholar] [CrossRef]
- Cheben, P.; Xu, D.X.; Janz, S.; Densmore, A. Subwavelength waveguide grating for mode conversion and light coupling in integrated optics. Opt. Express 2006, 14, 4695–4702. [Google Scholar] [CrossRef]
- Xiao, J.; Guo, Z. Ultracompact Polarization-Insensitive Power Splitter Using Subwavelength Gratings. IEEE Photonics Technol. Lett. 2018, 30, 529–532. [Google Scholar] [CrossRef]
- Yun, H.; Chrostowski, L.; Jaeger, N.A.F. Ultra-broadband 2 × 2 adiabatic 3 dB coupler using subwavelength-grating-assisted silicon-on-insulator strip waveguides. Opt. Lett. 2018, 43, 1935–1938. [Google Scholar] [CrossRef]
- Xu, L.; Wang, Y.; Kumar, A.; El-Fiky, E.; Mao, D.; Tamazin, H.; Jacques, M.; Xing, Z.; Saber, M.G.; Plant, D.V. Compact high-performance adiabatic 3-dB coupler enabled by subwavelength grating slot in the silicon-on-insulator platform. Opt. Express 2018, 26, 29873–29885. [Google Scholar] [CrossRef] [PubMed]
- Ye, C.; Dai, D. Ultra-Compact Broadband 2 × 2 3 dB Power Splitter Using a Subwavelength-Grating-Assisted Asymmetric Directional Coupler. J. Light. Technol. 2020, 38, 2370–2375. [Google Scholar] [CrossRef]
- Halir, R.; Maese-Novo, A.; Ortega-Moñux, A.; Molina-Fernández, I.; Wangüemert-Pérez, J.G.; Cheben, P.; Xu, D.X.; Schmid, J.H.; Janz, S. Colorless directional coupler with dispersion engineered sub-wavelength structure. Opt. Express 2012, 20, 13470–13477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halir, R.; Bock, P.J.; Cheben, P.; Ortega-Moñux, A.; Alonso-Ramos, C.; Schmid, J.H.; Lapointe, J.; Xu, D.-X.; Wangüemert-Pérez, J.G.; Molina-Fernández, Í.; et al. Waveguide sub-wavelength structures: A review of principles and applications. Laser Photonics Rev. 2015, 9, 25–49. [Google Scholar] [CrossRef]
- Huang, L.; Yan, H.; Xu, X.; Chakravarty, S.; Tang, N.; Tian, H.; Chen, R.T. Improving the detection limit for on-chip photonic sensors based on subwavelength grating racetrack resonators. Opt. Express 2017, 25, 10527–10535. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Pan, Z.; Chung, C.-J.; Chang, C.-W.; Yan, H.; Chen, R.T. Subwavelength grating metamaterial racetrack resonator for sensing and modulation. IEEE J. Sel. Top. Quantum Electron. 2019, 25, 1–8. [Google Scholar] [CrossRef]
- Yan, H.; Huang, L.; Xu, X.; Chakravarty, S.; Tang, N.; Tian, H.; Chen, R.T. Unique surface sensing property and enhanced sensitivity in microring resonator biosensors based on subwavelength grating waveguides. Opt. Express 2016, 24, 29724–29733. [Google Scholar] [CrossRef] [Green Version]
- Kazanskiy, N.L.; Khonina, S.N.; Butt, M.A. Subwavelength grating double slot waveguide racetrack ring resonator for refractive index sensing application. Sensors 2020, 20, 3416. [Google Scholar] [CrossRef]
- Butt, M.A.; Khonina, S.N.; Kazanskiy, N.L. A highly sensitive design of subwavelength grating double-slot waveguide microring resonator. Laser Phys. Lett. 2020, 17, 076201. [Google Scholar] [CrossRef]
- Steglich, P.; Bondarenko, S.; Mai, C.; Paul, M.; Weller, M.G.; Mai, A. CMOS-Compatible Silicon Photonic Sensor for Refractive Index Sensing Using Local Back-Side Release. IEEE Photonics Technol. Lett. 2020, 32, 1241–1244. [Google Scholar] [CrossRef]
- Tas, N.; Sonnenberg, T.; Jansen, H.; Legtenberg, R.; Elwenspoek, M. Stiction in surface micromachining. J. Micromech. Microeng. 1996, 6, 385–397. [Google Scholar] [CrossRef] [Green Version]
- Bachman, J. Improving the Performance of Nano-Optomechanical Systems for Use as Mass Sensors. Doctoral Dissertation, Department of Physics, University of Alberta, Edmonton, AB, Canada, 2019. [Google Scholar]
- Kim, C.-J.; Kim, J.Y.; Sridharan, B. Comparative evaluation of drying techniques for surface micromachining. Sens. Actuators A: Phys. 1998, 64, 17–26. [Google Scholar] [CrossRef]
- Ibrahim, M.; Schmid, J.H.; Aleali, A.; Cheben, P.; Lapointe, J.; Janz, S.; Bock, P.J.; Densmore, A.; Lamontagne, B.; Ma, R.; et al. Athermal silicon waveguides with bridged subwavelength gratings for TE and TM polarizations. Opt. Express 2012, 20, 18356–18361. [Google Scholar] [CrossRef] [PubMed]
- Povinelli, M.; Johnson, S.; Joannopoulos, J. Slow-light, band-edge waveguides for tunable time delays. Opt. Express 2005, 13, 7145–7159. [Google Scholar] [CrossRef] [Green Version]
- Bickford, J.R.; Cho, P.; Farrell, M.; Holthoff, E. Fish-bone subwavelength grating waveguide photonic integrated circuit sensor array. In Chemical, Biological, Radiological, Nuclear, and Explosives (CBRNE) Sensing XIX; Fountain, A.W., Guicheteau, J.A., Howle, C.R., Eds.; SPIE: Bellingham, WA, USA, 2018; Volume 10629, p. 35. [Google Scholar]
- Choice of Wavelength for RF over Fiber—1310 nm vs. 1550 nm|ViaLite Communications. Available online: https://www.vialite.com/resources/guides/choice-of-wavelength-for-rf-over-fiber-1310nm-vs-1550nm/ (accessed on 1 September 2022).
- Washburn, A.L.; Luchansky, M.S.; Bowman, A.L.; Bailey, R.C. Quantitative, Label-Free Detection of Five Protein Biomarkers Using Multiplexed Arrays of Silicon Photonic Microring Resonators. Anal. Chem. 2010, 82, 69–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arnfinnsdottir, N.B.; Chapman, C.A.; Bailey, R.C.; Aksnes, A.; Stokke, B.T. Impact of silanization parameters and antibody immobilization strategy on binding capacity of photonic ring resonators. Sensors 2020, 20, 3163. [Google Scholar] [CrossRef]
- Christenson, C.; Baryeh, K.; Ahadian, S.; Nasiri, R.; Dokmeci, M.R.; Goudie, M.; Khademhosseini, A.; Ye, J.Y. Enhancement of label-free biosensing of cardiac troponin I. Proc. SPIE 2020, 2020, 11251. [Google Scholar] [CrossRef]
- Zhang, B.; Tamez-Vela, J.M.; Solis, S.; Bustamante, G.; Peterson, R.; Rahman, S.; Morales, A.; Tang, L.; Ye, J.Y. Detection of Myoglobin with an Open-Cavity-Based Label-Free Photonic Crystal Biosensor. J. Med. Eng. 2013, 2013, 808056. [Google Scholar] [CrossRef] [Green Version]
- Cognetti, J.S.; Miller, B.L. Monitoring Serum Spike Protein with Disposable Photonic Biosensors Following SARS-CoV-2 Vaccination. Sensors 2021, 21, 5857. [Google Scholar] [CrossRef]
- Ramachandran, A.; Wang, S.; Clarke, J.; Ja, S.J.; Goad, D.; Wald, L.; Flood, E.M.; Knobbe, E.; Hryniewicz, J.V.; Chu, S.T.; et al. A universal biosensing platform based on optical micro-ring resonators. Biosens. Bioelectron. 2008, 23, 939–944. [Google Scholar] [CrossRef]
- Shia, W.W.; Bailey, R.C. Single domain antibodies for the detection of ricin using silicon photonic microring resonator arrays. Anal. Chem. 2013, 85, 805–810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chalyan, T.; Pasquardini, L.; Gandolfi, D.; Guider, R.; Samusenko, A.; Zanetti, M.; Pucker, G.; Pederzolli, C.; Pavesi, L. Aptamer- and Fab’- Functionalized Microring Resonators for Aflatoxin M1 Detection. IEEE J. Sel. Top. Quantum Electron. 2017, 23, 1–8. [Google Scholar] [CrossRef]
- Bandstructure of Photonic Crystal Waveguide—Line Defect—Ansys Optics. Available online: https://optics.ansys.com/hc/en-us/articles/360042038473-Bandstructure-of-photonic-crystal-waveguide-Line-defect (accessed on 23 August 2022).
- Sukhoivanov, I.A.; Guryev, I.V. FDTD method for band structure computation. In Photonic Crystals: Physics and Practical Modeling; Springer Series in Optical Sciences; Springer: Berlin/Heidelberg, Germany, 2009; Volume 152, pp. 163–175. ISBN 978-3-642-02645-4. [Google Scholar]
- Chrostowski, L.; Hochberg, M. Silicon Photonics Design; Cambridge University Press: Cambridge, UK, 2015; ISBN 9781316084168. [Google Scholar]
- Adams, C.S.; Hughes, I.G. Optics f2f: From Fourier to Fresnel; Oxford University Press: Oxford, UK, 2018; ISBN 0198786786. [Google Scholar]
- Material Database in FDTD and MODE—Ansys Optics. Available online: https://optics.ansys.com/hc/en-us/articles/360034394614-Material-Database-in-FDTD-and-MODE (accessed on 1 September 2022).
- Palik, E.D. Handbook of Optical Constants of Solids, Volume 2, 1st ed.; Academic Press: Boston, MA, USA, 1991; p. 1096. ISBN 978-0-12-544422-4. [Google Scholar]
- Tips for Improving the Quality of Optical Material Fits—Ansys Optics. Available online: https://optics.ansys.com/hc/en-us/articles/360034915053-Tips-for-improving-the-quality-of-optical-material-fits (accessed on 1 September 2022).
- Mason, W.P.; McSkimin, H.J. Attenuation and scattering of high frequency sound waves in metals and glasses. J. Acoust. Soc. Am. 1947, 19, 464–473. [Google Scholar] [CrossRef]
- Sarmiento-Merenguel, J.D.; Ortega-Moñux, A.; Fédéli, J.-M.; Wangüemert-Pérez, J.G.; Alonso-Ramos, C.; Durán-Valdeiglesias, E.; Cheben, P.; Molina-Fernández, Í.; Halir, R. Controlling leakage losses in subwavelength grating silicon metamaterial waveguides. Opt. Lett. 2016, 41, 3443–3446. [Google Scholar] [CrossRef] [PubMed]
- Ortega, D.; Aldariz, J.M.; Arnold, J.M.; Aitchison, J.S. Analysis of “quasi-modes” in periodic segmented waveguides. J. Light. Technol. 1999, 17, 369–375. [Google Scholar] [CrossRef]
- NanoSOI Design Center|Applied Nanotools Inc. Available online: https://www.appliednt.com/nanosoi/sys/resources/specs/ (accessed on 27 August 2022).
- Chrostowski, L.; Shoman, H.; Hammood, M.; Yun, H.; Jhoja, J.; Luan, E.; Lin, S.; Mistry, A.; Witt, D.; Jaeger, N.A.F.; et al. Silicon photonic circuit design using rapid prototyping foundry process design kits. IEEE J. Sel. Top. Quantum Electron. 2019, 25, 1–26. [Google Scholar] [CrossRef]
- Chrostowski, L.; Lu, Z.; Flueckiger, J.; Wang, X.; Klein, J.; Liu, A.; Jhoja, J.; Pond, J. Design and simulation of silicon photonic schematics and layouts. In Silicon Photonics and Photonic Integrated Circuits V; Vivien, L., Pavesi, L., Pelli, S., Eds.; SPIE Proceedings; SPIE: Bellingham, WA, USA, 2016; Volume 9891, p. 989114. [Google Scholar]
- Chrostowski, L.; Flueckiger, J.; Lin, C.; Hochberg, M.; Pond, J.; Klein, J.; Ferguson, J.; Cone, C. Design methodologies for silicon photonic integrated circuits. In Smart Photonic and Optoelectronic Integrated Circuits XVI; Eldada, L.A., Lee, E.-H., He, S., Eds.; SPIE Proceedings; SPIE: Bellingham, WA, USA, 2014; Volume 8989, p. 89890G. [Google Scholar]
- Bryk, P.; Korczeniewski, E.; Szymański, G.S.; Kowalczyk, P.; Terpiłowski, K.; Terzyk, A.P. What is the value of water contact angle on silicon? Materials 2020, 13, 1554. [Google Scholar] [CrossRef] [Green Version]
- SiEPIC/pyOptomip: Python Code for Silicon Photonics Automated Probe Stations. Available online: https://github.com/SiEPIC/pyOptomip (accessed on 28 August 2022).
- Cameron, T.C.; Randhawa, A.; Grist, S.M.; Bennet, T.; Hua, J.; Alde, L.G.; Caffrey, T.M.; Wellington, C.L.; Cheung, K.C. PDMS Organ-On-Chip Design and Fabrication: Strategies for Improving Fluidic Integration and Chip Robustness of Rapidly Prototyped Microfluidic In Vitro Models. Micromachines 2022, 13, 1573. [Google Scholar] [CrossRef]
- Carter, S.-S.D.; Atif, A.-R.; Kadekar, S.; Lanekoff, I.; Engqvist, H.; Varghese, O.P.; Tenje, M.; Mestres, G. PDMS leaching and its implications for on-chip studies focusing on bone regeneration applications. Organs--A-Chip 2020, 2, 100004. [Google Scholar] [CrossRef]
- Yousuf, S.; Kim, J.; Orozaliev, A.; Dahlem, M.S.; Song, Y.A.; Viegas, J. Label-Free Detection of Morpholino-DNA Hybridization Using a Silicon Photonics Suspended Slab Micro-Ring Resonator. IEEE Photonics J. 2021, 13, 1–9. [Google Scholar] [CrossRef]
- van Meer, B.J.; de Vries, H.; Firth, K.S.A.; van Weerd, J.; Tertoolen, L.G.J.; Karperien, H.B.J.; Jonkheijm, P.; Denning, C.; IJzerman, A.P.; Mummery, C.L. Small molecule absorption by PDMS in the context of drug response bioassays. Biochem. Biophys. Res. Commun. 2017, 482, 323–328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toepke, M.W.; Beebe, D.J. PDMS absorption of small molecules and consequences in microfluidic applications. Lab Chip 2006, 6, 1484–1486. [Google Scholar] [CrossRef]
- Han, J.; Kamber, M.; Pei, J. Getting to know your data. In Data Mining; Elsevier: Waltham, MA, USA, 2012; pp. 39–82. ISBN 9780123814791. [Google Scholar]
- Donzella, V.; Sherwali, A.; Flueckiger, J.; Talebi Fard, S.; Grist, S.M.; Chrostowski, L. Sub-wavelength grating components for integrated optics applications on SOI chips. Opt. Express 2014, 22, 21037–21050. [Google Scholar] [CrossRef] [PubMed]
- Mulloni, V.; Faes, A.; Margesin, B. Wet release technology for bulk-silicon resonators fabrication on silicon-on-insulator substrate. J. Micro/Nanolith. MEMS MOEMS 2013, 12, 041206. [Google Scholar] [CrossRef]
- Bañuls, M.-J.; Puchades, R.; Maquieira, Á. Chemical surface modifications for the development of silicon-based label-free integrated optical (IO) biosensors: A review. Anal. Chim. Acta 2013, 777, 1–16. [Google Scholar] [CrossRef]
- Spuller, M.T.; Hess, D.W. Incomplete Wetting of Nanoscale Thin-Film Structures. J. Electrochem. Soc. 2003, 150, G476. [Google Scholar] [CrossRef]
- Pereiro, I.; Fomitcheva Khartchenko, A.; Petrini, L.; Kaigala, G.V. Nip the bubble in the bud: A guide to avoid gas nucleation in microfluidics. Lab Chip 2019, 19, 2296–2314. [Google Scholar] [CrossRef] [PubMed]
- Morita, M.; Ohmi, T.; Hasegawa, E.; Kawakami, M.; Ohwada, M. Growth of native oxide on a silicon surface. J. Appl. Phys. 1990, 68, 1272–1281. [Google Scholar] [CrossRef]
- Qian, K.; Tang, J.; Guo, H.; Liu, W.; Liu, J.; Xue, C.; Zheng, Y.; Zhang, C. Under-Coupling Whispering Gallery Mode Resonator Applied to Resonant Micro-Optic Gyroscope. Sensors 2017, 17, 100. [Google Scholar] [CrossRef] [Green Version]
- Shi, W.; Yun, H.; Zhang, W.; Lin, C.; Chang, T.K.; Wang, Y.; Jaeger, N.A.F.; Chrostowski, L. Ultra-compact, high-Q silicon microdisk reflectors. Opt. Express 2012, 20, 21840–21846. [Google Scholar] [CrossRef]
- Chang, T.H.P. Proximity effect in electron-beam lithography. J. Vac. Sci. Technol. 1975, 12, 1271–1275. [Google Scholar] [CrossRef]
- Little, B.E.; Laine, J.P.; Chu, S.T. Surface-roughness-induced contradirectional coupling in ring and disk resonators. Opt. Lett. 1997, 22, 4–6. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Bogaerts, W. Backcoupling manipulation in silicon ring resonators. Photonics Res. 2018, 6, 620. [Google Scholar] [CrossRef] [Green Version]
- de Goede, M.; Dijkstra, M.; Chang, L.; Acharyya, N.; Kozyreff, G.; Obregón, R.; Martínez, E.; García-Blanco, S.M. Mode-splitting in a microring resonator for self-referenced biosensing. Opt. Express 2021, 29, 346–358. [Google Scholar] [CrossRef]
- Hunsperger, R.G. Integrated Optics; Springer: New York, NY, USA, 2009; ISBN 978-0-387-89774-5. [Google Scholar]
- Totzeck, M.; Ulrich, W.; Göhnermeier, A.; Kaiser, W. Pushing deep ultraviolet lithography to its limits. Nat. Photonics 2007, 1, 629–631. [Google Scholar] [CrossRef]
- Ahn, S.; Spuhler, P.S.; Chiari, M.; Cabodi, M.; Ünlü, M.S. Quantification of surface etching by common buffers and implications on the accuracy of label-free biological assays. Biosens. Bioelectron. 2012, 36, 222–229. [Google Scholar] [CrossRef]













| Ring Resonator Design | Λ (nm) | δ | w (nm) | wfb (nm) | neff | gc (nm) |
|---|---|---|---|---|---|---|
| C1 ╫ | 250 | 0.5 | 500 | 180 | 1.71 | 450, 500, 550, 600 |
| C2 ╫ | 250 | 0.6 | 500 | 100 | 1.70 | 450, 500, 550, 600 |
| C3 ╫ | 290 | 0.65 | 500 | 100 | 1.77 | 400, 450, 500, 550 |
| C4 | 290 | 0.65 | 500 | 0 | 1.71 | 450, 500, 550, 600 |
| C5 | 250 | 0.65 | 500 | 0 | 1.70 | 450, 500, 550, 600 |
| C6 | 250 | 0.7 | 500 | 0 | 1.77 | 400, 450, 500, 550 |
| O1 ╫ | 250 | 0.4 | 500 | 100 | 1.69 | 400, 450, 500, 550 |
| O2 ╫ | 250 | 0.4 | 500 | 140 | 1.77 | 350, 400, 450, 500 |
| O3 ╫ | 200 | 0.5 | 500 | 100 | 1.82 | 350, 400, 450, 500 |
| O4 | 200 | 0.5 | 500 | 0 | 1.72 | 400, 450, 500, 550 |
| Ring Resonator Design | ng | FSR (nm) | ||
|---|---|---|---|---|
| Simulation | Experiment | Simulation | Experiment | |
| C1 ╫ | 3.33 | 2.83 ± 0.06 | 3.83 | 4.51 ± 0.09 |
| C2 ╫ | 3.16 | 2.60 ± 0.05 | 4.04 | 4.91 ± 0.10 |
| C3 ╫ | 3.44 | 2.94 ± 0.05 | 3.71 | 4.33 ± 0.07 |
| C4 | 3.21 | 2.64 ± 0.04 | 3.97 | 4.82 ± 0.08 |
| C5 | 3.12 | 2.52 ± 0.05 | 4.09 | 5.05 ± 0.10 |
| C6 | 3.35 | 2.77 ± 0.05 | 3.81 | 4.61 ± 0.08 |
| O1 ╫ | 3.08 | 2.57 ± 0.07 | 2.96 | 3.54 ± 0.09 |
| O2 ╫ | 3.45 | 3.00 ± 0.08 | 2.64 | 3.04 ± 0.08 |
| O3 ╫ | 3.34 | 2.89 ± 0.07 | 2.73 | 3.15 ± 0.08 |
| O4 | 3.01 | 2.39 ± 0.07 | 3.02 | 3.80 ± 0.10 |
| Ring Resonator Design | Optimal gc (nm) | Extinction Ratio (dB) |
|---|---|---|
| C1 ╫ | 600 * | 26 ± 12 |
| C2 ╫ | 600 * | 27 ± 11 |
| C3 ╫ | 500 | 19 ± 5 |
| C4 | 600 * | 21 ± 4 |
| C5 | 600 * | 20 ± 7 |
| C6 | 550 * | 21 ± 4 |
| O1 ╫ | 500 | 15 ± 5 |
| O2 ╫ | 400 | 15 ± 4 |
| O3 ╫ | 400 | 16 ± 9 |
| O4 | 550 * | 19 ± 9 |
| Ring Resonator Design | Simulated Propagation Losses (dB/cm) | Q | |
|---|---|---|---|
| Simulation * | Experiment | ||
| C1 ╫ | 40.2 | 7.29 × 103 | (5.5 ± 1.0) × 103 |
| C2 ╫ | 40.7 | 6.82 × 103 | (4.7 ± 0.8) × 103 |
| C3 ╫ | 40.5 | 7.46 × 103 | (4.7 ± 0.5) × 103 |
| C4 | 40.4 | 7.00 × 103 | (4.9 ± 0.4) × 103 |
| C5 | 40.1 | 6.85 × 103 | (4.6 ± 0.3) × 103 |
| C6 | 39.9 | 7.37 × 103 | (5.2 ± 0.5) × 103 |
| O1 ╫ | 7.3 | 4.40 × 104 | (6.7 ± 1.5) × 103 |
| O2 ╫ | 7.5 | 4.79 × 104 | (7.6 ± 1.7) × 103 |
| O3 ╫ | 7.1 | 4.93 × 104 | (6.9 ± 1.6) × 103 |
| O4 | 6.1 | 5.11 × 104 | (7.8 ± 2.0) × 103 |
| Ring Resonator Design | Sb (nm/RIU) | iLoD (RIU) | |||
|---|---|---|---|---|---|
| Simulation | Experiment, Ch. 1 | Experiment, Ch. 2 | Simulation | Experiment | |
| C1 ╫ | 381 | 414 ± 8 | 389 ± 10 | 5.58 × 10−4 | (7.1 ± 0.9) × 10−4 |
| C2 ╫ | 395 | 438 ± 8 | 416 ± 11 | 5.75 × 10−4 | (7.7 ± 0.9) × 10−4 |
| C3 ╫ | 357 | 349 ± 6 | 323 ± 6 | 5.82 × 10−4 | (9.8 ± 0.7) × 10−4 |
| C4 | 382 | 354 ± 5 | 343 ± 7 | 5.80 × 10−4 | (9.1 ± 0.6) × 10−4 |
| C5 | 392 | 427 ± 8 | 392 ± 10 | 5.77 × 10−4 | (8.3 ± 0.4) × 10−4 |
| C6 | 369 | 413 ± 8 | 389 ± 10 | 5.69 × 10−4 | (7.5 ± 0.6) × 10−4 |
| O1 ╫ | 354 | 349 ± 11 | 349 ± 8 | 8.42 × 10−5 | (5.6 ± 0.9) × 10−4 |
| O2 ╫ | 325 | 330 ± 15 | 344 ± 7 | 8.41 × 10−5 | (5.1 ± 0.8) × 10−4 |
| O3 ╫ | 357 | 331 ± 6 | 333 ± 8 | 8.31 × 10−5 | (5.8 ± 0.9) × 10−4 |
| O4 | 382 | 364 ± 7 | 383 ± 7 | 7.69 × 10−5 | (4.5 ± 0.8) × 10−4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puumala, L.S.; Grist, S.M.; Wickremasinghe, K.; Al-Qadasi, M.A.; Chowdhury, S.J.; Liu, Y.; Mitchell, M.; Chrostowski, L.; Shekhar, S.; Cheung, K.C. An Optimization Framework for Silicon Photonic Evanescent-Field Biosensors Using Sub-Wavelength Gratings. Biosensors 2022, 12, 840. https://doi.org/10.3390/bios12100840
Puumala LS, Grist SM, Wickremasinghe K, Al-Qadasi MA, Chowdhury SJ, Liu Y, Mitchell M, Chrostowski L, Shekhar S, Cheung KC. An Optimization Framework for Silicon Photonic Evanescent-Field Biosensors Using Sub-Wavelength Gratings. Biosensors. 2022; 12(10):840. https://doi.org/10.3390/bios12100840
Chicago/Turabian StylePuumala, Lauren S., Samantha M. Grist, Kithmin Wickremasinghe, Mohammed A. Al-Qadasi, Sheri Jahan Chowdhury, Yifei Liu, Matthew Mitchell, Lukas Chrostowski, Sudip Shekhar, and Karen C. Cheung. 2022. "An Optimization Framework for Silicon Photonic Evanescent-Field Biosensors Using Sub-Wavelength Gratings" Biosensors 12, no. 10: 840. https://doi.org/10.3390/bios12100840
APA StylePuumala, L. S., Grist, S. M., Wickremasinghe, K., Al-Qadasi, M. A., Chowdhury, S. J., Liu, Y., Mitchell, M., Chrostowski, L., Shekhar, S., & Cheung, K. C. (2022). An Optimization Framework for Silicon Photonic Evanescent-Field Biosensors Using Sub-Wavelength Gratings. Biosensors, 12(10), 840. https://doi.org/10.3390/bios12100840