Design and Preparation of Sensing Surfaces for Capacitive Biodetection
Abstract
:1. Introduction
2. Methodologies for Capacitive Biosensor Detection
2.1. Potentiostatic Capacitance
2.2. Interdigitated Electrodes
3. Current State of Capacitive Biosensors Used in Clinical Applications
3.1. Infections
3.2. Cancer, Chronic and Inflammatory Diseases
3.3. Unicellular Organisms
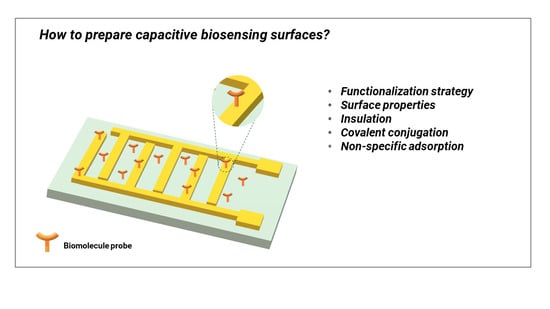
4. Capacitive Sensing Surfaces Preparation, and Limitations
4.1. Capacitive Sensors Limitations
4.2. Biomolecules Immobilization Techniques
4.2.1. Self-Assembled Monolayer (SAM) Formation on Metal Electrodes
4.2.2. Covalent Immobilization on an Insulating Layer
4.2.3. Influence of the Conjugation Strategy on the Sensor Performance
4.3. Impact of Surface Cleanliness and Contamination
4.4. Non-Specific Adsorption
4.5. Surface Insulation and Coverage
5. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ertürk, G.; Mattiasson, B. Capacitive Biosensors and Molecularly Imprinted Electrodes. Sensors 2017, 17, 390. [Google Scholar] [CrossRef] [Green Version]
- Victorious, A.; Saha, S.; Pandey, R.; Didar, T.F.; Soleymani, L. Affinity-Based Detection of Biomolecules Using Photo-Electrochemical Readout. Front. Chem. 2019, 7, 617. [Google Scholar] [CrossRef]
- Tsouti, V.; Boutopoulos, C.; Zergioti, I.; Chatzandroulis, S. Capacitive Microsystems for Biological Sensing. Biosens. Bioelectron. 2011, 27, 1–11. [Google Scholar] [CrossRef]
- Wang, L.; Veselinovic, M.; Yang, L.; Geiss, B.J.; Dandy, D.S.; Chen, T. A Sensitive DNA Capacitive Biosensor Using Interdigitated Electrodes. Biosens. Bioelectron. 2017, 87, 646–653. [Google Scholar] [CrossRef] [Green Version]
- Magar, H.S.; Hassan, R.Y.A.; Mulchandani, A. Electrochemical Impedance Spectroscopy (EIS): Principles, Construction, and Biosensing Applications. Sensors 2021, 21, 6578. [Google Scholar] [CrossRef]
- Assaifan, A.K.; Alqahtani, F.A.; Alnamlah, S.; Almutairi, R.; Alkhammash, H.I. Detection and Real-Time Monitoring of LDL-Cholesterol by Redox-Free Impedimetric Biosensors. BioChip J. 2022, 16, 197–206. [Google Scholar] [CrossRef]
- Darain, F.; Park, D.-S.; Park, J.-S.; Shim, Y.-B. Development of an Immunosensor for the Detection of Vitellogenin Using Impedance Spectroscopy. Biosens. Bioelectron. 2004, 19, 1245–1252. [Google Scholar] [CrossRef]
- Qureshi, A.; Gurbuz, Y.; Niazi, J.H. Label-Free Capacitance Based Aptasensor Platform for the Detection of HER2/ErbB2 Cancer Biomarker in Serum. Sens. Actuators B Chem. 2015, 220, 1145–1151. [Google Scholar] [CrossRef]
- Alhoshany, A.; Sivashankar, S.; Mashraei, Y.; Omran, H.; Salama, K.N. A Biosensor-CMOS Platform and Integrated Readout Circuit in 0.18-Μm CMOS Technology for Cancer Biomarker Detection. Sensors 2017, 17, 1942. [Google Scholar] [CrossRef] [Green Version]
- Daniels, J.S.; Pourmand, N. Label-Free Impedance Biosensors: Opportunities and Challenges. Electroanalysis 2007, 19, 1239–1257. [Google Scholar] [CrossRef]
- Qureshi, A.; Niazi, J.H.; Kallempudi, S.; Gurbuz, Y. Label-Free Capacitive Biosensor for Sensitive Detection of Multiple Biomarkers Using Gold Interdigitated Capacitor Arrays. Biosens. Bioelectron. 2010, 25, 2318–2323. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, A.; Gurbuz, Y.; Kallempudi, S.; Niazi, J.H. Label-Free RNA Aptamer-Based Capacitive Biosensor for the Detection of C-Reactive Protein. Phys. Chem. Chem. Phys. 2010, 12, 9176–9182. [Google Scholar] [CrossRef] [PubMed]
- Berggren, C.; Stålhandske, P.; Brundell, J.; Johansson, G. A Feasibility Study of a Capacitive Biosensor for Direct Detection of DNA Hybridization. Electroanalysis 1999, 11, 156–160. [Google Scholar] [CrossRef]
- Carrara, S.; Bhalla, V.; Stagni, C.; Benini, L.; Ferretti, A.; Valle, F.; Gallotta, A.; Riccò, B.; Samorì, B. Label-Free Cancer Markers Detection by Capacitance Biochip. Sens. Actuators B Chem. 2009, 136, 163–172. [Google Scholar] [CrossRef]
- Berggren, C.; Bjarnason, B.; Johansson, G. An Immunological Interleukine-6 Capacitive Biosensor Using Perturbation with a Potentiostatic Step. Biosens. Bioelectron. 1998, 13, 1061–1068. [Google Scholar] [CrossRef]
- Stagni, C.; Guiducci, C.; Benini, L.; Ricco, B.; Carrara, S.; Samori, B.; Paulus, C.; Schienle, M.; Augustyniak, M.; Thewes, R. CMOS DNA Sensor Array with Integrated A/D Conversion Based on Label-Free Capacitance Measurement. IEEE J. Solid-State Circuits 2006, 41, 2956–2964. [Google Scholar] [CrossRef]
- Stagni, C.; Guiducci, C.; Benini, L.; Ricco, B.; Carrara, S.; Paulus, C.; Schienle, M.; Thewes, R. A Fully Electronic Label-Free DNA Sensor Chip. IEEE Sens. J. 2007, 7, 577–585. [Google Scholar] [CrossRef]
- Love, J.C.; Estroff, L.A.; Kriebel, J.K.; Nuzzo, R.G.; Whitesides, G.M. Self-Assembled Monolayers of Thiolates on Metals as a Form of Nanotechnology. Chem. Rev. 2005, 105, 1103–1170. [Google Scholar] [CrossRef]
- Rafael Castiello, F.; Porter, J.; Modarres, P.; Tabrizian, M. Interfacial Capacitance Immunosensing Using Interdigitated Electrodes: The Effect of Insulation/Immobilization Chemistry. Phys. Chem. Chem. Phys. 2019, 21, 15787–15797. [Google Scholar] [CrossRef]
- Berney, H.; Alderman, J.; Lane, W.; Collins, J.K. A Differential Capacitive Biosensor Using Polyethylene Glycol to Overlay the Biolayer. Sens. Actuators B Chem. 1997, 44, 578–584. [Google Scholar] [CrossRef]
- Chen, H.-J.; Chen, R.L.C.; Hsieh, B.-C.; Hsiao, H.-Y.; Kung, Y.; Hou, Y.-T.; Cheng, T.-J. Label-Free and Reagentless Capacitive Aptasensor for Thrombin. Biosens. Bioelectron. 2019, 131, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Berggren, C.; Bjarnason, B.; Johansson, G. Capacitive Biosensors. Electroanalysis 2001, 13, 173–180. [Google Scholar] [CrossRef]
- Kirchhain, A.; Bonini, A.; Vivaldi, F.; Poma, N.; Di Francesco, F. Latest Developments in Non-Faradic Impedimetric Biosensors: Towards Clinical Applications. TrAC Trends Anal. Chem. 2020, 133, 116073. [Google Scholar] [CrossRef]
- Mattiasson, B.; Hedström, M. Capacitive Biosensors for Ultra-Sensitive Assays. TrAC Trends Anal. Chem. 2016, 79, 233–238. [Google Scholar] [CrossRef]
- Prodromidis, M.I. Impedimetric Immunosensors—A Review. Electrochim. Acta 2010, 55, 4227–4233. [Google Scholar] [CrossRef]
- Lum, J.; Wang, R.; Lassiter, K.; Srinivasan, B.; Abi-Ghanem, D.; Berghman, L.; Hargis, B.; Tung, S.; Lu, H.; Li, Y. Rapid Detection of Avian Influenza H5N1 Virus Using Impedance Measurement of Immuno-Reaction Coupled with RBC Amplification. Biosens. Bioelectron. 2012, 38, 67–73. [Google Scholar] [CrossRef]
- Wang, R.; Lin, J.; Lassiter, K.; Srinivasan, B.; Lin, L.; Lu, H.; Tung, S.; Hargis, B.; Bottje, W.; Berghman, L.; et al. Evaluation Study of a Portable Impedance Biosensor for Detection of Avian Influenza Virus. J. Virol. Methods 2011, 178, 52–58. [Google Scholar] [CrossRef]
- Rickert, J.; Göpel, W.; Beck, W.; Jung, G.; Heiduschka, P. A ‘Mixed’ Self-Assembled Monolayer for an Impedimetric Immunosensor. Biosens. Bioelectron. 1996, 11, 757–768. [Google Scholar] [CrossRef]
- Jung, H.-W.; Chang, Y.W.; Lee, G.; Cho, S.; Kang, M.-J.; Pyun, J.-C. A Capacitive Biosensor Based on an Interdigitated Electrode with Nanoislands. Anal. Chim. Acta 2014, 844, 27–34. [Google Scholar] [CrossRef]
- Nasrin, F.; Khoris, I.M.; Chowdhury, A.D.; Boonyakida, J.; Park, E.Y. Impedimetric Biosensor of Norovirus with Low Variance Using Simple Bioconjugation on Conductive Polymer-Au Nanocomposite. Sens. Actuators B Chem. 2022, 369, 132390. [Google Scholar] [CrossRef]
- Frias, I.A.M.; Vega Gonzales Gil, L.H.; Cordeiro, M.T.; Oliveira, M.D.L.; Andrade, C.A.S. Self-Enriching Electrospun Biosensors for Impedimetric Sensing of Zika Virus. ACS Appl. Mater. Interfaces 2022, 14, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-H.; Lee, G.-Y.; Song, Z.; Bong, J.-H.; Chang, Y.W.; Cho, S.; Kang, M.-J.; Pyun, J.-C. Capacitive Biosensor Based on Vertically Paired Electrodes for the Detection of SARS-CoV-2. Biosens. Bioelectron. 2022, 202, 113975. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Oueslati, R.; Wu, J.; Chen, J.; Eda, S. Capacitive DNA Sensor for Rapid and Sensitive Detection of Whole Genome Human Herpesvirus-1 DsDNA in Serum. Electrophoresis 2017, 38, 1617–1623. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Duarte, P.A.; Ma, Y.; Savchenko, O.; Shoute, L.; Khaniani, Y.; Babiuk, S.; Zhuo, R.; Abdelrasoul, G.N.; Charlton, C.; et al. An Impedimetric Biosensor for COVID-19 Serology Test and Modification of Sensor Performance via Dielectrophoresis Force. Biosens. Bioelectron. 2022, 213, 114476. [Google Scholar] [CrossRef] [PubMed]
- Figueroa-Miranda, G.; Liang, Y.; Suranglikar, M.; Stadler, M.; Samane, N.; Tintelott, M.; Lo, Y.; Tanner, J.A.; Vu, X.T.; Knoch, J.; et al. Delineating Charge and Capacitance Transduction in System-Integrated Graphene-Based BioFETs Used as Aptasensors for Malaria Detection. Biosens. Bioelectron. 2022, 208, 114219. [Google Scholar] [CrossRef]
- Figueroa-Miranda, G.; Chen, S.; Neis, M.; Zhou, L.; Zhang, Y.; Lo, Y.; Tanner, J.A.; Kreidenweiss, A.; Offenhäusser, A.; Mayer, D. Multi-Target Electrochemical Malaria Aptasensor on Flexible Multielectrode Arrays for Detection in Malaria Parasite Blood Samples. Sens. Actuators B Chem. 2021, 349, 130812. [Google Scholar] [CrossRef]
- Lenyk, B.; Figueroa-Miranda, G.; Pavlushko, I.; Lo, Y.; Tanner, J.A.; Offenhäusser, A.; Mayer, D. Dual-Transducer Malaria Aptasensor Combining Electrochemical Impedance and Surface Plasmon Polariton Detection on Gold Nanohole Arrays. ChemElectroChem 2020, 7, 4594–4600. [Google Scholar] [CrossRef]
- Figueroa-Miranda, G.; Feng, L.; Shiu, S.C.-C.; Dirkzwager, R.M.; Cheung, Y.-W.; Tanner, J.A.; Schöning, M.J.; Offenhäusser, A.; Mayer, D. Aptamer-Based Electrochemical Biosensor for Highly Sensitive and Selective Malaria Detection with Adjustable Dynamic Response Range and Reusability. Sens. Actuators B Chem. 2018, 255, 235–243. [Google Scholar] [CrossRef]
- Zhou, Y.-M.; Hu, S.-Q.; Cao, Z.-X.; Shen, G.-L.; Yu, R.-Q. Capacitive Immunosensor for the Determination of Schistosoma Japonicum Antigen. Anal. Lett. 2002, 35, 1919–1930. [Google Scholar] [CrossRef]
- Limbut, W.; Hedström, M.; Thavarungkul, P.; Kanatharana, P.; Mattiasson, B. Capacitive Biosensor for Detection of Endotoxin. Anal. Bioanal. Chem. 2007, 389, 517–525. [Google Scholar] [CrossRef]
- DeSilva, M.S.; Zhang, Y.; Hesketh, P.J.; Maclay, G.J.; Gendel, S.M.; Stetter, J.R. Impedance Based Sensing of the Specific Binding Reaction between Staphylococcus Enterotoxin B and Its Antibody on an Ultra-Thin Platinum Film. Biosens. Bioelectron. 1995, 10, 675–682. [Google Scholar] [CrossRef] [PubMed]
- Loyprasert, S.; Hedström, M.; Thavarungkul, P.; Kanatharana, P.; Mattiasson, B. Sub-Attomolar Detection of Cholera Toxin Using a Label-Free Capacitive Immunosensor. Biosens. Bioelectron. 2010, 25, 1977–1983. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A.; Creek, D.J. Discovery and Validation of Clinical Biomarkers of Cancer: A Review Combining Metabolomics and Proteomics. Proteomics 2019, 19, e1700448. [Google Scholar] [CrossRef] [PubMed]
- Durkin, T.J.; Barua, B.; Savagatrup, S. Rapid Detection of Sepsis: Recent Advances in Biomarker Sensing Platforms. ACS Omega 2021, 6, 31390–31395. [Google Scholar] [CrossRef]
- Wang, Y.; Ng, K.; Byrd, R.J.; Hu, J.; Ebadollahi, S.; Daar, Z.; deFilippi, C.; Steinhubl, S.R.; Stewart, W.F. Early Detection of Heart Failure with Varying Prediction Windows by Structured and Unstructured Data in Electronic Health Records. In Proceedings of the 2015 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Milan, Italy, 25–29 August 2015. [Google Scholar] [CrossRef] [Green Version]
- Arya, S.K.; Zhurauski, P.; Jolly, P.; Batistuti, M.R.; Mulato, M.; Estrela, P. Capacitive Aptasensor Based on Interdigitated Electrode for Breast Cancer Detection in Undiluted Human Serum. Biosens. Bioelectron. 2018, 102, 106–112. [Google Scholar] [CrossRef]
- Tanak, A.S.; Jagannath, B.; Tamrakar, Y.; Muthukumar, S.; Prasad, S. Non-Faradaic Electrochemical Impedimetric Profiling of Procalcitonin and C-Reactive Protein as a Dual Marker Biosensor for Early Sepsis Detection. Anal. Chim. Acta X 2019, 3, 100029. [Google Scholar] [CrossRef]
- Macwan, I.; Aphale, A.; Bhagvath, P.; Prasad, S.; Patra, P. Detection of Cardiovascular CRP Protein Biomarker Using a Novel Nanofibrous Substrate. Biosensors 2020, 10, 72. [Google Scholar] [CrossRef]
- Lin, K.-C.; Kunduru, V.; Bothara, M.; Rege, K.; Prasad, S.; Ramakrishna, B.L. Biogenic Nanoporous Silica-Based Sensor for Enhanced Electrochemical Detection of Cardiovascular Biomarkers Proteins. Biosens. Bioelectron. 2010, 25, 2336–2342. [Google Scholar] [CrossRef]
- Chaocharoen, W.; Suginta, W.; Limbut, W.; Ranok, A.; Numnuam, A.; Khunkaewla, P.; Kanatharana, P.; Thavarungkul, P.; Schulte, A. Electrochemical Detection of the Disease Marker Human Chitinase-3-like Protein 1 by Matching Antibody-Modified Gold Electrodes as Label-Free Immunosensors. Bioelectrochemistry 2015, 101, 106–113. [Google Scholar] [CrossRef]
- Chen, C.; Gopinath, S.C.B.; Anbu, P. Longitudinal Zeolite-Iron Oxide Nanocomposite Deposited Capacitance Biosensor for Interleukin-3 in Sepsis Detection. Nanoscale Res. Lett. 2021, 16, 68. [Google Scholar] [CrossRef]
- Liu, Z.; Huang, S.; Jiang, D.; Liu, B.; Kong, J. A Novel Capacitive Immunosensor Using Electropolymerized Insulating Poly (O-phenylenediamine) Film on a Glass Carbon Electrode for Probing Transferrin. Anal. Lett. 2004, 37, 2283–2301. [Google Scholar] [CrossRef]
- Park, J.-W.; Saravan Kallempudi, S.; Niazi, J.H.; Gurbuz, Y.; Youn, B.-S.; Gu, M.B. Rapid and Sensitive Detection of Nampt (PBEF/Visfatin) in Human Serum Using an SsDNA Aptamer-Based Capacitive Biosensor. Biosens. Bioelectron. 2012, 38, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.K.; Kim, E.-S.; Mishra, S.; Ganbold, E.; Seong, R.-S.; Kim, Y.M.; Jahng, G.-H.; Rhee, H.Y.; Han, H.-S.; Kim, D.H.; et al. Ultrasensitive Probeless Capacitive Biosensor for Amyloid Beta (Aβ1-42) Detection in Human Plasma Using Interdigitated Electrodes. Biosens. Bioelectron. 2022, 212, 114365. [Google Scholar] [CrossRef] [PubMed]
- Radke, S.M.; Alocilja, E.C. A High Density Microelectrode Array Biosensor for Detection of E. coli O157:H7. Biosens. Bioelectron. 2005, 20, 1662–1667. [Google Scholar] [CrossRef] [PubMed]
- Couniot, N.; Flandre, D.; Francis, L.A.; Afzalian, A. Signal-to-Noise Ratio Optimization for Detecting Bacteria with Interdigitated Microelectrodes. Sens. Actuators B Chem. 2013, 189, 43–51. [Google Scholar] [CrossRef]
- Brosel-Oliu, S.; Mergel, O.; Uria, N.; Abramova, N.; van Rijn, P.; Bratov, A. 3D Impedimetric Sensors as a Tool for Monitoring Bacterial Response to Antibiotics. Lab Chip 2019, 19, 1436–1447. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Liu, Y. A Digital Microfluidic Device Integrated with Electrochemical Impedance Spectroscopy for Cell-Based Immunoassay. Biosensors 2022, 12, 330. [Google Scholar] [CrossRef]
- Posseckardt, J.; Schirmer, C.; Kick, A.; Rebatschek, K.; Lamz, T.; Mertig, M. Monitoring of Saccharomyces Cerevisiae Viability by Non-Faradaic Impedance Spectroscopy Using Interdigitated Screen-Printed Platinum Electrodes. Sens. Actuators B Chem. 2018, 255, 3417–3424. [Google Scholar] [CrossRef]
- Hwang, C.; Park, N.; Kim, E.S.; Kim, M.; Kim, S.D.; Park, S.; Kim, N.Y.; Kim, J.H. Ultra-Fast and Recyclable DNA Biosensor for Point-of-Care Detection of SARS-CoV-2 (COVID-19). Biosens. Bioelectron. 2021, 185, 113177. [Google Scholar] [CrossRef]
- Shojaei, S.; Ali, M.S.; Suresh, M.; Upreti, T.; Mogourian, V.; Helewa, M.; Labouta, H.I. Dynamic Placenta-on-a-Chip Model for Fetal Risk Assessment of Nanoparticles Intended to Treat Pregnancy-Associated Diseases. Biochim. Biophys. Acta BBA—Mol. Basis Dis. 2021, 1867, 166131. [Google Scholar] [CrossRef]
- Figueroa-Miranda, G.; Wu, C.; Zhang, Y.; Nörbel, L.; Lo, Y.; Tanner, J.A.; Elling, L.; Offenhäusser, A.; Mayer, D. Polyethylene Glycol-Mediated Blocking and Monolayer Morphology of an Electrochemical Aptasensor for Malaria Biomarker Detection in Human Serum. Bioelectrochemistry 2020, 136, 107589. [Google Scholar] [CrossRef] [PubMed]
- Zhurauski, P.; Arya, S.K.; Jolly, P.; Tiede, C.; Tomlinson, D.C.; Ko Ferrigno, P.; Estrela, P. Sensitive and Selective Affimer-Functionalised Interdigitated Electrode-Based Capacitive Biosensor for Her4 Protein Tumour Biomarker Detection. Biosens. Bioelectron. 2018, 108, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Subramani, I.G.; Ayub, R.M.; Gopinath, S.C.B.; Perumal, V.; Fathil, M.F.M.; Md Arshad, M.K. Lectin Bioreceptor Approach in Capacitive Biosensor for Prostate-Specific Membrane Antigen Detection in Diagnosing Prostate Cancer. J. Taiwan Inst. Chem. Eng. 2021, 120, 9–16. [Google Scholar] [CrossRef]
- Liao, W.; Cui, X.T. Reagentless Aptamer Based Impedance Biosensor for Monitoring a Neuro-Inflammatory Cytokine PDGF. Biosens. Bioelectron. 2007, 23, 218–224. [Google Scholar] [CrossRef]
- Kallempudi, S.S.; Gurbuz, Y. A Nanostructured-Nickel Based Interdigitated Capacitive Transducer for Biosensor Applications. Sens. Actuators B Chem. 2011, 160, 891–898. [Google Scholar] [CrossRef]
- Bhalla, V.; Carrara, S.; Sharma, P.; Nangia, Y.; Raman Suri, C. Gold Nanoparticles Mediated Label-Free Capacitance Detection of Cardiac Troponin I. Sens. Actuators B Chem. 2012, 161, 761–768. [Google Scholar] [CrossRef]
- De Vasconcelos, E.A.; Peres, N.G.; Pereira, C.O.; da Silva, V.L.; da Silva, E.F.; Dutra, R.F. Potential of a Simplified Measurement Scheme and Device Structure for a Low Cost Label-Free Point-of-Care Capacitive Biosensor. Biosens. Bioelectron. 2009, 25, 870–876. [Google Scholar] [CrossRef]
- Díaz-Fernández, A.; Bernalte, E.; Fernández-Ramos, C.; Moise, S.; Estrela, P.; Di Lorenzo, M. An Impedimetric Immunosensor for the Selective Detection of CD34+ T-Cells in Human Serum. Sens. Actuators B Chem. 2022, 356, 131306. [Google Scholar] [CrossRef]
- Wu, Z.-S.; Li, J.-S.; Deng, T.; Luo, M.-H.; Shen, G.-L.; Yu, R.-Q. A Sensitive Immunoassay Based on Electropolymerized Films by Capacitance Measurements for Direct Detection of Immunospecies. Anal. Biochem. 2005, 337, 308–315. [Google Scholar] [CrossRef]
- Mirsky, V.M.; Riepl, M.; Wolfbeis, O.S. Capacitive Monitoring of Protein Immobilization and Antigen–Antibody Reactions on Monomolecular Alkylthiol Films on Gold Electrodes. Biosens. Bioelectron. 1997, 12, 977–989. [Google Scholar] [CrossRef]
- Quoc, T.V.; Ngoc, V.N.; Bui, T.T.; Jen, C.-P.; Duc, T.C. High-Frequency Interdigitated Array Electrode-Based Capacitive Biosensor for Protein Detection. BioChip J. 2019, 13, 403–415. [Google Scholar] [CrossRef]
- Luka, G.; Samiei, E.; Dehghani, S.; Johnson, T.; Najjaran, H.; Hoorfar, M. Label-Free Capacitive Biosensor for Detection of Cryptosporidium. Sensors 2019, 19, 258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, K.-H.; Lee, J.-O.; Sohn, M.-J.; Lee, B.; Choi, S.-H.; Kim, S.K.; Yoon, J.-B.; Cho, G.-H. One-Chip Electronic Detection of DNA Hybridization Using Precision Impedance-Based CMOS Array Sensor. Biosens. Bioelectron. 2010, 26, 1373–1379. [Google Scholar] [CrossRef] [PubMed]
- Dijksma, M.; Kamp, B.; Hoogvliet, J.C.; van Bennekom, W.P. Development of an Electrochemical Immunosensor for Direct Detection of Interferon-γ at the Attomolar Level. Anal. Chem. 2001, 73, 901–907. [Google Scholar] [CrossRef] [PubMed]
- Niyomdecha, S.; Limbut, W.; Numnuam, A.; Kanatharana, P.; Charlermroj, R.; Karoonuthaisiri, N.; Thavarungkul, P. Phage-Based Capacitive Biosensor for Salmonella Detection. Talanta 2018, 188, 658–664. [Google Scholar] [CrossRef]
- Wongkittisuksa, B.; Limsakul, C.; Kanatharana, P.; Limbut, W.; Asawatreratanakul, P.; Dawan, S.; Loyprasert, S.; Thavarungkul, P. Development and Application of a Real-Time Capacitive Sensor. Biosens. Bioelectron. 2011, 26, 2466–2472. [Google Scholar] [CrossRef]
- Limbut, W.; Kanatharana, P.; Mattiasson, B.; Asawatreratanakul, P.; Thavarungkul, P. A Comparative Study of Capacitive Immunosensors Based on Self-Assembled Monolayers Formed from Thiourea, Thioctic Acid, and 3-Mercaptopropionic Acid. Biosens. Bioelectron. 2006, 22, 233–240. [Google Scholar] [CrossRef]
- Hedström, M.; Galaev, I.Y.; Mattiasson, B. Continuous Measurements of a Binding Reaction Using a Capacitive Biosensor. Biosens. Bioelectron. 2005, 21, 41–48. [Google Scholar] [CrossRef]
- Yagati, A.K.; Behrent, A.; Beck, S.; Rink, S.; Goepferich, A.M.; Min, J.; Lee, M.-H.; Baeumner, A.J. Laser-Induced Graphene Interdigitated Electrodes for Label-Free or Nanolabel-Enhanced Highly Sensitive Capacitive Aptamer-Based Biosensors. Biosens. Bioelectron. 2020, 164, 112272. [Google Scholar] [CrossRef]
- Varlan, A.R.; Suls, J.; Sansen, W.; Veelaert, D.; De Loof, A. Capacitive Sensor for the Allatostatin Direct Immunoassay. Sens. Actuators B Chem. 1997, 44, 334–340. [Google Scholar] [CrossRef]
- Mantzila, A.G.; Prodromidis, M.I. Performance of Impedimetric Biosensors Based on Anodically Formed Ti/TiO2 Electrodes. Electroanalysis 2005, 17, 1878–1885. [Google Scholar] [CrossRef]
- Choudhury, S.; Nautiyal, R.; Thakkar, D.K.; Betty, C.A. Thickness Dependence of Nanocrystalline Tin Oxide Thin Films in Capacitive Biosensor Characterization. J. Electroanal. Chem. 2020, 877, 114742. [Google Scholar] [CrossRef]
- Wei, F.; Sun, B.; Guo, Y.; Zhao, X.S. Monitoring DNA Hybridization on Alkyl Modified Silicon Surface through Capacitance Measurement. Biosens. Bioelectron. 2003, 18, 1157–1163. [Google Scholar] [CrossRef] [PubMed]
- Betty, C.A. Highly Sensitive Capacitive Immunosensor Based on Porous Silicon-Polyaniline Structure: Bias Dependence on Specificity. Biosens. Bioelectron. 2009, 25, 338–343. [Google Scholar] [CrossRef] [PubMed]
- Gebbert, A.; Alvarez-Icaza, M.; Stoecklein, W.; Schmid, R.D. Real-Time Monitoring of Immunochemical Interactions with a Tantalum Capacitance Flow-through Cell. Anal. Chem. 1992, 64, 997–1003. [Google Scholar] [CrossRef]
- Vericat, C.; Vela, M.E.; Benitez, G.; Carro, P.; Salvarezza, R.C. Self-Assembled Monolayers of Thiols and Dithiols on Gold: New Challenges for a Well-Known System. Chem. Soc. Rev. 2010, 39, 1805–1834. [Google Scholar] [CrossRef]
- Numnuam, A.; Kanatharana, P.; Mattiasson, B.; Asawatreratanakul, P.; Wongkittisuksa, B.; Limsakul, C.; Thavarungkul, P. Capacitive Biosensor for Quantification of Trace Amounts of DNA. Biosens. Bioelectron. 2009, 24, 2559–2565. [Google Scholar] [CrossRef]
- Oliverio, M.; Perotto, S.; Messina, G.C.; Lovato, L.; De Angelis, F. Chemical Functionalization of Plasmonic Surface Biosensors: A Tutorial Review on Issues, Strategies, and Costs. ACS Appl. Mater. Interfaces 2017, 9, 29394–29411. [Google Scholar] [CrossRef] [Green Version]
- Radzicka, A.; Wolfenden, R. Rates of Uncatalyzed Peptide Bond Hydrolysis in Neutral Solution and the Transition State Affinities of Proteases. J. Am. Chem. Soc. 1996, 118, 6105–6109. [Google Scholar] [CrossRef]
- Feng, L.; Lyu, Z.; Offenhäusser, A.; Mayer, D. Electrochemically Triggered Aptamer Immobilization via Click Reaction for Vascular Endothelial Growth Factor Detection. Eng. Life Sci. 2016, 16, 550–559. [Google Scholar] [CrossRef]
- Hayat, A.; Sassolas, A.; Marty, J.-L.; Radi, A.-E. Highly Sensitive Ochratoxin A Impedimetric Aptasensor Based on the Immobilization of Azido-Aptamer onto Electrografted Binary Film via Click Chemistry. Talanta 2013, 103, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Moshari, M.; Koirala, D.; Allen, P.B. Electrochemical Biosensors Based on Divinyl Sulfone Conjugation of DNA to Graphene Oxide Electrodes. J. Solid State Electrochem. 2021, 25, 1667–1678. [Google Scholar] [CrossRef]
- Bhalla, V.; Carrara, S.; Stagni, C.; Samorì, B. Chip Cleaning and Regeneration for Electrochemical Sensor Arrays. Thin Solid Film. 2010, 518, 3360–3366. [Google Scholar] [CrossRef]
- Lichtenberg, J.Y.; Ling, Y.; Kim, S. Non-Specific Adsorption Reduction Methods in Biosensing. Sensors 2019, 19, 2488. [Google Scholar] [CrossRef] [Green Version]
- Zander, Z.K.; Becker, M.L. Antimicrobial and Antifouling Strategies for Polymeric Medical Devices. ACS Macro Lett. 2018, 7, 16–25. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Noy, A. Antifouling Strategies for Protecting Bioelectronic Devices. APL Mater. 2021, 9, 020701. [Google Scholar] [CrossRef]
- Bevilacqua, P.; Nuzzo, S.; Torino, E.; Condorelli, G.; Salvatore, M.; Grimaldi, A.M. Antifouling Strategies of Nanoparticles for Diagnostic and Therapeutic Application: A Systematic Review of the Literature. Nanomaterials 2021, 11, 780. [Google Scholar] [CrossRef]
- Ramburrun, P.; Pringle, N.A.; Dube, A.; Adam, R.Z.; D’Souza, S.; Aucamp, M. Recent Advances in the Development of Antimicrobial and Antifouling Biocompatible Materials for Dental Applications. Materials 2021, 14, 3167. [Google Scholar] [CrossRef]
- Dykstra, P.H.; Roy, V.; Byrd, C.; Bentley, W.E.; Ghodssi, R. Microfluidic Electrochemical Sensor Array for Characterizing Protein Interactions with Various Functionalized Surfaces. Anal. Chem. 2011, 83, 5920–5927. [Google Scholar] [CrossRef]
- Wang, K.; Jiang, D.; Kong, J.; Zhang, S.; Liu, B.; Lu, T. Sensitively Detecting Recombinant Hirudin Variant-2 with Capacitive Immunoassay Based on Self-Assembled Monolayers. Anal. Lett. 2003, 36, 2571–2583. [Google Scholar] [CrossRef]
- Ianeselli, L.; Grenci, G.; Callegari, C.; Tormen, M.; Casalis, L. Development of Stable and Reproducible Biosensors Based on Electrochemical Impedance Spectroscopy: Three-Electrode versus Two-Electrode Setup. Biosens. Bioelectron. 2014, 55, 1–6. [Google Scholar] [CrossRef] [PubMed]






| Target | Sensor Type | Electrode Material | Sensor Preparation | Detection Range | LoD | Ref. | |
|---|---|---|---|---|---|---|---|
| Viral infection | Foot and Mouth disease | Immunosensor | Gold | SAM formation of thiol-modified epitope | N/D | N/D | [28] |
| Hepatitis B | Immunosensor | Gold nanoislands | Parylene coating, followed by glutaraldehyde cross-linking | 0.1–1000 ng/mL | <100 pg/mL in both buffer and serum | [29] | |
| Influenza H5N2 | Immunosensor | Gold | Magnetic nanobeads coated with antibodies | 1.5·101–1.5.105 ELD50/ml | 1.6·102 ELD50/mL of purified virus | [27] | |
| Influenza H5N1 | Immunosensor | Gold | Antibody immobilization through adsorbed Protein A | 101–105 EID50/mL | 103 EID50/mL in buffer | [26] | |
| Norovirus | Immunosensor | Gold | Polyaniline followed by streptavidin coupling and biotinylated Ab immobilization | 1 fg/mL–1 ng/mL | 60 ag/mL in buffer | [30] | |
| Zika virus | Immunosensor | Polyvinyl alcohol, Alignate and Polyaniline | EDC/NHS coupling of antibodies on alginate | N/D | 6.6 × 103 PFU/mL in buffer | [31] | |
| SARS-CoV-2 virus | Immunosensor | Poly(3,4-ethylenedioxythiophene) polystyrene sulfonate | Antibodies adsorption | N/D | 147 TCID50/mL of virus from culture fluid | [32] | |
| Genosensor | Platinium/Titanium | APTES modification, followed by phosphoramidite linkage | 5 µM–10 nM | 10 nM in PBS | [60] | ||
| SARS-CoV-2 antibodies | Immunosensor | Gold | APTES modification, followed by EDC/NHS coupling of antibodies | N/D | 200 ng/mL and 2 μg/mL in buffer (for AuNP and DEP enhancements, respectively) | [34] | |
| Cytomegalovirus | Genosensor | Gold | SAM formation of thiolated oligonucleotides | N/D | 0.2 aM of pure fragment in buffer | [13] | |
| West Nile virus | Genosensor | Gold | SAM formation of thiolated oligonucleotides | 3–33 aM | 1.5 aM in buffer | [4] | |
| Herpes 1 virus | Genosensor | Aluminum | SAM formation of thiolated oligonucleotides | 0.2 fM–0.2 pM in serum | 10.7 aM in buffer 0.21 fM in neat serum | [33] | |
| Papillomavirus | Genosensor | Indium oxide | SAM formation of thiolated oligonucleotides | 0.1 pM–0.1 μM | 20 fM in buffer | [61] | |
| Pathogen markers | Malaria enzyme marker | Aptasensor | Graphene oxide | Graphene modified aptasensors linked to grapehene surface via π- π stacking | 0.78 fM–100 nM | 0.78 fM in diluted human serum | [35] |
| Aptasensor | Gold | Thiolated-aptamers Polyethylene glycol layer added to reduce non-specific adsorption | 1 pM–100 nM | 0.77 fM in 50% human serum 1.49 pM in whole human serum | [62] | ||
| Schistosoma japonicum antigen | Immunosensor | Gold | Antibody immobilization through adsorbed Protein A | 0.4–18 ng/mL | 0.1 ng/mL in PBS | [39] | |
| Cancer biomarkers | SSAT | Immunosensor | Gold/Titanium | Parylene coating, followed by glutaraldehyde cross-linking | 1.25–10 mg/L | 1.25 mg/mL in buffer | [9] |
| Her2 | Aptasensor | Gold | SAM formation of thiol-modified aptamers | 1 pM–100 nM | 0.1 ng/mL in non-diluted serum | [46] | |
| Aptasensor | Gold | SAM formation of mercaptopropionic acid followed by peptide coupling | 0.2–2 ng/mL | 0.2 ng/mL in diluted serum | [8] | ||
| Her4 | Affimer-based sensor | Gold | SAM formation of cysteine-modified aptamers | 1 pM–100 nM | 1 pM in non-diluted serum | [63] | |
| PMSA | Protein-affinity-based sensor | Aluminum electrodes | Carboxylic-modified gold nanoparticles layer formed via thiol-gold bond, followed by peptide coupling | 10 pM–100 nM | 10 pM pure antigens in buffer | [64] | |
| Platelet derived growth factor | Aptasensor | Silicium | APTES modification followed by phosphoramidite bond | 1–50 µg/mL | 1 µg/mL in buffer | [65] | |
| Squamous carcinoma antigen | Immunosensor | Gold | Carboxylic acids introduced via SAM formation, followed by peptide coupling | N/D | 2.43 μg/mL in buffer | [14] | |
| Chronic or inflammatory diseases | Protein C reactive | Immunosensor | Gold | Carboxylic acids introduced via SAM formation, followed by peptide coupling | 25 pg/mL–25 ng/ml | 25 pg/mL in PBS | [11] |
| Aptasensor | Gold | SAM formation of thiol-modified aptamers | 200 pg/mL–2 ng/mL | 200 pg/mL in PBS | [12] | ||
| Immunosensor | Gold | ZnO thin film deposition, followed by succinimidyl propionate crosslinking | 0.01–20 µg/mL. | 0.10 µg/mL in human serum and whole blood | [47] | ||
| Immunosensor | Gold | Carbon fibers sputtered on the electrode, followed by dithiobis(succinimidyl) propionate cross-linking | 1 fg/mL–1 ng/mL | 10 fg/mL in PBS and serum buffer | [48] | ||
| Immunosensor | Nickel | SAM formation of carboxylic acids, followed by | 1–250 ng/ml | 1 ng/mL of purified antigens | [66] | ||
| Myeloperoxidase | Immunosensor | Gold | Immobilization of streptavidin via SAM formation, followed by biotinylated-antibodies conjugation | 1 pg/mL to 1 μg/ml | ~1 pg/mL in buffer | [49] | |
| Troponin | Immunosensor | Screen printed electrode | Gold nanoparticles spread on the electrode, followed by adsorption of the antibody | 0.1–12.5 | 0.2 ng/mL in buffer | [67] | |
| Immunosensor | Alumiium | Amino groups introduced via SAM formation, followed by cross-linking with glutaraldehyde | 0.01–5 ng/mL in PBS buffer 0.07–6.83 ng/mL in human blood serum | 0.01 ng/mL in PBS 0.07 ng/mL in human serum | [68] | ||
| Human chitinase-3-like protein 1 | Immunosensor | Gold | Thiourea introduction via SAM formation, followed by glutaraldehyde cross-liking | 0.1 μg/L–1 mg/L | 0.07 μg/L in buffer | [50] | |
| Transferrin | Immunosensor | Silicon doped semiconductor | Introduction of amine followed by glutaraldehyde cross-linking | N/D | N/D | [20] | |
| Immunosensor | Glass carbon | Electropolymerization of phenylenediamine, followed by glutaraldehyde cross-coupling | 0.1–45.0 ng/mL in | 0.061 ng/mL in PBS | [52] | ||
| Interleukin-3 | Immunosensor | Zeolite-Iron | Amine introduction followed by peptide coupling | 3–100 pg/mL | 3 pg/mL in buffer | [51] | |
| Interleukin-6 | Immunosensor | Gold | Carboxylic acids introduced via SAM formation, followed by peptide coupling | 25 pg/mL–25 ng/ml | 25 pg/mL in PBS | [11] | |
| Nampt | Aptasensor | Gold | SAM thiol aptamers | 1–50 ng/mL | 1 ng/mL in diluted serum | [53] | |
| Amyloid beta | Aptasensor | Platinium/Titanium | Amine introduction followed by peptide coupling | 0.001–10 μM | 1 fg/mL in buffer | [54] | |
| LDL-Cholesterol | Immunosensor | Gold | Amino groups introduced via SAM formation, followed by cross-linking with glutaraldehyde | N/D | 120 pg/mL of pure antigens | [6] | |
| Unicellular organisms | Peripheral blood mononuclear cell | Immunosensor | Gold | Carboxylic groups introduced via SAM formation followed by peptide coupling | N/D | 104 cells/mL in buffer | [34] |
| CD34+ T-cells | Immunosensor | Gold | Carboxylic acids introduced via SAM formation, followed by peptide coupling | 50–1 × 105 cells/mL | 44 cells/mL in diluted serum | [69] | |
| Bacteria answers to antibiotics | / | Tantalum silicide | Polyethyleneimine layer for bacteria adsorption (non-specific) | N/D | N/D | [57] |
| Function Introduced | Coupling Agent | Capture Molecule | Chemical Linkage | Reference |
|---|---|---|---|---|
 3-Triethoxysilylpropylamine |  N-Cyclohexyl-N′-(2-morpholinoethyl)carbodiimide |  Antibody |  Peptide bond | [86] |
 3-Triethoxysilylpropylamine |  EDC/NHS |  Antibody |  Peptide bond | [19] |
 3-Triethoxysilylpropylamine |  N-Cyclohexyl-N′-(2-morpholinoethyl)carbodiimide |  Aptamer |  Peptide bond | [65] |
 3-Triethoxysilylpropylamine |  N-Cyclohexyl-N′-(2-morpholinoethyl)carbodiimide |  ssDNA |  Peptide bond | [60] |
 3-Triethoxysilylpropylamine, Aminobutyldimethylmethoxysilane |  Glutaraldehyde |  Antibody |  Peptide bond | [20] |
 3-Triethoxysilylpropylamine |  Glutaraldehyde |  Avidin |  Peptide bond | [82] |
 Polytyramine |  Glutaraldehyde |  Antibody |  Peptide bond | [70] |
 Polytyramine |  Glutaraldehyde |  Phage |  Peptide bond | [76] |
 Polyaniline |  Glutaraldehyde |  Antibody |  Peptide bond | [85] |
 Polyaniline |  Glutaraldehyde |  Antibody |  Peptide bond | [83] |
 3-Triethoxysilylpropylamine, Aminobutyldimethylmethoxysilane |  Glutaraldehyde |  Antibody |  Peptide bond | [81] |
 Glycidoxypropyldimethylethoxysilane | None |  Antibody |  Epoxide ring-opening | |
 Mercaptopropylmethyldimethoxysilane |  N-y-maleimidobutyryloxy succinimide ester |  Antibody |  Peptide bond | [55] |
 Mercaptopropyltrimethoxysilane |  N-y-maleimidobutyloxy succinimide ester |  Antibody |  Peptide bond | [41] |
 Polydimethylsiloxane |  Dithiobis (succinimidyl propionate) Dithiobis (succinimidyl propionate) |  Antibody |  Peptide bond | [47] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Robin, P.; Gerber-Lemaire, S. Design and Preparation of Sensing Surfaces for Capacitive Biodetection. Biosensors 2023, 13, 17. https://doi.org/10.3390/bios13010017
Robin P, Gerber-Lemaire S. Design and Preparation of Sensing Surfaces for Capacitive Biodetection. Biosensors. 2023; 13(1):17. https://doi.org/10.3390/bios13010017
Chicago/Turabian StyleRobin, Perrine, and Sandrine Gerber-Lemaire. 2023. "Design and Preparation of Sensing Surfaces for Capacitive Biodetection" Biosensors 13, no. 1: 17. https://doi.org/10.3390/bios13010017
APA StyleRobin, P., & Gerber-Lemaire, S. (2023). Design and Preparation of Sensing Surfaces for Capacitive Biodetection. Biosensors, 13(1), 17. https://doi.org/10.3390/bios13010017