Continuous In Vivo Monitoring of Indole-3-Acetic Acid and Salicylic Acid in Tomato Leaf Veins Based on an Electrochemical Microsensor
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Material Preparation
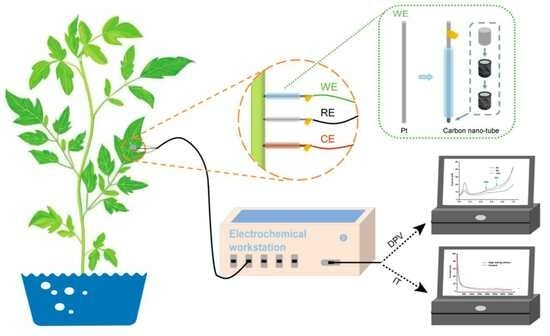
2.3. Preparation of Electrochemical Microsensor
2.4. Electrochemical Measurements
2.5. qRT-PCR Analysis of Gene Expression
3. Results and Discussion
3.1. Characterization of theModified Electrodes
3.2. Performance of the Electrochemical Microsensor for Detecting IAA and SA
3.3. Continuous In Vivo Electrochemical Monitoring of IAA and SA in the Veinsof Tomato Leaves
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Santner, A.; Calderon-Villalobos, L.I.; Estelle, M. Plant hormones are versatile chemical regulators of plant growth. Nat. Chem. Biol. 2009, 5, 301–307. [Google Scholar] [CrossRef]
- Fenn, M.A.; Giovannoni, J.J. Phytohormones in fruit development and maturation. Plant J. 2021, 105, 446–458. [Google Scholar] [CrossRef] [PubMed]
- Blázquez, M.A.; Nelson, D.C.; Weijers, D. Evolution of Plant Hormone Response Pathways. Annu. Rev. Plant Biol. 2020, 71, 327–353. [Google Scholar] [CrossRef]
- Waadt, R.; Seller, C.A.; Hsu, P.K.; Takahashi, Y.; Munemasa, S.; Schroeder, J.I. Plant hormone regulation of abiotic stress responses. Nat. Rev. Mol. Cell Biol. 2022, 23, 680–694. [Google Scholar] [CrossRef] [PubMed]
- Ku, Y.S.; Sintaha, M.; Cheung, M.Y.; Lam, H.M. Plant Hormone Signaling Crosstalks between Biotic and Abiotic Stress Responses. Int. J. Mol. Sci. 2018, 19, 3206. [Google Scholar] [CrossRef] [PubMed]
- Berens, M.L.; Berry, H.M.; Mine, A.; Argueso, C.T.; Tsuda, K. Evolution of Hormone Signaling Networks in Plant Defense. Annu. Rev. Phytopathol. 2017, 55, 401–425. [Google Scholar] [CrossRef]
- Peng, Y.J.; Yang, J.F.; Li, X.; Zhang, Y.L. Salicylic Acid: Biosynthesis and Signaling. Annu. Rev. Plant Biol. 2021, 72, 761–791. [Google Scholar] [CrossRef]
- Ding, P.T.; Ding, Y.L. Stories of Salicylic Acid: A Plant Defense Hormone. Trends Plant Sci. 2020, 25, 549–565. [Google Scholar] [CrossRef]
- Koo, Y.M.; Heo, A.Y.; Choi, H.W. Salicylic Acid as a Safe Plant Protector and Growth Regulator. Plant Pathol. J. 2020, 36, 1–10. [Google Scholar] [CrossRef]
- Pasternak, T.; Groot, E.P.; Kazantsev, F.V.; Teale, W.; Omelyanchuk, N.; Kovrizhnykh, V.; Palme, K.; Mironova, V.V. Salicylic Acid Affects Root Meristem Patterning via Auxin Distribution in a Concentration-Dependent Manner. Plant Physiol. 2019, 180, 1725–1739. [Google Scholar] [CrossRef]
- Enders, T.A.; Strader, L.C. Auxin activity: Past, present, and future. Am. J. Bot. 2015, 102, 180–196. [Google Scholar] [CrossRef]
- Fukui, K.; Hayashi, K.I. Manipulation and Sensing of Auxin Metabolism, Transport and Signaling. Plant Cell Physiol. 2018, 59, 1500–1510. [Google Scholar] [CrossRef] [PubMed]
- Leyser, O. Auxin Signaling. Plant Physiol. 2018, 176, 465–479. [Google Scholar] [CrossRef] [PubMed]
- Gomes, G.L.B.; Scortecci, K.C. Auxin and its role in plant development: Structure, signalling, regulation and response mechanisms. Plant Biol. 2021, 23, 894–904. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Wang, S. Insights into auxin signaling in plant-pathogen interactions. Front. Plant Sci. 2011, 2, 74. [Google Scholar] [CrossRef]
- Rivas-San Vicente, M.; Plasencia, J. Salicylic acid beyond defence: Its role in plant growth and development. J. Exp. Bot. 2011, 62, 3321–3338. [Google Scholar] [CrossRef]
- Wang, D.; Pajerowska-Mukhtar, K.; Culler, A.H.; Dong, X. Salicylic acid inhibits pathogen growth in plants through repression of the auxin signaling pathway. Curr. Biol. 2007, 17, 1784–1790. [Google Scholar] [CrossRef]
- Park, J.E.; Park, J.Y.; Kim, Y.S.; Staswick, P.E.; Jeon, J.; Yun, J.; Kim, S.Y.; Kim, J.; Lee, Y.H.; Park, C.M. GH3-mediated auxin homeostasis links growth regulation with stress adaptation response in Arabidopsis. J. Biol. Chem. 2007, 282, 10036–10046. [Google Scholar] [CrossRef]
- Kong, X.; Zhang, C.; Zheng, H.; Sun, M.; Zhang, F.; Zhang, M.; Cui, F.; Lv, D.; Liu, L.; Guo, S.; et al. Antagonistic Interaction between Auxin and SA Signaling Pathways Regulates Bacterial Infection through Lateral Root in Arabidopsis. Cell Rep. 2020, 32, 108060. [Google Scholar] [CrossRef]
- Pérez-Llorca, M.; Muñoz, P.; Müller, M.; Munné-Bosch, S. Biosynthesis, Metabolism and Function of Auxin, Salicylic Acid and Melatonin in Climacteric and Non-climacteric Fruits. Front. Plant Sci. 2019, 10, 136. [Google Scholar] [CrossRef]
- Du, F.; Ruan, G.; Liu, H. Analytical methods for tracing plant hormones. Anal. Bioanal. Chem. 2012, 403, 55–74. [Google Scholar] [CrossRef]
- Xiao, F.; Wang, L.; Duan, H. Nanomaterial based electrochemical sensors for in vitro detection of small molecule metabolites. Biotechnol. Adv. 2016, 34, 234–249. [Google Scholar] [CrossRef]
- Hashemi, P.; Karimian, N.; Khoshsafar, H.; Arduini, F.; Mesri, M.; Afkhami, A.; Bagheri, H. Reduced graphene oxide decorated on Cu/CuO-Ag nanocomposite as a high-performance material for the construction of a non-enzymatic sensor: Application to the determination of carbaryl and fenamiphos pesticides. Mater. Sci. Eng. C 2019, 102, 764–772. [Google Scholar] [CrossRef] [PubMed]
- Khanmohammadi, A.; Ghazizadeh, A.J.; Hashemi, P.; Afkhami, A.; Arduini, F.; Bagheri, H. An overview to electrochemical biosensors and sensors for the detection of environmental contaminants. J. Iran. Chem. Soc. 2020, 17, 2429–2447. [Google Scholar] [CrossRef]
- Sun, L.J.; Feng, Q.M.; Yan, Y.F.; Pan, Z.Q.; Li, X.H.; Song, F.M.; Yang, H.; Xu, J.J.; Bao, N.; Gu, H.Y. Paper-based electroanalytical devices for in situ determination of salicylic acid in living tomato leaves. Biosens. Bioelectron. 2014, 60, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.J.; Xie, Y.; Yan, Y.F.; Yang, H.; Gu, H.Y.; Bao, N. Paper-based analytical devices for direct electrochemical detection of free IAA and SA in plant samples with the weight of several milligrams. Sens. Actuator. B Chem. 2017, 247, 336–342. [Google Scholar] [CrossRef]
- Sun, L.J.; Zhou, J.J.; Pan, J.L.; Liang, Y.Y.; Fang, Z.J.; Xie, Y.; Yang, H.; Gu, H.Y.; Bao, N. Electrochemical mapping of indole-3-acetic acid and salicylic acid in whole pea seedlings under normal conditions and salinity. Sens. Actuator. B Chem. 2018, 276, 543–551. [Google Scholar] [CrossRef]
- Huo, D.D.; Li, D.D.; Xu, S.Z.; Tang, Y.J.; Xie, X.Q.; Li, D.Y.; Song, F.M.; Zhang, Y.L.; Li, A.; Sun, L.J. Disposable Stainless-Steel Wire-Based Electrochemical Microsensor for In Vivo Continuous Monitoring of Hydrogen Peroxide in Vein of Tomato Leaf. Biosensors 2022, 12, 35. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.T.; Wang, S.C.; Huang, Z.J.; Zhang, S.B.; Liao, Q.G.; Zhang, C.Z.; Lin, T.; Qin, M.; Peng, M.; Yang, C.K.; et al. Rewiring of the Fruit Metabolome in Tomato Breeding. Cell 2018, 172, 249–261. [Google Scholar] [CrossRef] [PubMed]
- Verma, V.; Ravindran, P.; Kumar, P.P. Plant hormone-mediated regulation of stress responses. BMC Plant Biol. 2016, 16, 86. [Google Scholar] [CrossRef]
- Javid, M.G.; Sorooshzadeh, A.; ModarresSanavy, S.A.; Allahdadi, I.; Moradi, F. Effects of the exogenous application of auxin and cytokinin on carbohydrate accumulation in grains of rice under salt stress. Plant Growth Regul. 2011, 65, 305–313. [Google Scholar] [CrossRef]
- Wang, Y.; Mopper, S.; Hasenstein, K.H. Effects of salinity on endogenous ABA, IAA, JA, and SA in Iris hexagona. J. Chem. Ecol. 2001, 27, 327–342. [Google Scholar] [CrossRef] [PubMed]
- Javid, M.G.; Sorooshzadeh, A.; Moradi, F.; Sanavy, S.A.; Allahdadi, I. The Role of Phytohormones in Alleviating Salt Stress in Crop Plants. Aust. J. Crop Sci. 2011, 5, 726–734. [Google Scholar]
- Yu, Z.; Duan, X.; Luo, L.; Dai, S.; Ding, Z.; Xia, G. How Plant Hormones Mediate Salt Stress Responses. Trends Plant Sci. 2020, 25, 1117–1130. [Google Scholar] [CrossRef] [PubMed]
- Iglesias, M.J.; Terrile, M.C.; Bartoli, C.G.; D’Ippólito, S.; Casalongué, C.A. Auxin signaling participates in the adaptative response against oxidative stress and salinity by interacting with redox metabolism in Arabidopsis. Plant Mol. Biol. 2010, 74, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Li, R.J.; Han, T.T.; Cai, W.; Fu, Z.W.; Lu, Y.T. Salt stress reduces root meristem size by nitric oxide-mediated modulation of auxin accumulation and signaling in Arabidopsis. Plant Physiol. 2015, 168, 343–356. [Google Scholar] [CrossRef] [PubMed]
- Iglesias, M.J.; Terrile, M.C.; Windels, D.; Lombardo, M.C.; Bartoli, C.G.; Vazquez, F.; Estelle, M.; Casalongué, C.A. MiR393 regulation of auxin signaling and redox-related components during acclimation to salinity in Arabidopsis. PLoS ONE 2014, 9, e107678. [Google Scholar] [CrossRef]
- Ahanger, M.A.; Aziz, U.; Alsahli, A.A.; Alyemeni, M.N.; Ahmad, P. Influence of Exogenous Salicylic Acid and Nitric Oxide on Growth, Photosynthesis, and Ascorbate-Glutathione Cycle in Salt Stressed Vignaangularis. Biomolecules 2019, 10, 42. [Google Scholar] [CrossRef]
- Palma, F.; López-Gómez, M.; Tejera, N.A.; Lluch, C. Salicylic acid improves the salinity tolerance of Medicago sativa in symbiosis with Sinorhizobiummeliloti by preventing nitrogen fixation inhibition. Plant Sci. 2013, 208, 75–82. [Google Scholar] [CrossRef]
- Borsani, O.; Valpuesta, V.; Botella, M.A. Evidence for a role of salicylic acid in the oxidative damage generated by NaCl and osmotic stress in Arabidopsis seedlings. Plant Physiol. 2001, 126, 1024–1030. [Google Scholar] [CrossRef]
- Hao, L.; Zhao, Y.; Jin, D.D.; Zhang, L.; Bi, X.H.; Chen, H.X.; Xu, Q.; Ma, C.Y.; Li, G.Z. Salicylic acid-altering Arabidopsis mutants response to salt stress. Plant Soil. 2011, 354, 81–95. [Google Scholar] [CrossRef]
- Kwon, H.D.; Song, H.G. Expression of Auxin Response Genes SlIAA1 and SlIAA9 in Solanum lycopersicum During Interaction with Acinetobacter guillouiae SW5. J. Ind. Microbiol. Biot. 2015, 25, 903–909. [Google Scholar] [CrossRef] [PubMed]
- El Oirdi, M.; El Rahman, T.A.; Rigano, L.; El Hadrami, A.; Rodriguez, M.C.; Daayf, F.; Vojnov, A.; Bouarab, K. Botrytis cinerea manipulates the antagonistic effects between immune pathways to promote disease development in tomato. Plant Cell 2011, 23, 2405–2421. [Google Scholar] [CrossRef] [PubMed]








| Gene Name | Primer Set | |
|---|---|---|
| Forward Primer (5′-3′) | Reverse Primer (5′-3′) | |
| SLIAA1 | ATGATGTTTTTCCTGTTAGATCTCACT | TGAATCTAAGTCAAGTTCTGATCATGTC |
| SLNPR1 | GGGAAAGATAGCAGCACG | GTCCACACAAACACACACATC |
| β-actin | GTCCTCTTCCAGCCATCCAT | ACCACTGAGCACAATGTTACCG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, L.; Li, D.; Liu, W.; Sun, Y.; Dai, Y.; Cui, W.; Geng, X.; Li, D.; Song, F.; Sun, L. Continuous In Vivo Monitoring of Indole-3-Acetic Acid and Salicylic Acid in Tomato Leaf Veins Based on an Electrochemical Microsensor. Biosensors 2023, 13, 1002. https://doi.org/10.3390/bios13121002
Tang L, Li D, Liu W, Sun Y, Dai Y, Cui W, Geng X, Li D, Song F, Sun L. Continuous In Vivo Monitoring of Indole-3-Acetic Acid and Salicylic Acid in Tomato Leaf Veins Based on an Electrochemical Microsensor. Biosensors. 2023; 13(12):1002. https://doi.org/10.3390/bios13121002
Chicago/Turabian StyleTang, Lingjuan, Daodong Li, Wei Liu, Yafang Sun, Ying Dai, Wenjing Cui, Xinliu Geng, Dayong Li, Fengming Song, and Lijun Sun. 2023. "Continuous In Vivo Monitoring of Indole-3-Acetic Acid and Salicylic Acid in Tomato Leaf Veins Based on an Electrochemical Microsensor" Biosensors 13, no. 12: 1002. https://doi.org/10.3390/bios13121002
APA StyleTang, L., Li, D., Liu, W., Sun, Y., Dai, Y., Cui, W., Geng, X., Li, D., Song, F., & Sun, L. (2023). Continuous In Vivo Monitoring of Indole-3-Acetic Acid and Salicylic Acid in Tomato Leaf Veins Based on an Electrochemical Microsensor. Biosensors, 13(12), 1002. https://doi.org/10.3390/bios13121002