Viscosity-Sensitive Solvatochromic Fluorescent Probes for Lipid Droplets Staining
Abstract
:1. Introduction
2. Synthesis of Probes
2.1. Reagents and Materials
2.2. Synthesis of Couoxo-LD
3. Result and Discussion
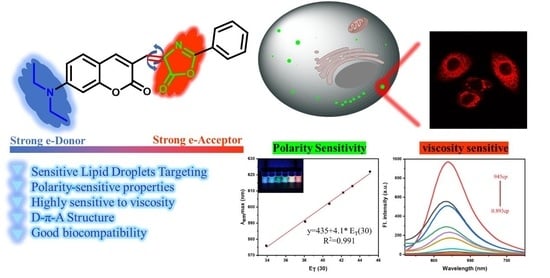
3.1. Probe Design and Discussion
3.2. Study of Photophysical Properties of Probes
3.3. The Emission Behavior of the Probe in PBS and Dioxane
3.4. Probe Emission Behavior in Viscous Environments
3.5. Probe Stability Study
3.6. Probe Couoxo-LD for Bioimaging Applications
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Storey, S.M.; McIntosh, A.L.; Senthivinayagam, S.; Moon, K.C.; Atshaves, B.P. The phospholipid monolayer associated with perilipin-enriched lipid droplets is a highly organized rigid membrane structure. J. Appl. Physiol. Endocrinol. Metab. 2011, 301, E991–E1003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krahmer, N.; Farese, R.V.; Walther, T.C. Balancing the fat: Lipid droplets and human disease. EMBO Mol. Med. 2013, 5, 973–983. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Li, P.; Tang, B. Recent progresses in fluorescent probes for detection of polarity. Coordin. Chem. Rev. 2021, 427, 213582. [Google Scholar] [CrossRef]
- Olzmann, J.A.; Richter, C.M.; Kopito, R.R. Spatial regulation of UBXD8 and p97/VCP controls ATGL-mediated lipid droplet turnover. Proc. Natl. Acad. Sci. USA 2013, 110, 1345–1350. [Google Scholar] [CrossRef] [Green Version]
- Onal, G.; Kutlu, O.; Gozuacik, D.; Dokmeci Emre, S. Lipid Droplets in Health and Disease. Lipids Health Dis. 2017, 16, 128. [Google Scholar] [CrossRef] [Green Version]
- Zheng, X.; Zhu, W.; Ni, F.; Ai, H.; Yang, C. A specific bioprobe for super-resolution fluorescence imaging of lipid droplets. Sens. Actuat. B-Chem. 2018, 255, 3148–3154. [Google Scholar] [CrossRef]
- Beloribi-Djefaflia, S.; Vasseur, S.; Guillaumond, F. Lipid metabolic reprogramming in cancer cells. Oncogenesis 2016, 5, e189. [Google Scholar] [CrossRef] [Green Version]
- Qiu, B.; Ackerman, D.; Sanchez, D.J.; Li, B.; Ochocki, J.D.; Grazioli, A.; Bobrovnikova-Marjon, E.; Diehl, J.A.; Keith, B.; Simon, M.C. HIF2α-Dependent Lipid Storage Promotes Endoplasmic Reticulum Homeostasis in Clear-Cell Renal Cell Carcinoma. Cancer Discov. 2015, 5, 652–667. [Google Scholar] [CrossRef] [Green Version]
- Farese, R.V., Jr.; Walther, T.C. Lipid droplets finally get a little R-E-S-P-E-C-T. Cell 2009, 139, 855–860. [Google Scholar] [CrossRef] [Green Version]
- Martin, S.; Parton, R.G. Lipid droplets: A unified view of a dynamic organelle. Nat. Rev. Mol. Cell Biol. 2006, 7, 373–378. [Google Scholar] [CrossRef]
- Chowdhury, R.; Jana, B.; Saha, A.; Ghosh, S.; Bhattacharyya, K. Confocal microscopy of cytoplasmic lipid droplets in a live cancer cell: Number, polarity, diffusion and solvation dynamics. Med. Chem. Comm. 2014, 5, 536–539. [Google Scholar] [CrossRef]
- Chen, S.; Hong, Y.; Zeng, Y.; Sun, Q.; Liu, Y.; Zhao, E.; Bai, G.; Qu, J.; Hao, J.; Tang, B.Z. Mapping live cell viscosity with an aggregation-induced emission fluorogen by means of two-photon fluorescence lifetime imaging. Chem. Eur. J. 2015, 21, 4315–4320. [Google Scholar] [CrossRef]
- Biegas, K.J.; Swarts, B.M. Chemical probes for tagging mycobacterial lipids. Curr. Opin. Chem. Biol. 2021, 65, 57–65. [Google Scholar] [CrossRef]
- Zhan, Z.; Zhuang, W.; Lei, Q.; Li, S.; Mao, W.; Chen, M.; Li, W. A smart probe for simultaneous imaging of the lipid/water microenvironment in atherosclerosis and fatty liver. Chem. Commun. 2022, 58, 4020–4023. [Google Scholar] [CrossRef]
- Yu, C.; Guo, X.; Fang, X.; Chen, N.; Wu, Q.; Hao, E.; Jiao, L. Efficiently emissive, strongly solvatochromic and lipid droplet-specific, fluorescent probes for mapping polarity in vitro. Dyes Pigments 2022, 197, 109838. [Google Scholar] [CrossRef]
- Tan, P.; Zhuang, W.; Li, S.; Zhang, J.; Xu, H.; Yang, L.; Liao, Y.; Chen, M.; Wei, Q. A lipid droplet targeted fluorescent probe for high-efficiency image-guided photodynamic therapy of renal cell carcinoma. Chem. Commun. 2021, 57, 1046–1049. [Google Scholar] [CrossRef]
- Tian, H.; Sedgwick, A.C.; Han, H.H.; Sen, S.; Chen, G.R.; Zang, Y.; Sessler, J.L.; James, T.D.; Li, J.; He, X.P. Fluorescent probes for the imaging of lipid droplets in live cells. Coord. Chem. Rev. 2021, 427, 213577. [Google Scholar] [CrossRef]
- Hu, R.; Chen, B.; Wang, Z.; Qin, A.; Zhao, Z.; Lou, X.; Tang, B.Z. Intriguing “chameleon” fluorescent bioprobes for the visualization of lipid droplet-lysosome interplay. Biomaterials 2019, 203, 43–51. [Google Scholar] [CrossRef]
- Guo, L.; Tian, M.; Zhang, Z.; Lu, Q.; Liu, Z.; Niu, G.; Yu, X. Simultaneous Two-Color Visualization of Lipid Droplets and Endoplasmic Reticulum and Their Interplay by Single Fluorescent Probes in Lambda Mode. J. Am. Chem. Soc. 2021, 143, 3169–3179. [Google Scholar] [CrossRef]
- Ye, M.; Hu, W.; He, M.; Li, C.; Zhai, S.; Liu, Z.; Wang, Y.; Zhang, H.; Li, C. Deep imaging for visualizing nitric oxide in lipid droplets: Discovering the relationship between nitric oxide and resistance to cancer chemotherapy drugs. Chem. Commun. 2020, 56, 6233–6236. [Google Scholar] [CrossRef]
- Cho, M.K.; Seo, M.J.; Juvekar, V.; Jo, J.H.; Kim, W.; Choi, K.S.; Kim, H.M. Screening of Drug-Induced Steatosis and Phospholipidosis Using Lipid Droplet-Selective Two-Photon Probes. Anal. Chem. 2020, 92, 11223–11231. [Google Scholar] [CrossRef]
- Jiang, G.; Jin, Y.; Li, M.; Wang, H.; Xiong, M.; Zeng, W.; Yuan, H.; Liu, C.; Ren, Z.; Liu, C. Faster and More Specific: Excited-State Intramolecular Proton Transfer-Based Dyes for High-Fidelity Dynamic Imaging of Lipid Droplets within Cells and Tissues. Anal. Chem. 2020, 92, 10342–10349. [Google Scholar] [CrossRef]
- Zhang, X.; Yuan, L.; Jiang, J.; Hu, J.; du Rietz, A.; Cao, H.; Zhang, R.; Tian, X.; Zhang, F.; Ma, Y.; et al. Light-Up Lipid Droplets Dynamic Behaviors Using a Red-Emitting Fluorogenic Probe. Anal. Chem. 2020, 92, 3613–3619. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.; Ma, S.; Ma, Y.; Zhao, Y.; Xing, M.; Zhou, L.; Cao, D.; Lin, W. Aurone Derivative Revealing the Metabolism of Lipid Droplets and Monitoring Oxidative Stress in Living Cells. Anal. Chem. 2020, 92, 6631–6636. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Qi, F.; Jiang, Z.; Yan, M.; Xu, L. Highly fluorescent bisboron complexes in both solution and solid-state: Synthesis, photophysical properties and lipid droplet imaging in living cells. Dyes Pigments 2021, 186, 108999. [Google Scholar] [CrossRef]
- Ren, W.; Wang, D.; Huang, W.; Li, J.; Tian, X.; Liu, Z.; Han, G.; Liu, B.; Han, M.Y.; Zhang, Z.; et al. Real-time tracking of lipid droplets interactions with other organelles by a high signal/noise probe. Dyes Pigments 2021, 191, 109366. [Google Scholar] [CrossRef]
- Dong, B.; Song, W.; Lu, Y.; Sun, Y.; Lin, W. Revealing the Viscosity Changes in Lipid Droplets during Ferroptosis by the Real-Time and In Situ Near-Infrared Imaging. ACS Sens. 2021, 6, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Collot, M.; Fam, T.K.; Pichandi, A.; Faklaris, O.; Galli, T.; Danglot, L.; Klymchenko, A. Ultrabright and Fluorogenic Probes for Multicolor Imaging and Tracking of Lipid Droplets in Cells and Tissues. J. Am. Chem. Soc. 2018, 140, 5401–5411. [Google Scholar] [CrossRef] [Green Version]
- Luo, J.; Xie, Z.; Lam, J.W.; Cheng, L.; Chen, H.; Qiu, C.; Kwok, H.S.; Zhan, X.; Liu, Y.; Zhu, D.; et al. Aggregation–induced emission of 1-methyl-1,2,3,4,5-pentaphenylsilole. Chem. Commun. 2001, 18, 1740–1741. [Google Scholar] [CrossRef]
- Perkin, W.H., VI. On the artificial production of coumarin and formation of its homologues. J. Chem. Soc. 1868, 21, 53–63. [Google Scholar] [CrossRef]
- Jiang, J.; Tian, X.; Xu, C.; Wang, S.; Feng, Y.; Chen, M.; Yu, H.; Zhu, M.; Meng, X. A two-photon fluorescent probe for real-time monitoring of autophagy by ultrasensitive detection of the change in lysosomal polarity. Chem. Commun. 2017, 53, 3645–3648. [Google Scholar] [CrossRef]
- Bai, Y.; Shi, X.; Chen, Y.; Zhu, C.; Jiao, Y.; Han, Z.; He, W.; Guo, Z. Coumarin/BODIPY Hybridisation for Ratiometric Sensing of Intracellular Polarity Oscillation. Chem. Eur. J. 2018, 24, 7513–7524. [Google Scholar] [CrossRef]
- Jimenez-Sanchez, A.; Lei, E.K.; Kelley, S.O. A Multifunctional Chemical Probe for the Measurement of Local Micropolarity and Microviscosity in Mitochondria. Angew. Chem. Int. Ed. 2018, 57, 8891–8895. [Google Scholar] [CrossRef]
- Kahveci, B.; Menteşe, E. Microwave Assisted Synthesis of Coumarins: A Review From 2007 to 2018. Curr. Micro. Chem. 2019, 5, 162–178. [Google Scholar] [CrossRef]
- Long, L.; Huang, M.; Wang, N.; Wu, Y.; Wang, K.; Gong, A.; Zhang, Z.; Sessler, J.L. A Mitochondria-Specific Fluorescent Probe for Visualizing Endogenous Hydrogen Cyanide Fluctuations in Neurons. J. Am. Chem. Soc. 2018, 140, 1870–1875. [Google Scholar] [CrossRef]
- Chen, X.; Wang, H.; Ma, X.; Wang, M.; Zhang, Y.; Gao, G.; Liu, J.; Hou, S. Colorimetric and fluorescent probe for real-time detection of palladium (II) ion in aqueous medium and live cell imaging. Dyes Pigments 2018, 148, 286–291. [Google Scholar] [CrossRef]
- Ding, Y.; Zhao, S.; Wang, Q.; Yu, X.; Zhang, W. Construction of a coumarin based fluorescent sensing platform for palladium and hydrazine detection. Sens. Actuat. B-Chem. 2018, 256, 1107–1113. [Google Scholar] [CrossRef]
- Chen, W.; Pacheco, A.; Takano, Y.; Day, J.J.; Hanaoka, K.; Xian, M. A Single Fluorescent Probe to Visualize Hydrogen Sulfide and Hydrogen Polysulfides with Different Fluorescence Signals. Angew. Chem. Int. Ed. 2016, 55, 9993–9996. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Su, Y.; Geng, Y.; Zhang, Y.; Ren, X.; He, L.; Song, X. A Triple-Emission Fluorescent Probe for Discriminatory Detection of Cysteine/Homocysteine, Glutathione/Hydrogen Sulfide, and Thiophenol in Living Cells. ACS Sens. 2018, 3, 1863–1869. [Google Scholar] [CrossRef]
- Kwon, N.; Hu, Y.; Yoon, J. Fluorescent Chemosensors for Various Analytes Including Reactive Oxygen Species, Biothiol, Metal Ions, and Toxic Gases. ACS Omega 2018, 3, 13731–13751. [Google Scholar] [CrossRef]
- Wang, K.N.; Liu, L.Y.; Mao, D.; Xu, S.; Tan, C.P.; Cao, Q.; Mao, Z.W.; Liu, B. A Polarity-Sensitive Ratiometric Fluorescence Probe for Monitoring Changes in Lipid Droplets and Nucleus during Ferroptosis. Angew. Chem. Int. Ed. 2021, 60, 15095–15100. [Google Scholar] [CrossRef]
- Cui, W.L.; Wang, M.H.; Chen, X.Q.; Zhang, Z.H.; Qu, J.; Wang, J.Y. A novel polarity-sensitive fluorescent probe for lighting up lipid droplets and its application in discriminating dead and living zebrafish. Dyes Pigments 2022, 204, 110433. [Google Scholar] [CrossRef]
- Punna Rao, A.M.L.; Sridhar Rao, A.; Saratchandra Babu, M.; Krishnaji Rao, M. Triphenylphosphine (PPh3) Catalyzed Erlenmeyer Reaction for Azlactones under Solvent-free Conditions. J. Heterocyclic. Chem. 2017, 54, 429–435. [Google Scholar] [CrossRef]
- Sasaki, S.; Drummen, G.P.C.; Konishi, G.i. Recent advances in twisted intramolecular charge transfer (TICT) fluorescence and related phenomena in materials chemistry. J. Mater. Chem. C 2016, 4, 2731–2743. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.J.; Li, X.Y.; Zhu, T.; Zhao, M.; Song, Z.; Li, S.; Shan, G.G.; Niu, G. Exploiting the Twisted Intramolecular Charge Transfer Effect to Construct a Wash-Free Solvatochromic Fluorescent Lipid Droplet Probe for Fatty Liver Disease Diagnosis. Anal. Chem. 2022, 94, 3881–3887. [Google Scholar] [CrossRef]
- Marwani, H.M.; Asiri, A.M.; Khana, S.A. Spectral, stoichiometric ratio, physicochemical, polarity and photostability studies of newly synthesized chalcone dye in organized media. J. Lumin. 2013, 136, 296–302. [Google Scholar] [CrossRef]










| Toluene | Dioxane | THF | EtOAc | DCM | Acetone | DMF | DMSO | MeCN | |
|---|---|---|---|---|---|---|---|---|---|
| λabs (nm) | 533 | 514 | 513 | 524 | 531 | 528 | 543 | 548 | 531 |
| λem (nm) | 576 | 581 | 592 | 591 | 602 | 609 | 613 | 622 | 607 |
| ET(30) 1 | 33.9 | 36 | 37.4 | 38.1 | 40.7 | 42.2 | 43.2 | 45.1 | 45.6 |
| Stokes shift (nm/cm–1) | 43/1401 | 67/2244 | 61/1940 | 67/2163 | 71/2221 | 81/2519 | 70/2103 | 74/2071 | 76/2358 |
| ΦF 2 | 5.2% | 6.1% | 3.4% | 3.4% | 1.3% | 19.0% | 11.0% | 11.3% | 4.3% |
| (Logεmax) 3 | 4.74 | 4.33 | 4.78 | 4.76 | 4.74 | 4.24 | 4.75 | 4.78 | 4.76 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, M.-H.; Cui, W.-L.; Yang, Y.-H.; Wang, J.-Y. Viscosity-Sensitive Solvatochromic Fluorescent Probes for Lipid Droplets Staining. Biosensors 2022, 12, 851. https://doi.org/10.3390/bios12100851
Wang M-H, Cui W-L, Yang Y-H, Wang J-Y. Viscosity-Sensitive Solvatochromic Fluorescent Probes for Lipid Droplets Staining. Biosensors. 2022; 12(10):851. https://doi.org/10.3390/bios12100851
Chicago/Turabian StyleWang, Mao-Hua, Wei-Long Cui, Yun-Hao Yang, and Jian-Yong Wang. 2022. "Viscosity-Sensitive Solvatochromic Fluorescent Probes for Lipid Droplets Staining" Biosensors 12, no. 10: 851. https://doi.org/10.3390/bios12100851
APA StyleWang, M. -H., Cui, W. -L., Yang, Y. -H., & Wang, J. -Y. (2022). Viscosity-Sensitive Solvatochromic Fluorescent Probes for Lipid Droplets Staining. Biosensors, 12(10), 851. https://doi.org/10.3390/bios12100851