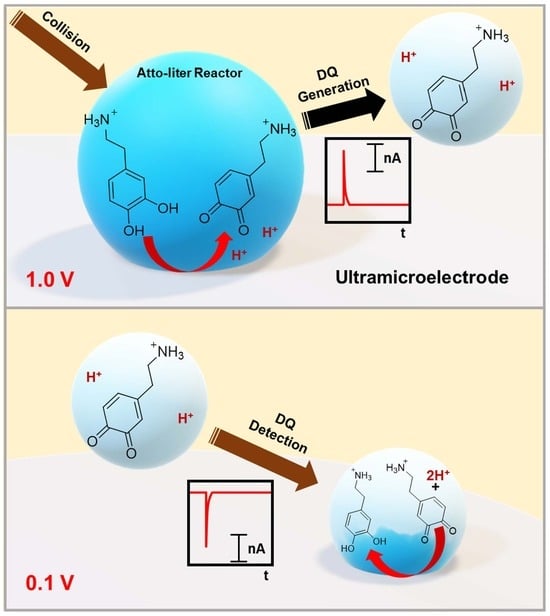
Electrochemical Characterization of Neurotransmitters in a Single Submicron Droplet
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Materials
2.3. Instruments
2.4. Preparation of Metal UMEs
2.5. Preparation of Inverse Emulsion
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Grace, A.A. Dysregulation of the dopamine system in the pathophysiology of schizophrenia and depression. Nat. Rev. Neurosci. 2016, 17, 524–532. [Google Scholar] [CrossRef]
- Speranza, L.; di Porzio, U.; Viggiano, D.; de Donato, A.; Volpicelli, F. Dopamine: The Neuromodulator of Long-Term Synaptic Plasticity, Reward and Movement Control. Cells 2021, 10, 735. [Google Scholar] [CrossRef]
- Bucher, M.L.; Barrett, C.W.; Moon, C.J.; Mortimer, A.D.; Burton, E.A.; Greenamyre, J.T.; Hastings, T.G. Acquired dysregulation of dopamine homeostasis reproduces features of Parkinson’s disease. npj Parkinsons Dis. 2020, 6, 34. [Google Scholar] [CrossRef]
- Ko, M.; Mendecki, L.; Eagleton, A.M.; Durbin, C.G.; Stolz, R.M.; Meng, Z.; Mirica, K.A. Employing Conductive Metal-Organic Frameworks for Voltammetric Detection of Neurochemicals. J. Am. Chem. Soc. 2020, 142, 11717–11733. [Google Scholar] [CrossRef]
- Kalinke, C.; Neumsteir, N.V.; Aparecido, G.D.; Ferraz, T.V.D.; dos Santos, P.L.; Janegitz, B.C.; Bonacin, J.A. Comparison of activation processes for 3D printed PLA-graphene electrodes: Electrochemical properties and application for sensing of dopamine. Analyst 2020, 145, 1207–1218. [Google Scholar] [CrossRef]
- Chang, Y.N.; Shen, C.H.; Huang, C.W.; Tsai, M.D.; Kung, C.W. Defective Metal-Organic Framework Nanocrystals as Signal Amplifiers for Electrochemical Dopamine Sensing. ACS Appl. Nano. Mater. 2023, 6, 3675–3684. [Google Scholar] [CrossRef]
- Tyburski, R.; Liu, T.F.; Glover, S.D.; Hammarström, L. Proton-Coupled Electron Transfer Guidelines, Fair and Square. J. Am. Chem. Soc. 2021, 143, 560–576. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.K.; Wang, D.C.; Zhou, M.; Bae, J.H.; Yu, Y.; Xin, H.L.; Mirkin, M.V. Ultrasensitive Detection of Dopamine with Carbon Nanopipets. Anal. Chem. 2019, 91, 12935–12941. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Park, J.H.; Hwang, S.; Kwak, J. Bench-top fabrication and electrochemical applications of a micro-gap electrode using a microbead spacer. Electrochem. Commun. 2016, 68, 76–80. [Google Scholar] [CrossRef]
- Li, X.; Dunevall, J.; Ewing, A.G. Quantitative Chemical Measurements of Vesicular Transmitters with Electrochemical Cytometry. Acc. Chem. Res. 2016, 49, 2347–2354. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.P.; Perry, D.; Teahan, J.; McPherson, I.J.; Edmondson, J.; Kang, M.; Valavanis, D.; Frenguelli, B.G.; Unwin, P.R. Artificial Synapse: Spatiotemporal Heterogeneities in Dopamine Electrochemistry at a Carbon Fiber Ultramicroelectrode. ACS Meas. Sci. Au 2021, 1, 6–10. [Google Scholar] [CrossRef]
- Bai, J.; Wang, X.J.; Meng, Y.N.; Zhang, H.M.; Qu, L.T. Fabrication of Graphene Coated Carbon Fiber Microelectrode for Highly Sensitive Detection Application. Anal. Sci. 2014, 30, 903–909. [Google Scholar] [CrossRef]
- Zhou, L.; Yang, R.J.; Li, X.R.; Dong, N.; Zhu, B.Y.; Wang, J.J.; Lin, X.Y.; Su, B. COF-Coated Microelectrode for Space-Confined Electrochemical Sensing of Dopamine in Parkinson’s Disease Model Mouse Brain. J. Am. Chem. Soc. 2023, 145, 23727–23738. [Google Scholar] [CrossRef] [PubMed]
- Sáenz, H.S.C.; Hernández-Saravia, L.P.; Selva, J.S.G.; Sukeri, A.; Espinoza-Montero, P.J.; Bertotti, M. Electrochemical dopamine sensor using a nanoporous gold microelectrode: A proof-of-concept study for the detection of dopamine release by scanning electrochemical microscopy. Microchim. Acta 2018, 185, 367. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.Z.; Li, M.N.; Zhang, F.; Zhu, A.W.; Shi, G.Y. Development of Au Disk Nanoelectrode Down to 3 nm in Radius for Detection of Dopamine Release from a Single Cell. Anal. Chem. 2015, 87, 5531–5538. [Google Scholar] [CrossRef]
- Oja, S.M.; Fan, Y.S.; Armstrong, C.M.; Defnet, P.; Zhang, B. Nanoscale Electrochemistry Revisited. Anal. Chem. 2016, 88, 414–430. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.H.T.; Lee, J.; Kim, H.Y.; Nam, K.M.; Kim, B.K. Current research on single-entity electrochemistry for soft nanoparticle detection: Introduction to detection methods and applications. Biosens. Bioelectron. 2020, 151, 111999. [Google Scholar] [CrossRef]
- Xiao, X.; Fan, F.-R.F.; Zhou, J.; Bard, A.J. Current transients in single nanoparticle collision events. J. Am. Chem. Soc. 2008, 130, 16669–16677. [Google Scholar] [CrossRef]
- Kwon, S.J.; Fan, F.R.F.; Bard, A.J. Observing Iridium Oxide (IrOx) Single Nanoparticle Collisions at Ultramicroelectrodes. J. Am. Chem. Soc. 2010, 132, 13165–13167. [Google Scholar] [CrossRef]
- Zhou, H.; Fan, F.-R.F.; Bard, A.J. Observation of discrete Au nanoparticle collisions by electrocatalytic amplification using Pt ultramicroelectrode surface modification. J. Phys. Chem. Lett. 2010, 1, 2671–2674. [Google Scholar] [CrossRef]
- Kwon, S.J.; Zhou, H.J.; Fan, F.R.F.; Vorobyev, V.; Zhang, B.; Bard, A.J. Stochastic electrochemistry with electrocatalytic nanoparticles at inert ultramicroelectrodes-theory and experiments. Phys. Chem. Chem. Phys. 2011, 13, 5394–5402. [Google Scholar] [CrossRef]
- Zhou, Y.G.; Rees, N.V.; Compton, R.G. Nanoparticle–electrode collision processes: The underpotential deposition of thallium on silver nanoparticles in aqueous solution. Chemphyschem 2011, 12, 2085–2087. [Google Scholar] [CrossRef]
- Haddou, B.; Rees, N.V.; Compton, R.G. Nanoparticle–electrode impacts: The oxidation of copper nanoparticles has slow kinetics. Phys. Chem. Chem. Phys. 2012, 14, 13612–13617. [Google Scholar] [CrossRef]
- Sardesai, N.P.; Andreescu, D.; Andreescu, S. Electroanalytical Evaluation of Antioxidant Activity of Cerium Oxide Nanoparticles by Nanoparticle Collisions at Microelectrodes. J. Am. Chem. Soc. 2013, 135, 16770–16773. [Google Scholar] [CrossRef]
- Ma, H.; Chen, J.F.; Wang, H.F.; Hu, P.J.; Ma, W.; Long, Y.T. Exploring dynamic interactions of single nanoparticles at interfaces for surface-confined electrochemical behavior and size measurement. Nat. Commun. 2020, 11, 2307. [Google Scholar] [CrossRef]
- Lin, Y.; Trouillon, R.; Svensson, M.I.; Keighron, J.D.; Cans, A.S.; Ewing, A.G. Carbon-ring microelectrode arrays for electrochemical imaging of single cell exocytosis: Fabrication and characterization. Anal. Chem. 2012, 84, 2949–2954. [Google Scholar] [CrossRef]
- Lee, J.Y.; Kim, B.K.; Kang, M.; Park, J.H. Label-Free Detection of Single Living Bacteria via Electrochemical Collision Event. Sci. Rep. 2016, 6, 30022. [Google Scholar] [CrossRef]
- Bentley, C.L.; Kang, M.; Unwin, P.R. Nanoscale Surface Structure-Activity in Electrochemistry and Electrocatalysis. J. Am. Chem. Soc. 2019, 141, 2179–2193. [Google Scholar] [CrossRef]
- Layman, B.R.; Dick, J.E. Through-Space Electrochemiluminescence Reveals Bubble Forces at Remote Phase Boundaries. J. Am. Chem. Soc. 2024, 146, 707–713. [Google Scholar] [CrossRef]
- Yakushenko, A.; Schnitker, J.; Wolfrum, B. Printed Carbon Microelectrodes for Electrochemical Detection of Single Vesicle Release from PC12 Cells. Anal. Chem. 2012, 84, 4613–4617. [Google Scholar] [CrossRef]
- Lebegue, E.; Anderson, C.M.; Dick, J.E.; Webb, L.J.; Bard, A.J. Electrochemical Detection of Single Phospholipid Vesicle Collisions at a Pt Ultramicroelectrode. Langmuir 2015, 31, 11734–11739. [Google Scholar] [CrossRef]
- Kim, P.; Moon, H.; Park, J.H. Electrochemical Detection of Surfactant-Encapsulated Aqueous Nanodroplets in Organic Solution. Chemosensors 2023, 11, 112. [Google Scholar] [CrossRef]
- Thorgaard, S.N.; Jenkins, S.; Tarach, A.R. Influence of Electroosmotic Flow on Stochastic Collisions at Ultramicroelectrodes. Anal. Chem. 2020, 92, 12663–12669. [Google Scholar] [CrossRef]
- Yang, H.J.; Kwon, H.; Kim, B.K.; Park, J.H. Electrochemical detection of single attoliter aqueous droplets in electrolyte-free organic solvent via collision events. Electrochim. Acta 2019, 320, 134620. [Google Scholar] [CrossRef]
- Jeun, Y.E.; Baek, B.; Lee, M.W.; Ahn, H.S. Surfactant-free electrochemical synthesis of metallic nanoparticles via stochastic collisions of aqueous nanodroplet reactors. Chem. Commun. 2018, 54, 10052–10055. [Google Scholar] [CrossRef]
- Glasscott, M.W.; Pendergast, A.D.; Dick, J.E. A Universal Platform for the Electrodeposition of Ligand-Free Metal Nanoparticles from a Water-in-Oil Emulsion System. ACS Appl. Nano. Mater. 2018, 1, 5702–5711. [Google Scholar] [CrossRef]
- Zhang, H.Z.; Sepunaru, L.; Sokolov, S.V.; Laborda, E.; Batchelor-McAuley, C.; Compton, R.G. Electrochemistry of single droplets of inverse (water-in-oil) emulsions. Phys. Chem. Chem. Phys. 2017, 19, 15662–15666. [Google Scholar] [CrossRef]
- Kim, B.K.; Kim, J.; Bard, A.J. Electrochemistry of a Single Attoliter Emulsion Droplet in Collisions. J. Am. Chem. Soc. 2015, 137, 2343–2349. [Google Scholar] [CrossRef]
- Kim, B.K.; Boika, A.; Kim, J.; Dick, J.E.; Bard, A.J. Characterizing Emulsions by Observation of Single Droplet Collisions-Attoliter Electrochemical Reactors. J. Am. Chem. Soc. 2014, 136, 4849–4852. [Google Scholar] [CrossRef]
- Binks, B.P.; Cho, W.G.; Fletcher, P.D.I.; Petsev, D.N. Stability of oil-in-water emulsions in a low interfacial tension system. Langmuir 2000, 16, 1025–1034. [Google Scholar] [CrossRef]
- Sondaghuethorst, J.A.M.; Fokkink, L.G.J. Potential-Dependent Wetting of Octadecanethiol-Modified Polycrystalline Gold Electrodes. Langmuir 1992, 8, 2560–2566. [Google Scholar] [CrossRef]
- Willman, K.W.; Murray, R.W. Contact-Angle between Water and a Poly(Vinylferrocene) Film on a Potential-Controlled Platinum-Electrode. Anal. Chem. 1983, 55, 1139–1142. [Google Scholar] [CrossRef]
- Madawala, H.; Sabaragamuwe, S.G.; Elangovan, S.; Kim, J. In Situ Measuring Partition Coefficient at Intact Nanoemulsions: A New Application of Single-Entity Electrochemistry. Anal. Chem. 2021, 93, 1154–1160. [Google Scholar] [CrossRef]
- Moon, H.; Park, J.H. Electrochemical Analysis of Attoliter Water Droplets in Organic Solutions through Partitioning Equilibrium. Sensors 2023, 23, 2157. [Google Scholar] [CrossRef]
- Moon, H.; Park, J.H. In Situ Probing Liquid/Liquid Interfacial Kinetics through Single Nanodroplet Electrochemistry. Anal. Chem. 2021, 93, 16915–16921. [Google Scholar] [CrossRef]
- Bacil, R.P.; Chen, L.F.; Serrano, S.H.P.; Compton, R.G. Dopamine oxidation at gold electrodes: Mechanism and kinetics near neutral pH. Phys. Chem. Chem. Phys. 2020, 22, 607–614. [Google Scholar] [CrossRef]
- Park, H.; Park, J.H. In Situ Monitoring of Collision and Recollision Events of Single Attoliter Droplets via Single-Entity Electrochemistry. J. Phys. Chem. Lett. 2020, 11, 10250–10255. [Google Scholar] [CrossRef]
- Delmo, N.; Mostafiz, B.; Ross, A.E.; Suni, J.; Peltola, E. Developing an electrochemical sensor for the measurements of dopamine. Sens. Diagn. 2023, 2, 559–581. [Google Scholar] [CrossRef]
- Dinu, L.A.; Kurbanoglu, S.; Romanitan, C.; Pruneanu, S.; Serban, A.B.; Stoian, M.C.; Pachiu, C.; Craciun, G. Electrodeposited copper nanocubes on multi-layer graphene: A novel nanozyme for ultrasensitive dopamine detection from biological samples. Appl. Surf. Sci. 2022, 604, 154392. [Google Scholar] [CrossRef]
- Moradpour, H.; Beitollahi, H. Simultaneous Electrochemical Sensing of Dopamine, Ascorbic Acid, and Uric Acid Using Nitrogen-Doped Graphene Sheet-Modified Glassy Carbon Electrode. C 2022, 8, 50. [Google Scholar] [CrossRef]
- Ta’alia, S.A.H.; Rohaeti, E.; Putra, B.R.; Wahyuni, W.T. Electrochemical sensors for simultaneous detection of dopamine and uric acid based on a composite of electrochemically reduced graphene oxide and PEDOT:PSS-modified glassy carbon electrode. Results Chem. 2023, 6, 101024. [Google Scholar] [CrossRef]
- Aronson, M.P.; Petko, M.F. Highly Concentrated Water-in-Oil Emulsions—Influence of Electrolyte on Their Properties and Stability. J. Colloid Interface Sci. 1993, 159, 134–149. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods: Fundamentals and Applications, 2nd ed.; Wiley: Hoboken, NJ, USA, 2000; pp. 417–418. [Google Scholar]
- Li, Y.; Deng, H.Q.; Dick, J.E.; Bard, A.J. Analyzing Benzene and Cyclohexane Emulsion Droplet Collisions on Ultramicroelectrodes. Anal. Chem. 2015, 87, 11013–11021. [Google Scholar] [CrossRef]
- Zhou, M.; Gan, S.Y.; Zhong, L.J.; Su, B.; Niu, L. Ion Transfer Voltammetry by a Simple Two Polarized Interfaces Setup. Anal. Chem. 2010, 82, 7857–7860. [Google Scholar] [CrossRef]
- Markin, V.S.; Volkov, A.G. The Gibbs Free-Energy of Ion Transfer between 2 Immiscible Liquids. Electrochim. Acta 1989, 34, 93–107. [Google Scholar] [CrossRef]






| Electrode Material | Focus | Ref. |
|---|---|---|
| Carbon fiber | Scanning ion conductance microscope | [11] |
| Carbon fiber | Graphene-coated microelectrodes | [12] |
| Carbon fiber | Covalent organic framework modified electrode | [13] |
| Gold | Nanoporous electrode | [14] |
| Gold | Nanoscale electrode | [15] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, H.; Park, J.H. Electrochemical Characterization of Neurotransmitters in a Single Submicron Droplet. Biosensors 2024, 14, 102. https://doi.org/10.3390/bios14020102
Park H, Park JH. Electrochemical Characterization of Neurotransmitters in a Single Submicron Droplet. Biosensors. 2024; 14(2):102. https://doi.org/10.3390/bios14020102
Chicago/Turabian StylePark, Heekyung, and Jun Hui Park. 2024. "Electrochemical Characterization of Neurotransmitters in a Single Submicron Droplet" Biosensors 14, no. 2: 102. https://doi.org/10.3390/bios14020102
APA StylePark, H., & Park, J. H. (2024). Electrochemical Characterization of Neurotransmitters in a Single Submicron Droplet. Biosensors, 14(2), 102. https://doi.org/10.3390/bios14020102